Complement Inhibitors Vitronectin and Clusterin Are Recruited from Human Serum to the Surface of Coronavirus OC43-Infected Lung Cells through Antibody-Dependent Mechanisms
Abstract
1. Introduction
2. Materials and Methods
2.1. Cells, Viruses, and Infections
2.2. Flow Cytometry
2.3. Cell Viability Assays
2.4. Western Blotting
2.5. Immunostaining
2.6. Human C5a ELISA
2.7. Statistical Analyses
3. Results
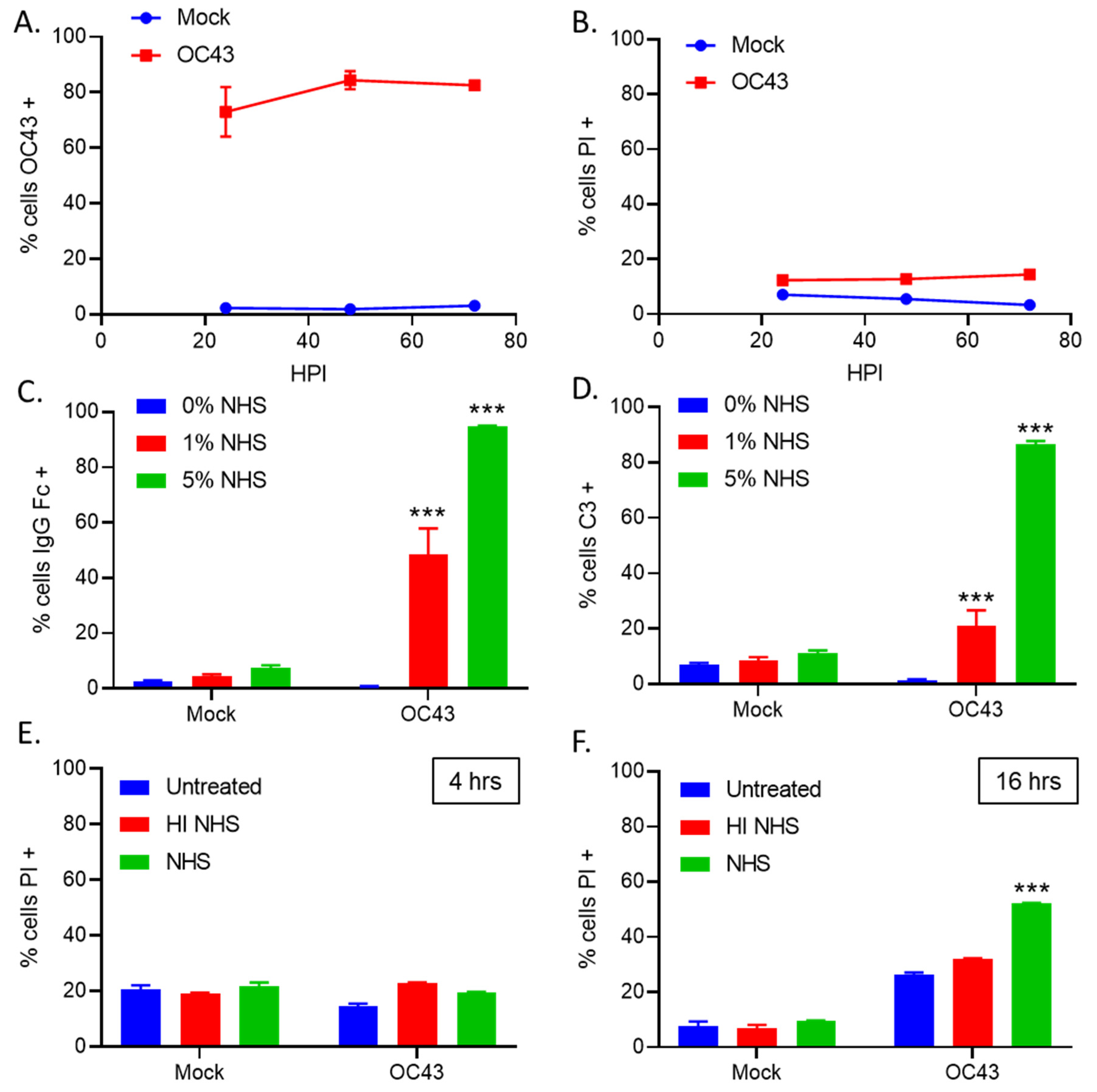
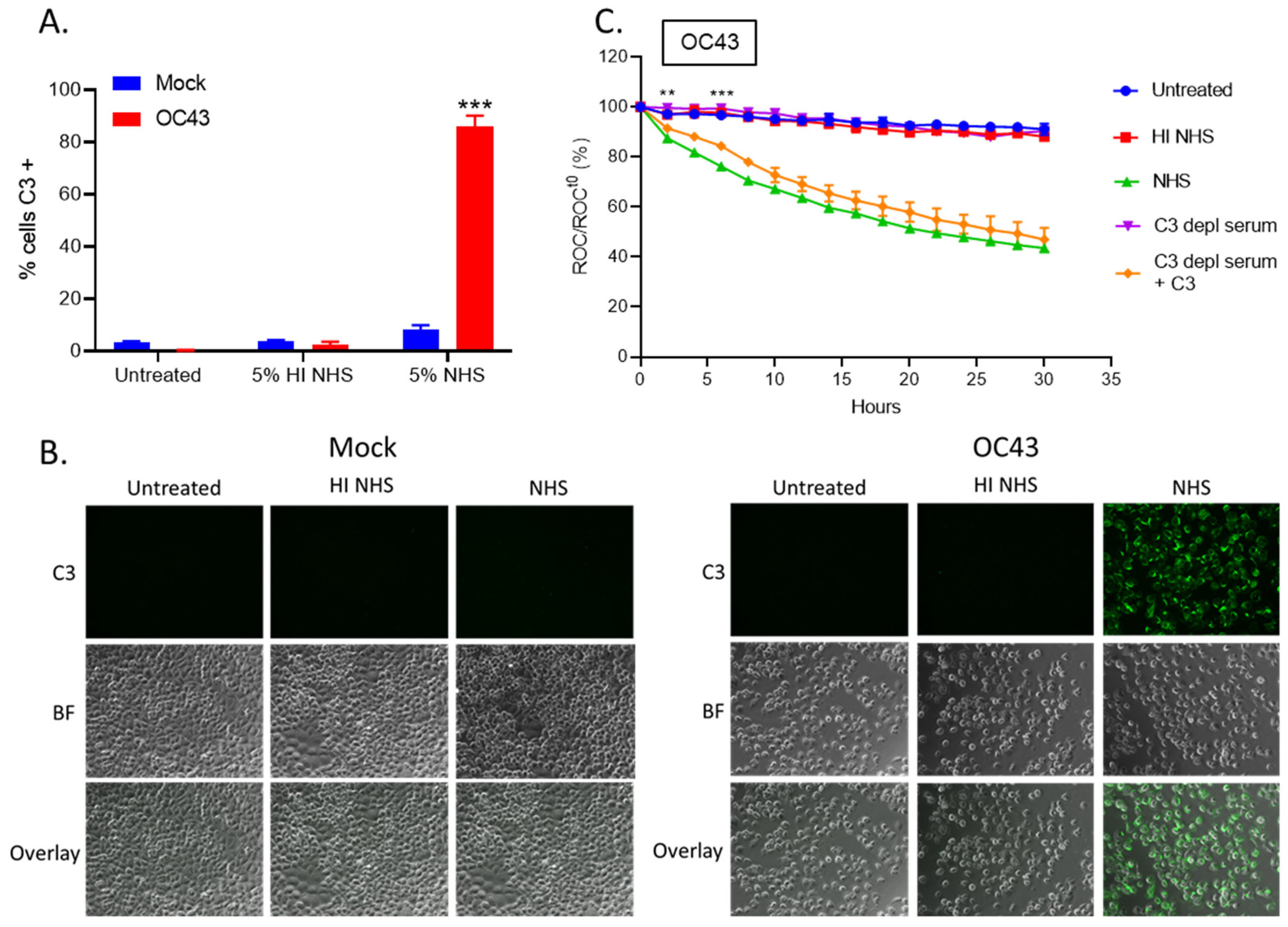
3.1. Treatment of OC43-Infected Human Lung Cells with NHS Results in Deposition of Antibodies and C3
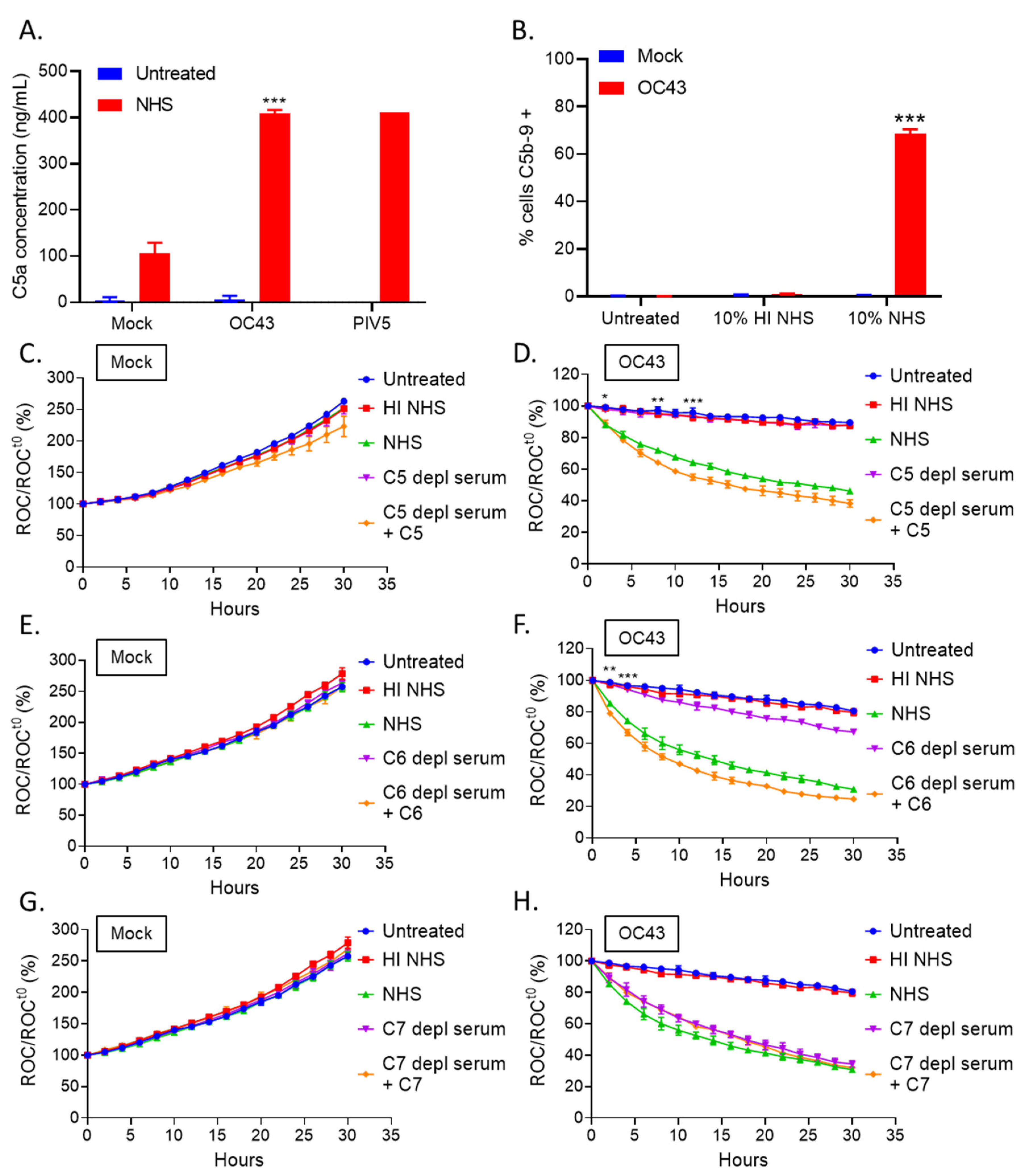
3.2. Real Time Cell Killing Assays Reveal That NHS Lyses OC43 Infected Lung Cells at a Slower Rate Than Parainfluenza Virus Infected Lung Cells and Require Complement C3
3.3. Complement-Mediate Lysis of OC43-Infected Cells Requires C5 and C6, but Not C7
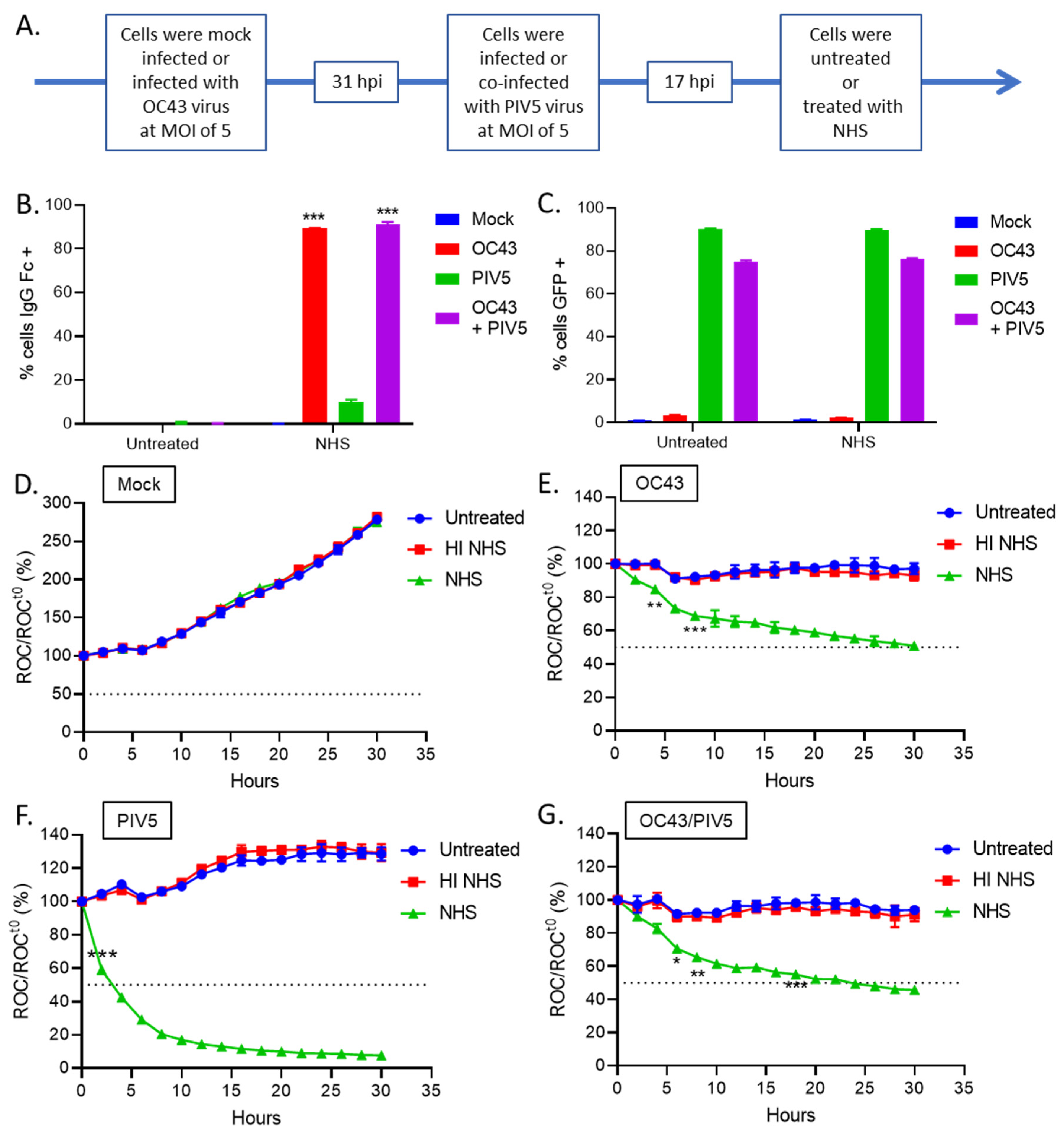
3.4. OC43 Infected Lung Cells Actively Delay C’-Mediated Lysis
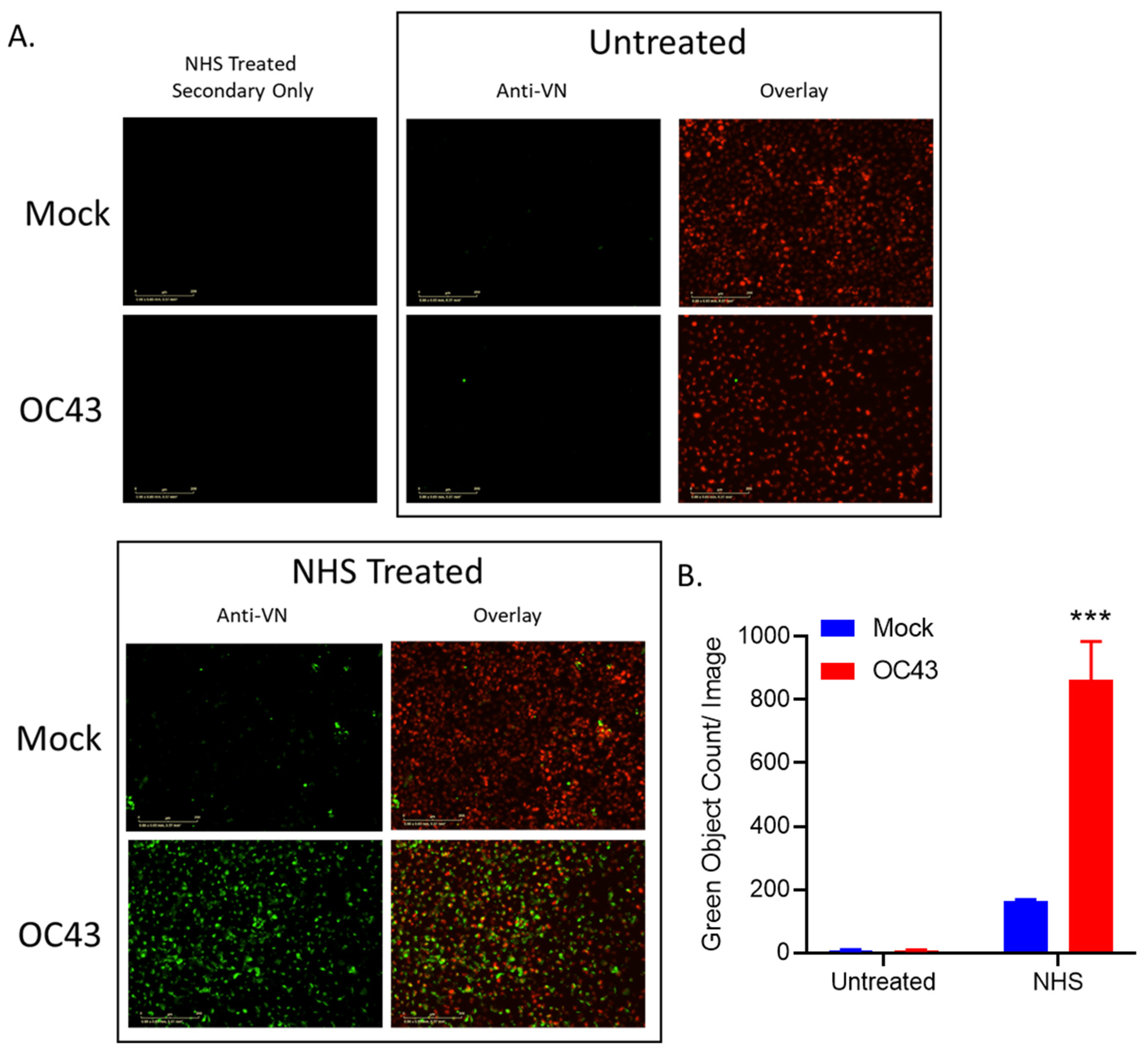
3.5. NHS Treatment of OC43-Infected Human Lung Cells Results in Deposition of C’ Inhibitors VN and CLU
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fendrick, A.M.; Monto, A.S.; Nightengale, B.; Sarnes, M. The economic burden of non-influenza-related viral respiratory tract infection in the United States. Arch. Intern. Med. 2003, 163, 487–494. [Google Scholar] [CrossRef] [PubMed]
- Rucinski, S.L.; Binnicker, M.J.; Thomas, A.S.; Patel, R. Seasonality of Coronavirus 229E, HKU1, NL63, and OC43 From 2014 to 2020. Mayo Clin. Proc. 2020, 95, 1701–1703. [Google Scholar] [CrossRef]
- Blue, C.E.; Spiller, O.B.; Blackbourn, D.J. The relevance of complement to virus biology. Virology 2004, 319, 176–184. [Google Scholar] [CrossRef] [PubMed]
- Carroll, M.C. The complement system in regulation of adaptive immunity. Nat. Immunol. 2004, 5, 981–986. [Google Scholar] [CrossRef] [PubMed]
