IFN-γ Attenuates Eosinophilic Inflammation but Is Not Essential for Protection against RSV-Enhanced Asthmatic Comorbidity in Adult Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Murine Model of Viral and Bacterial Infection
2.2. Ovalbumin (OVA)-Induced Asthma
2.3. House Dust Mite (HDM)-Induced Asthma
2.4. Plaque Assay
2.5. BALF Collection
2.6. Flow Cytometry
2.7. Evaluation of Airway Damage
2.8. Statistical Analysis
3. Results
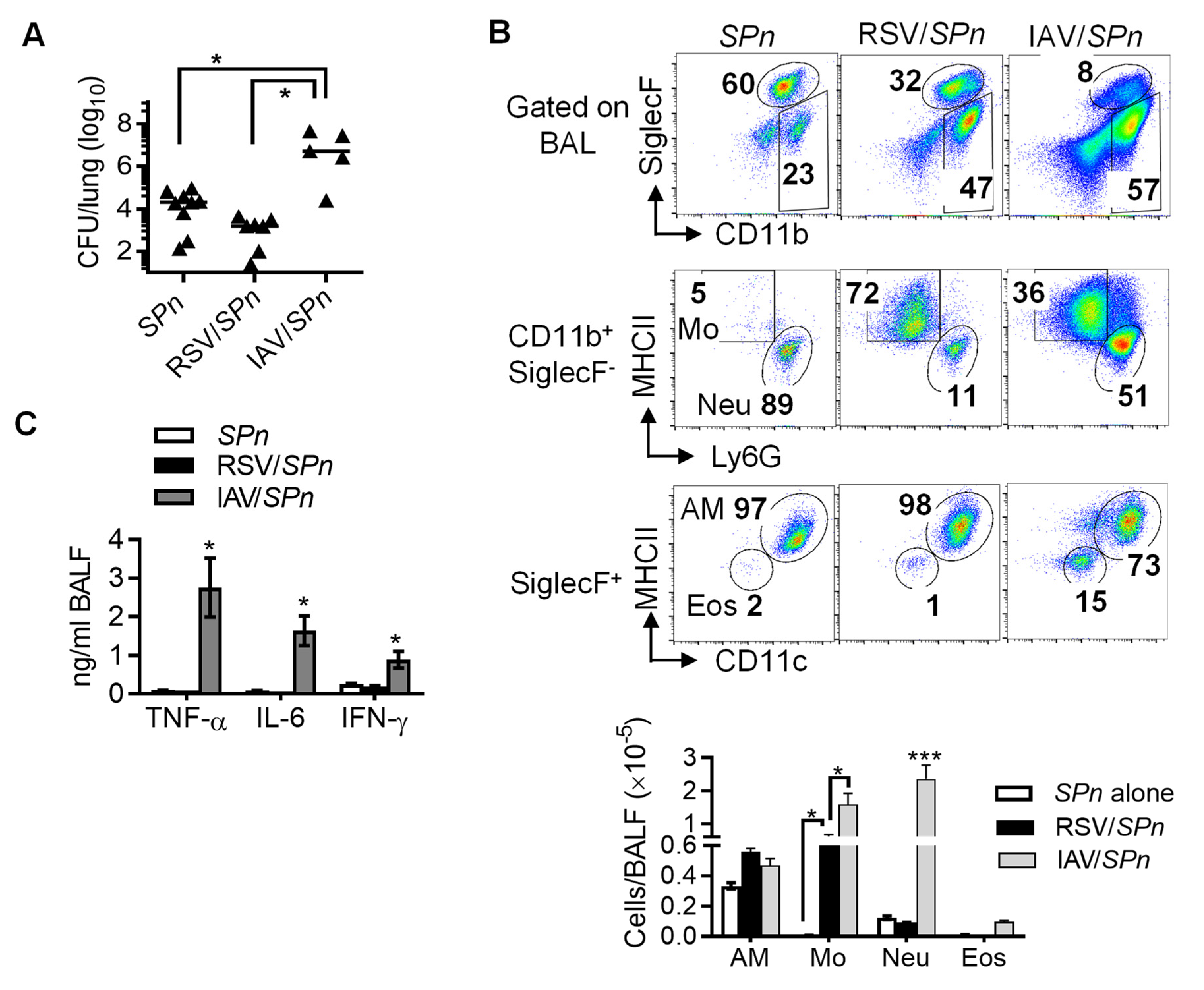
3.1. Prior RSV Infection Does not Have a Significant Impact on Lung Bacterial Clearance during Secondary Pneumococcal Infection
3.2. RSV Infection Induces Neutrophil Recruitment and Increases Acute Lung Damage in OVA-Induced Asthmatic Mice
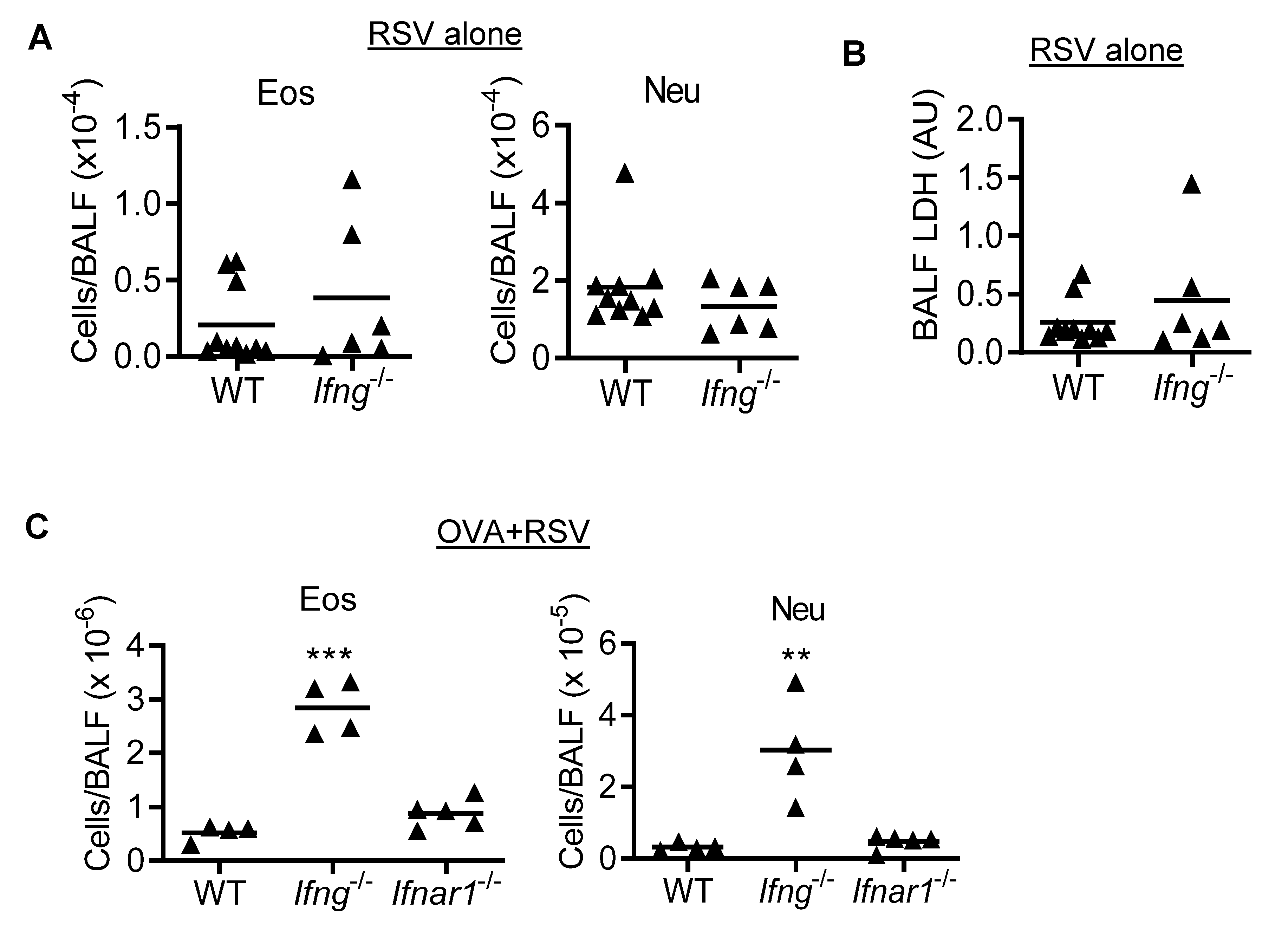
3.3. IFN-γ Inhibits Eosinophilic and Neutrophilic Inflammation during RSV Infection of OVA-Induced Asthmatic Mice
3.4. IFN-γ Inhibits Eosinophil Recruitment during Asthmatic Inflammation in the Absence of RSV Infection
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Calvo, C.; Garcia-Garcia, M.L.; Blanco, C.; Pozo, F.; Flecha, I.C.; Perez-Brena, P. Role of rhinovirus in hospitalized infants with respiratory tract infections in Spain. Pediatr. Infect. Dis. J. 2007, 26, 904–908. [Google Scholar] [CrossRef]
- Hall, C.B.; Weinberg, G.A.; Blumkin, A.K.; Edwards, K.M.; Staat, M.A.; Schultz, A.F.; Poehling, K.A.; Szilagyi, P.G.; Griffin, M.R.; Williams, J.V.; et al. Respiratory syncytial virus-associated hospitalizations among children less than 24 months of age. Pediatrics 2013, 132, e341–e348. [Google Scholar] [CrossRef]
- Falsey, A.R.; Hennessey, P.A.; Formica, M.A.; Cox, C.; Walsh, E.E. Respiratory syncytial virus infection in elderly and high-risk adults. N. Engl. J. Med. 2005, 352, 1749–1759. [Google Scholar] [CrossRef]
- Kitsantas, P.; Nirmalraj, L. Effects of Respiratory Syncytial Virus Infection in Infancy on Asthma and Respiratory Allergy in 6-Year-Old Children. South Med. J. 2018, 111, 698–702. [Google Scholar] [CrossRef]