- Kemper, C.; Atkinson, J.P. T-cell regulation: With complements from innate immunity. Nat. Rev. Immunol. 2007, 7, 9–18. [Google Scholar] [CrossRef]
- Mehlhop, E.; Nelson, S.; Jost, C.A.; Gorlatov, S.; Johnson, S.; Fremont, D.H.; Diamond, M.S.; Pierson, T.C. Complement protein C1q reduces the stoichiometric threshold for antibody-mediated neutralization of West Nile virus. Cell Host Microbe 2009, 6, 381–391. [Google Scholar] [CrossRef]
- Morrison, T.E.; Fraser, R.J.; Smith, P.N.; Mahalingam, S.; Heise, M.T. Complement contributes to inflammatory tissue destruction in a mouse model of Ross River virus-induced disease. J. Virol. 2007, 81, 5132–5143. [Google Scholar] [CrossRef]
- Delgado, M.F.; Polack, F.P. Involvement of antibody, complement and cellular immunity in the pathogenesis of enhanced respiratory syncytial virus disease. Expert Rev. Vaccines 2004, 3, 693–700. [Google Scholar] [CrossRef]
- Stoermer, K.A.; Morrison, T.E. Complement and viral pathogenesis. Virology 2011, 411, 362–373. [Google Scholar] [CrossRef]
- Johnson, J.B.; Aguilar, H.C.; Lee, B.; Parks, G.D. Interactions of human complement with virus particles containing the Nipah virus glycoproteins. J. Virol. 2011, 85, 5940–5948. [Google Scholar] [CrossRef]
- Bergmann-Leitner, E.S.; Leitner, W.W.; Tsokos, G.C. Complement 3d: From molecular adjuvant to target of immune escape mechanisms. Clin. Immunol. 2006, 121, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Schauber-Plewa, C.; Simmons, A.; Tuerk, M.J.; Pacheco, C.D.; Veres, G. Complement regulatory proteins are incorporated into lentiviral vectors and protect particles against complement inactivation. Gene Ther. 2005, 12, 238–245. [Google Scholar] [CrossRef] [PubMed]
- Markiewski, M.M.; Lambris, J.D. The role of complement in inflammatory diseases from behind the scenes into the spotlight. Am. J. Pathol. 2007, 171, 715–727. [Google Scholar] [CrossRef] [PubMed]
- Roozendaal, R.; Carroll, M.C. Emerging patterns in complement-mediated pathogen recognition. Cell 2006, 125, 29–32. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kerr, M.A. The human complement system: Assembly of the classical pathway C3 convertase. Biochem. J. 1980, 189, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, J.P.; Liszewski, M.K.; Richards, A.; Kavanagh, D.; Moulton, E.A. Hemolytic uremic syndrome: An example of insufficient complement regulation on self-tissue. Ann. N. Y. Acad. Sci. 2005, 1056, 144–152. [Google Scholar] [CrossRef]
- Pangburn, M.K.; Schreiber, R.D.; Müller-Eberhard, H.J. Deficiency of an erythrocyte membrane protein with complement regulatory activity in paroxysmal nocturnal hemoglobinuria. Proc. Natl. Acad. Sci. USA 1983, 80, 5430–5434. [Google Scholar] [CrossRef]
- Ahmad, M.; Pyaram, K.; Mullick, J.; Sahu, A. Viral complement regulators: The expert mimicking swindlers. Indian J. Biochem. Biophys. 2007, 44, 331–343. [Google Scholar]
- Bernet, J.; Mullick, J.; Singh, A.K.; Sahu, A. Viral mimicry of the complement system. J. Biosci. 2003, 28, 249–264. [Google Scholar] [CrossRef]
- Lambris, J.D.; Ricklin, D.; Geisbrecht, B.V. Complement evasion by human pathogens. Nat. Rev. Microbiol. 2008, 6, 132–142. [Google Scholar] [CrossRef]
- Cummings, K.L.; Waggoner, S.N.; Tacke, R.; Hahn, Y.S. Role of complement in immune regulation and its exploitation by virus. Viral Immunol. 2007, 20, 505–524. [Google Scholar] [CrossRef] [PubMed]
- Saifuddin, M.; Hedayati, T.; Atkinson, J.P.; Holguin, M.H.; Parker, C.J.; Spear, G.T. Human immunodeficiency virus type 1 incorporates both glycosyl phosphatidylinositol-anchored CD55 and CD59 and integral membrane CD46 at levels that protect from complement-mediated destruction. J. Gen. Virol. 1997, 78 Pt 8, 1907–1911. [Google Scholar] [CrossRef] [PubMed]