- Nguyen-Van-Tam, J.; Wyffels, V.; Smulders, M.; Mazumder, D.; Tyagi, R.; Gupta, N.; Gavart, S.; Fleischhackl, R. Cumulative incidence of post-infection asthma or wheezing among young children clinically diagnosed with respiratory syncytial virus infection in the United States: A retrospective database analysis. Influenza Other Respir. Viruses 2020, 14, 730–738. [Google Scholar] [CrossRef]
- Mori, H.; Parker, N.S.; Rodrigues, D.; Hulland, K.; Chappell, D.; Hincks, J.S.; Bright, H.; Evans, S.M.; Lamb, D.J. Differences in respiratory syncytial virus and influenza infection in a house-dust-mite-induced asthma mouse model: Consequences for steroid sensitivity. Clin. Sci. (Lond) 2013, 125, 565–574. [Google Scholar] [CrossRef][Green Version]
- Oo, S.W.C.; Khoo, S.K.; Cox, D.W.; Chidlow, G.; Franks, K.; Prastanti, F.; Bochkov, Y.A.; Borland, M.L.; Zhang, G.; Gern, J.E.; et al. Defining Age-specific Relationships of Respiratory Syncytial Virus and Rhinovirus Species in Hospitalized Children with Acute Wheeze. Pediatr. Infect. Dis. J. 2021, 40, 873–879. [Google Scholar] [CrossRef] [PubMed]
- Raita, Y.; Perez-Losada, M.; Freishtat, R.J.; Harmon, B.; Mansbach, J.M.; Piedra, P.A.; Zhu, Z.; Camargo, C.A.; Hasegawa, K. Integrated omics endotyping of infants with respiratory syncytial virus bronchiolitis and risk of childhood asthma. Nat. Commun. 2021, 12, 3601. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.M.; Miyahara, N.; Takeda, K.; Prpich, J.; Oh, A.; Balhorn, A.; Joetham, A.; Gelfand, E.W.; Dakhama, A. IFN-gamma production during initial infection determines the outcome of reinfection with respiratory syncytial virus. Am. J. Respir. Crit. Care Med. 2008, 177, 208–218. [Google Scholar] [CrossRef] [PubMed]
- Glezen, W.P.; Taber, L.H.; Frank, A.L.; Kasel, J.A. Risk of primary infection and reinfection with respiratory syncytial virus. Am. J. Dis. Child. 1986, 140, 543–546. [Google Scholar] [CrossRef] [PubMed]
- Shibata, T.; Makino, A.; Ogata, R.; Nakamura, S.; Ito, T.; Nagata, K.; Terauchi, Y.; Oishi, T.; Fujieda, M.; Takahashi, Y.; et al. Respiratory syncytial virus infection exacerbates pneumococcal pneumonia via Gas6/Axl-mediated macrophage polarization. J. Clin. Investig. 2020, 130, 3021–3037. [Google Scholar] [CrossRef] [PubMed]