- Mazumdar, B.; Kim, H.; Meyer, K.; Bose, S.K.; Di Bisceglie, A.M.; Ray, R.B.; Diamond, M.S.; Atkinson, J.P.; Ray, R. Hepatitis C virus infection upregulates CD55 expression on the hepatocyte surface and promotes association with virus particles. J. Virol. 2013, 87, 7902–7910. [Google Scholar] [CrossRef]
- Biswas, M.; Johnson, J.B.; Kumar, S.R.; Parks, G.D.; Elankumarana, S.; Subbiah, E. Incorporation of host complement regulatory proteins into Newcastle disease virus enhances complement evasion. J. Virol. 2012, 86, 12708–12716. [Google Scholar] [CrossRef] [PubMed]
- Johnson, J.B.; Grant, K.; Parks, G.D. The paramyxoviruses simian virus 5 and mumps virus recruit host cell CD46 to evade complement-mediated neutralization. J. Virol. 2009, 83, 7602–7611. [Google Scholar] [CrossRef]
- Li, Y.; Parks, G.D. Relative Contribution of Cellular Complement Inhibitors CD59, CD46, and CD55 to Parainfluenza Virus 5 Inhibition of Complement-Mediated Neutralization. Viruses 2018, 10, 219. [Google Scholar] [CrossRef]
- Li, Y.; Johnson, J.B.; Parks, G.D. Parainfluenza virus 5 upregulates CD55 expression to produce virions with enhanced resistance to complement-mediated neutralization. Virology 2016, 497, 305–313. [Google Scholar] [CrossRef]
- Hierholzer, J.C.; Killington, R.A. Virus isolation and quantitation. In Virology Methods Manual; Kangro, H.O., Ed.; Academic Press, Inc.: San Diego, CA, USA, 1996; pp. 25–46. [Google Scholar]
- Manuse, M.J.; Parks, G.D. Role for the paramyxovirus genomic promoter in limiting host cell antiviral responses and cell killing. J. Virol. 2009, 83, 9057–9067. [Google Scholar] [CrossRef]
- Varudkar, N.; Oyer, J.L.; Copik, A.; Parks, G.D. Oncolytic parainfluenza virus combines with NK cells to mediate killing of infected and non-infected lung cancer cells within 3D spheroids: Role of type I and type III interferon signaling. J. Immunother. Cancer 2021, 9. [Google Scholar] [CrossRef]
- Johnson, J.B.; Lyles, D.S.; Alexander-Miller, M.A.; Parks, G.D. Virion-associated complement regulator CD55 is more potent than CD46 in mediating resistance of mumps virus and vesicular stomatitis virus to neutralization. J. Virol. 2012, 86, 9929–9940. [Google Scholar] [CrossRef]
- Johnson, J.B.; Schmitt, A.P.; Parks, G.D. Point mutations in the paramyxovirus F protein that enhance fusion activity shift the mechanism of complement-mediated virus neutralization. J. Virol. 2013, 87, 9250–9259. [Google Scholar] [CrossRef][Green Version]
- Parks, G.D.; Manuse, M.J.; Johnson, J.B. The parainfluenza virus siman virus 5. In The Paramyxoviruses; Samal, S., Ed.; Horizon Scientific Press: Norwich, UK, 2010; pp. 37–68. [Google Scholar]
- Plumb, M.E.; Sodetz, J.M. Proteins of the membrane attack complex. In The Human Complement System in Health and Disease; Volanakis, J.E., Frank, M.M., Eds.; Marcel Dekker, Inc.: New York, NY, USA, 1998; pp. 119–148. [Google Scholar]
- Latuszek, A.; Liu, Y.; Olsen, O.; Foster, R.; Cao, M.; Lovric, I.; Yuan, M.; Liu, N.; Chen, H.; Zhang, Q.; et al. Inhibition of complement pathway activation with Pozelimab, a fully human antibody to complement component C5. PLoS ONE 2020, 15, e0231892. [Google Scholar] [CrossRef]
- Liszewski, M.K.; Atkinson, J.P. Regulatory proteins of complement. In The Human Complement System in Health and Disease; Volanakis, J.E., Frank, M.M., Eds.; Marcel Dekker, Inc.: New York, NY, USA, 1998; pp. 149–165. [Google Scholar]
- Gonzalez-Quintela, A.; Alende, R.; Gude, F.; Campos, J.; Rey, J.; Meijide, L.M.; Fernandez-Merino, C.; Vidal, C. Serum levels of immunoglobulins (IgG, IgA, IgM) in a general adult population and their relationship with alcohol consumption, smoking and common metabolic abnormalities. Clin. Exp. Immunol. 2008, 151, 42–50. [Google Scholar] [CrossRef] [PubMed]