- Sun, K.; Metzger, D.W. Influenza and Staphylococcus aureus Coinfection: TLR9 at Play. Trends Microbiol. 2019, 27, 383–384. [Google Scholar] [CrossRef] [PubMed]
- Metzger, D.W.; Sun, K. Immune dysfunction and bacterial coinfections following influenza. J. Immunol. 2013, 191, 2047–2052. [Google Scholar] [CrossRef] [PubMed]
- Rynda-Apple, A.; Harmsen, A.; Erickson, A.S.; Larson, K.; Morton, R.V.; Richert, L.E.; Harmsen, A.G. Regulation of IFN-gamma by IL-13 dictates susceptibility to secondary postinfluenza MRSA pneumonia. Eur. J. Immunol. 2014, 44, 3263–3272. [Google Scholar] [CrossRef]
- Sun, K.; Metzger, D.W. Inhibition of pulmonary antibacterial defense by interferon-gamma during recovery from influenza infection. Nat. Med. 2008, 14, 558–564. [Google Scholar] [CrossRef]
- Rutigliano, J.A.; Sharma, S.; Morris, M.Y.; Oguin, T.H., 3rd; McClaren, J.L.; Doherty, P.C.; Thomas, P.G. Highly pathological influenza A virus infection is associated with augmented expression of PD-1 by functionally compromised virus-specific CD8+ T cells. J. Virol. 2014, 88, 1636–1651. [Google Scholar] [CrossRef]
- Sun, K.; Johansen, F.E.; Eckmann, L.; Metzger, D.W. An important role for polymeric Ig receptor-mediated transport of IgA in protection against Streptococcus pneumoniae nasopharyngeal carriage. J. Immunol. 2004, 173, 4576–4581. [Google Scholar] [CrossRef] [PubMed]
- Bansal, S.; Yajjala, V.K.; Bauer, C.; Sun, K. IL-1 Signaling Prevents Alveolar Macrophage Depletion during Influenza and Streptococcus pneumoniae Coinfection. J. Immunol. 2018, 200, 1425–1433. [Google Scholar] [CrossRef]
- Kim, D.I.; Song, M.K.; Lee, K. Comparison of asthma phenotypes in OVA-induced mice challenged via inhaled and intranasal routes. BMC Pulm. Med. 2019, 19, 241. [Google Scholar] [CrossRef]
- Woo, L.N.; Guo, W.Y.; Wang, X.; Young, A.; Salehi, S.; Hin, A.; Zhang, Y.; Scott, J.A.; Chow, C.W. A 4-Week Model of House Dust Mite (HDM) Induced Allergic Airways Inflammation with Airway Remodeling. Sci. Rep. 2018, 8, 6925. [Google Scholar] [CrossRef]
- Draijer, C.; Robbe, P.; Boorsma, C.E.; Hylkema, M.N.; Melgert, B.N. Characterization of macrophage phenotypes in three murine models of house-dust-mite-induced asthma. Mediat. Inflamm. 2013, 2013, 632049. [Google Scholar] [CrossRef] [PubMed]
- Polack, F.P.; Teng, M.N.; Collins, P.L.; Prince, G.A.; Exner, M.; Regele, H.; Lirman, D.D.; Rabold, R.; Hoffman, S.J.; Karp, C.L.; et al. A role for immune complexes in enhanced respiratory syncytial virus disease. J. Exp. Med. 2002, 196, 859–865. [Google Scholar] [CrossRef]
- Araujo-Jorge, T.; Rivera, M.T.; el Bouhdidi, A.; Daeron, M.; Carlier, Y. An Fc gamma RII-, Fc gamma RIII-specific monoclonal antibody (2.4G2) decreases acute Trypanosoma cruzi infection in mice. Infect. Immun. 1993, 61, 4925–4928. [Google Scholar] [CrossRef] [PubMed]
- Aun, M.V.; Bonamichi-Santos, R.; Arantes-Costa, F.M.; Kalil, J.; Giavina-Bianchi, P. Animal models of asthma: Utility and limitations. J. Asthma Allergy 2017, 10, 293–301. [Google Scholar] [CrossRef]
- Blume, C.; Davies, D.E. In vitro and ex vivo models of human asthma. Eur. J Pharm Biopharm 2013, 84, 394–400. [Google Scholar] [CrossRef] [PubMed]
- Zosky, G.R.; Sly, P.D. Animal models of asthma. Clin. Exp. Allergy 2007, 37, 973–988. [Google Scholar] [CrossRef]
- Aberle, J.H.; Aberle, S.W.; Rebhandl, W.; Pracher, E.; Kundi, M.; Popow-Kraupp, T. Decreased interferon-gamma response in respiratory syncytial virus compared to other respiratory viral infections in infants. Clin. Exp. Immunol. 2004, 137, 146–150. [Google Scholar] [CrossRef] [PubMed]
- Russell, C.D.; Unger, S.A.; Walton, M.; Schwarze, J. The Human Immune Response to Respiratory Syncytial Virus Infection. Clin. Microbiol. Rev. 2017, 30, 481–502. [Google Scholar] [CrossRef]
- Aoyagi, M.; Shimojo, N.; Sekine, K.; Nishimuta, T.; Kohno, Y. Respiratory syncytial virus infection suppresses IFN-gamma production of gammadelta T cells. Clin. Exp. Immunol. 2003, 131, 312–317. [Google Scholar] [CrossRef]
- Zheng, X.Y.; Xu, Y.J.; Guan, W.J.; Lin, L.F. Regional, age and respiratory-secretion-specific prevalence of respiratory viruses associated with asthma exacerbation: A literature review. Arch. Virol. 2018, 163, 845–853. [Google Scholar] [CrossRef]