- Podack, E.R.; Kolb, W.P.; Müller-Eberhard, H.J. The SC5b-7 complex: Formation, isolation, properties, and subunit composition. J. Immunol. 1977, 119, 2024–2029. [Google Scholar] [PubMed]
- Milis, L.; Morris, C.A.; Sheehan, M.C.; Charlesworth, J.A.; Pussell, B.A. Vitronectin-mediated inhibition of complement: Evidence for different binding sites for C5b-7 and C9. Clin. Exp. Immunol. 1993, 92, 114–119. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.; Su, Y.C.; Riesbeck, K. Vitronectin in bacterial pathogenesis: A host protein used in complement escape and cellular invasion. Mol. Microbiol. 2010, 78, 545–560. [Google Scholar] [CrossRef] [PubMed]
- Arko, R.J.; Chen, C.Y.; Schalla, W.O.; Sarafian, S.K.; Taylor, C.L.; Knapp, J.S.; Morse, S.A. Binding of S protein by Neisseria gonorrhoeae and potential role in invasion. J. Clin. Microbiol. 1991, 29, 70–75. [Google Scholar] [CrossRef]
- Attia, A.S.; Ram, S.; Rice, P.A.; Hansen, E.J. Binding of vitronectin by the Moraxella catarrhalis UspA2 protein interferes with late stages of the complement cascade. Infect. Immun. 2006, 74, 1597–1611. [Google Scholar] [CrossRef]
- Griffiths, N.J.; Hill, D.J.; Borodina, E.; Sessions, R.B.; Devos, N.I.; Feron, C.M.; Poolman, J.T.; Virji, M. Meningococcal surface fibril (Msf) binds to activated vitronectin and inhibits the terminal complement pathway to increase serum resistance. Mol. Microbiol. 2011, 82, 1129–1149. [Google Scholar] [CrossRef]
- Leroy-Dudal, J.; Gagnière, H.; Cossard, E.; Carreiras, F.; Di Martino, P. Role of alphavbeta5 integrins and vitronectin in Pseudomonas aeruginosa PAK interaction with A549 respiratory cells. Microbes Infect. 2004, 6, 875–881. [Google Scholar] [CrossRef]
- Singh, B.; Jalalvand, F.; Mörgelin, M.; Zipfel, P.; Blom, A.M.; Riesbeck, K. Haemophilus influenzae protein E recognizes the C-terminal domain of vitronectin and modulates the membrane attack complex. Mol. Microbiol. 2011, 81, 80–98. [Google Scholar] [CrossRef] [PubMed]