- Gregory, L.G.; Lloyd, C.M. Orchestrating house dust mite-associated allergy in the lung. Trends Immunol. 2011, 32, 402–411. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, V.D.; Davidson, C.; Asaduzzaman, M.; Nahirney, D.; Vliagoftis, H. House dust mite interactions with airway epithelium: Role in allergic airway inflammation. Curr. Allergy Asthma Rep. 2013, 13, 262–270. [Google Scholar] [CrossRef] [PubMed]
- Masoli, M.; Fabian, D.; Holt, S.; Beasley, R.; Global Initiative for Asthma, P. The global burden of asthma: Executive summary of the GINA Dissemination Committee report. Allergy 2004, 59, 469–478. [Google Scholar] [CrossRef] [PubMed]
- Stark, J.M.; Stark, M.A.; Colasurdo, G.N.; LeVine, A.M. Decreased bacterial clearance from the lungs of mice following primary respiratory syncytial virus infection. J. Med. Virol. 2006, 78, 829–838. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muralidharan, A.; Uddin, M.B.; Bauer, C.; Wu, W.; Bao, X.; Sun, K. IFN-γ Attenuates Eosinophilic Inflammation but Is Not Essential for Protection against RSV-Enhanced Asthmatic Comorbidity in Adult Mice. Viruses 2022, 14, 147. https://doi.org/10.3390/v14010147
Muralidharan A, Uddin MB, Bauer C, Wu W, Bao X, Sun K. IFN-γ Attenuates Eosinophilic Inflammation but Is Not Essential for Protection against RSV-Enhanced Asthmatic Comorbidity in Adult Mice. Viruses. 2022; 14(1):147. https://doi.org/10.3390/v14010147
Chicago/Turabian StyleMuralidharan, Abenaya, Md Bashir Uddin, Christopher Bauer, Wenzhe Wu, Xiaoyong Bao, and Keer Sun. 2022. "IFN-γ Attenuates Eosinophilic Inflammation but Is Not Essential for Protection against RSV-Enhanced Asthmatic Comorbidity in Adult Mice" Viruses 14, no. 1: 147. https://doi.org/10.3390/v14010147
APA StyleMuralidharan, A., Uddin, M. B., Bauer, C., Wu, W., Bao, X., & Sun, K. (2022). IFN-γ Attenuates Eosinophilic Inflammation but Is Not Essential for Protection against RSV-Enhanced Asthmatic Comorbidity in Adult Mice. Viruses, 14(1), 147. https://doi.org/10.3390/v14010147