- Hallström, T.; Uhde, M.; Singh, B.; Skerka, C.; Riesbeck, K.; Zipfel, P.F. Pseudomonas aeruginosa Uses Dihydrolipoamide Dehydrogenase (Lpd) to Bind to the Human Terminal Pathway Regulators Vitronectin and Clusterin to Inhibit Terminal Pathway Complement Attack. PLoS ONE 2015, 10, e0137630. [Google Scholar] [CrossRef] [PubMed]
- Akesson, P.; Sjöholm, A.G.; Björck, L. Protein SIC, a novel extracellular protein of Streptococcus pyogenes interfering with complement function. J. Biol. Chem. 1996, 271, 1081–1088. [Google Scholar] [CrossRef]
- Li, D.Q.; Ljungh, A. Binding of human clusterin by Staphylococcus epidermidis. FEMS Immunol. Med. Microbiol. 2001, 31, 197–202. [Google Scholar] [CrossRef] [PubMed]
- Partridge, S.R.; Baker, M.S.; Walker, M.J.; Wilson, M.R. Clusterin, a putative complement regulator, binds to the cell surface of Staphylococcus aureus clinical isolates. Infect. Immun. 1996, 64, 4324–4329. [Google Scholar] [CrossRef]
- Su, H.R.; Boackle, R.J. Heparin mediates binding of S-protein/vitronectin to the envelope glycoprotein of the human immunodeficiency virus and CD4. Int. Arch. Allergy Immunol. 1994, 105, 238–244. [Google Scholar] [CrossRef]
- Huang, Y.P.; Cheng, J.; Zhang, S.L.; Wang, L.; Guo, J.; Liu, Y.; Yang, Y.; Zhang, L.Y.; Bai, G.Q.; Gao, X.S.; et al. Screening of hepatocyte proteins binding to F protein of hepatitis C virus by yeast two-hybrid system. World J. Gastroenterol. 2005, 11, 5659–5665. [Google Scholar] [CrossRef]
- Conde, J.N.; da Silva, E.M.; Allonso, D.; Coelho, D.R.; Andrade, I.D.S.; de Medeiros, L.N.; Menezes, J.L.; Barbosa, A.S.; Mohana-Borges, R. Inhibition of the Membrane Attack Complex by Dengue Virus NS1 through Interaction with Vitronectin and Terminal Complement Proteins. J. Virol. 2016, 90, 9570–9581. [Google Scholar] [CrossRef]
- Kurosu, T.; Chaichana, P.; Yamate, M.; Anantapreecha, S.; Ikuta, K. Secreted complement regulatory protein clusterin interacts with dengue virus nonstructural protein 1. Biochem. Biophys. Res. Commun. 2007, 362, 1051–1056. [Google Scholar] [CrossRef]
- Lin, C.-Y.; Wolf, J.; Brice, D.C.; Sun, Y.; Locke, M.; Cherry, S.; Castellaw, A.H.; Wehenkel, M.; Crawford, J.C.; Zarnitsyna, V.I.; et al. Pre-existing humoral immunity to human common cold coronaviruses negatively impacts the protective SARS-CoV-2 antibody response. Cell Host Microbe 2021, in press. [Google Scholar] [CrossRef]
- Wratil, P.R.; Schmacke, N.A.; Karakoc, B.; Dulovic, A.; Junker, D.; Becker, M.; Rothbauer, U.; Osterman, A.; Spaeth, P.M.; Ruhle, A.; et al. Evidence for increased SARS-CoV-2 susceptibility and COVID-19 severity related to pre-existing immunity to seasonal coronaviruses. Cell Rep. 2021, 110169. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fox, C.R.; Parks, G.D. Complement Inhibitors Vitronectin and Clusterin Are Recruited from Human Serum to the Surface of Coronavirus OC43-Infected Lung Cells through Antibody-Dependent Mechanisms. Viruses 2022, 14, 29. https://doi.org/10.3390/v14010029
Fox CR, Parks GD. Complement Inhibitors Vitronectin and Clusterin Are Recruited from Human Serum to the Surface of Coronavirus OC43-Infected Lung Cells through Antibody-Dependent Mechanisms. Viruses. 2022; 14(1):29. https://doi.org/10.3390/v14010029
Chicago/Turabian StyleFox, Candace R., and Griffith D. Parks. 2022. "Complement Inhibitors Vitronectin and Clusterin Are Recruited from Human Serum to the Surface of Coronavirus OC43-Infected Lung Cells through Antibody-Dependent Mechanisms" Viruses 14, no. 1: 29. https://doi.org/10.3390/v14010029
APA StyleFox, C. R., & Parks, G. D. (2022). Complement Inhibitors Vitronectin and Clusterin Are Recruited from Human Serum to the Surface of Coronavirus OC43-Infected Lung Cells through Antibody-Dependent Mechanisms. Viruses, 14(1), 29. https://doi.org/10.3390/v14010029





