High SARS-CoV-2 Seroprevalence among Healthcare Workers in Bamako, Mali
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Population
2.2. SARS-CoV-2 IgG ELISA
2.3. Validation of the Assay and Determination of Index Values
2.4. Statistical Analysis
3. Results
3.1. Study Population
3.2. Participant Sociodemographic and Clinical Characteristics
3.3. Impact of Occupational Exposure of SARS-CoV-2, Trends among Healthcare Workers in Bamako, Mali
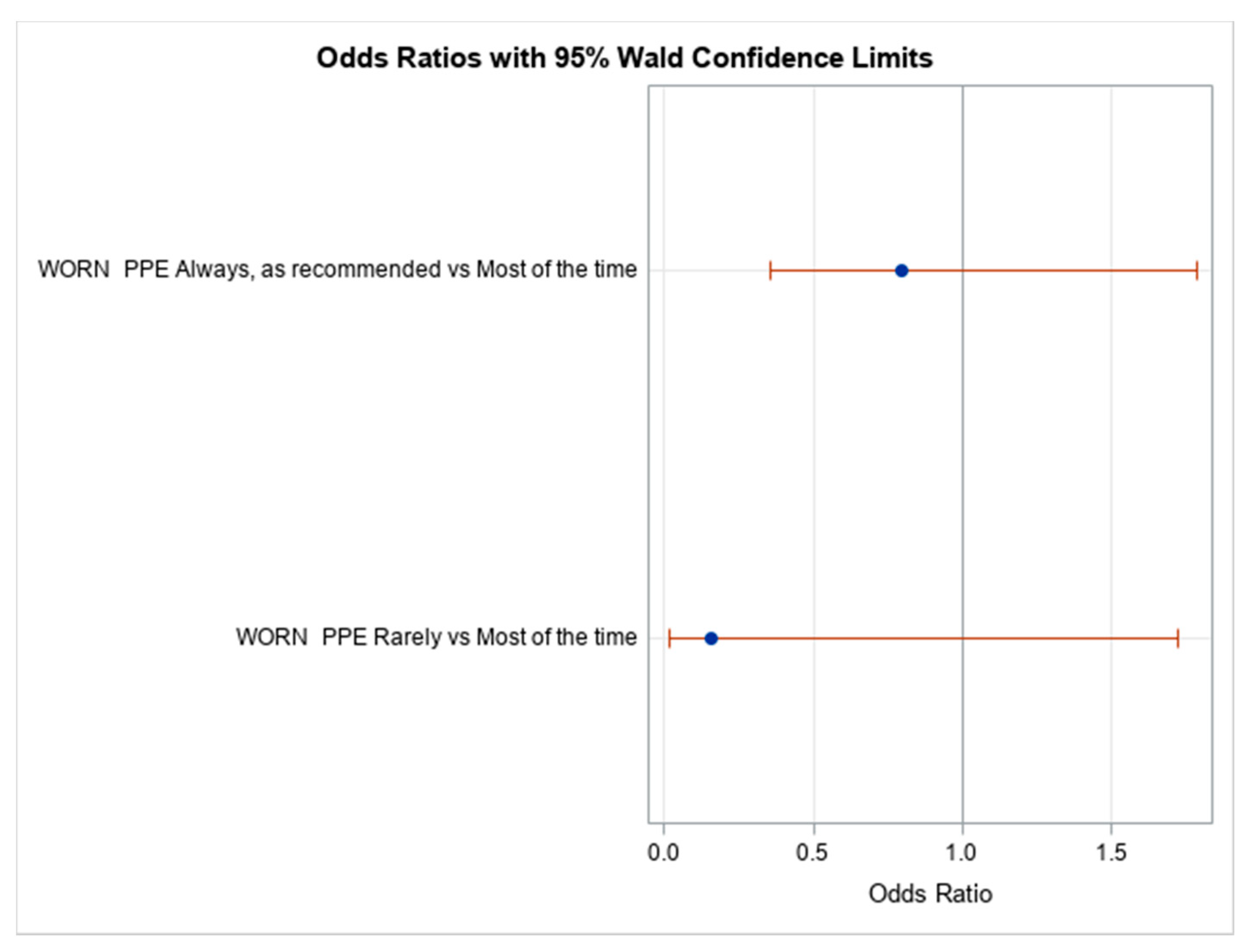
3.3.1. Self-Reported Occupational Exposure to SARS-CoV-2
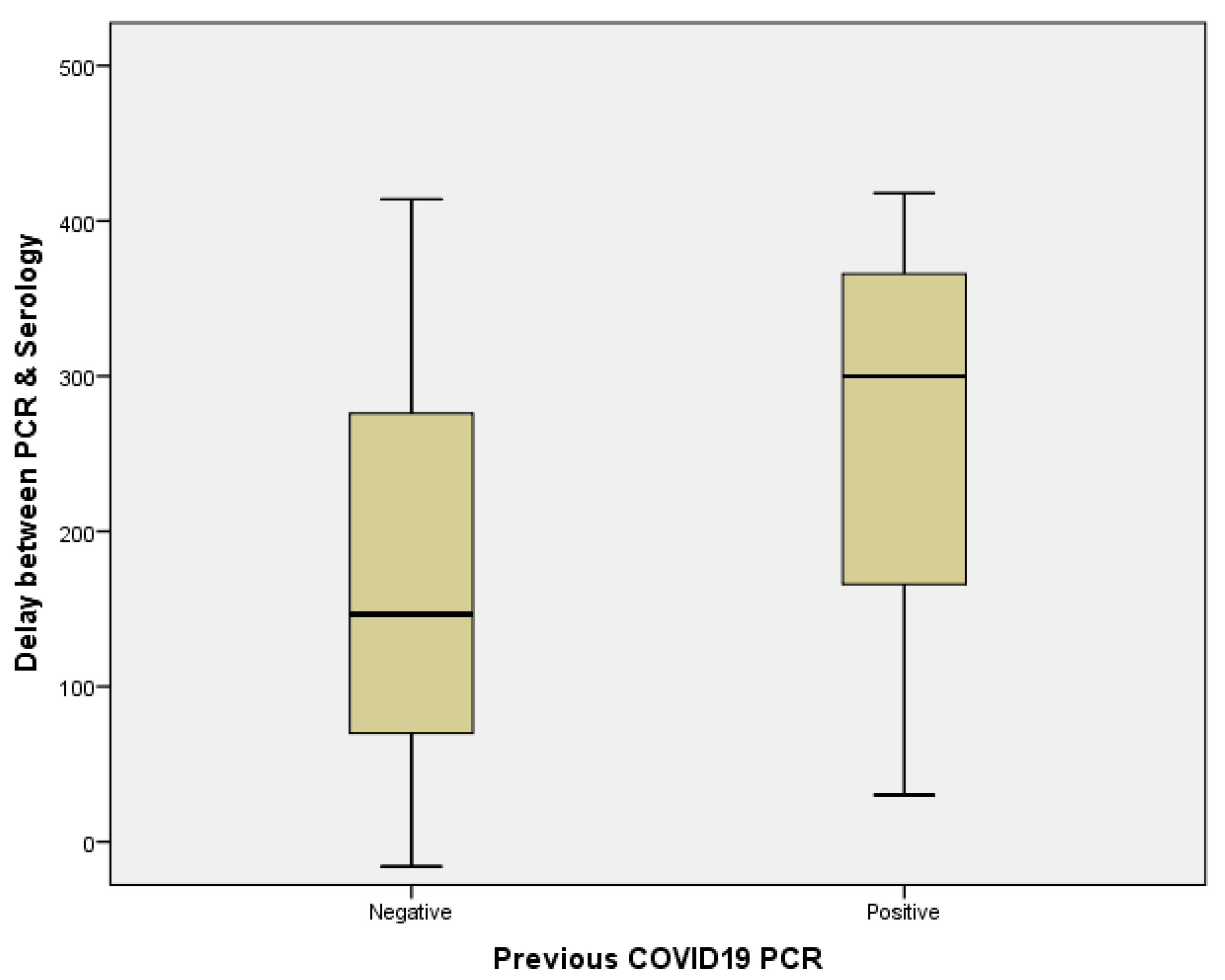
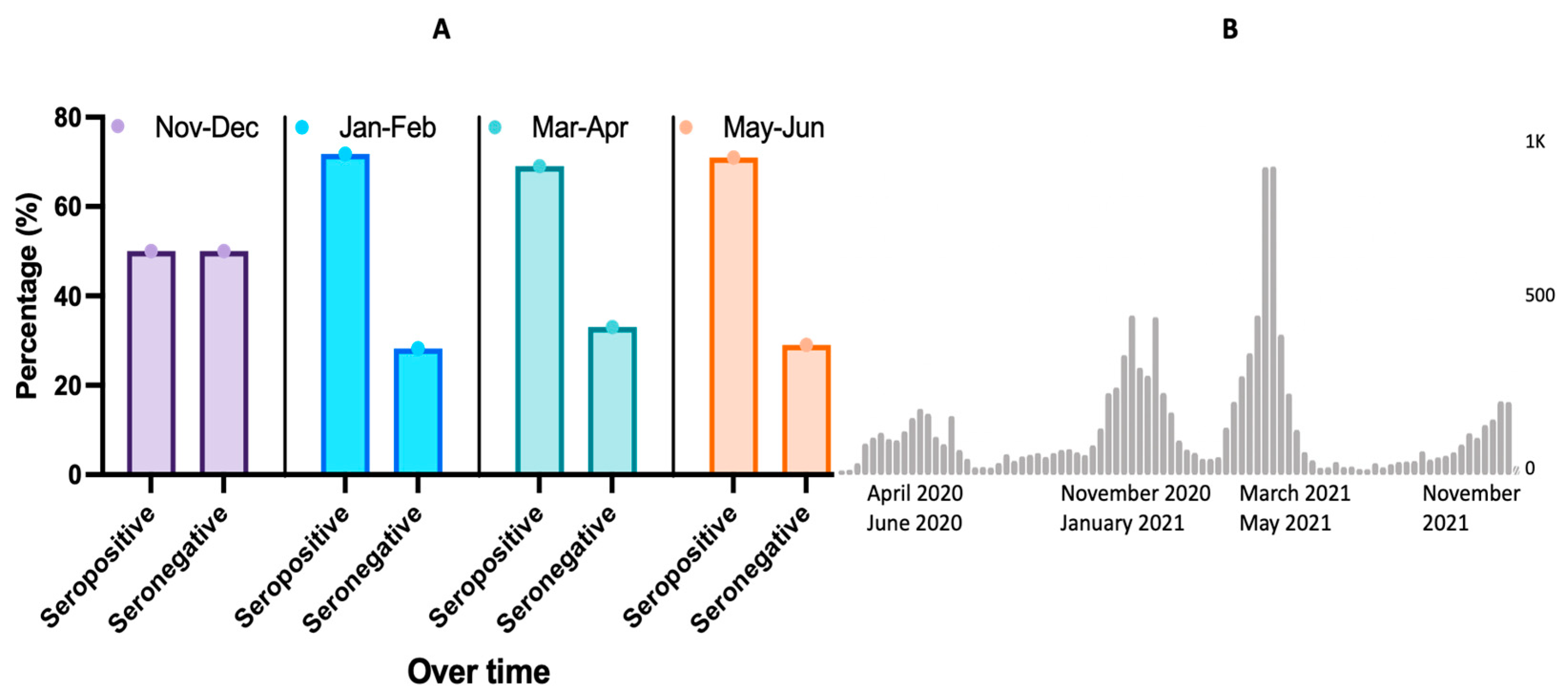
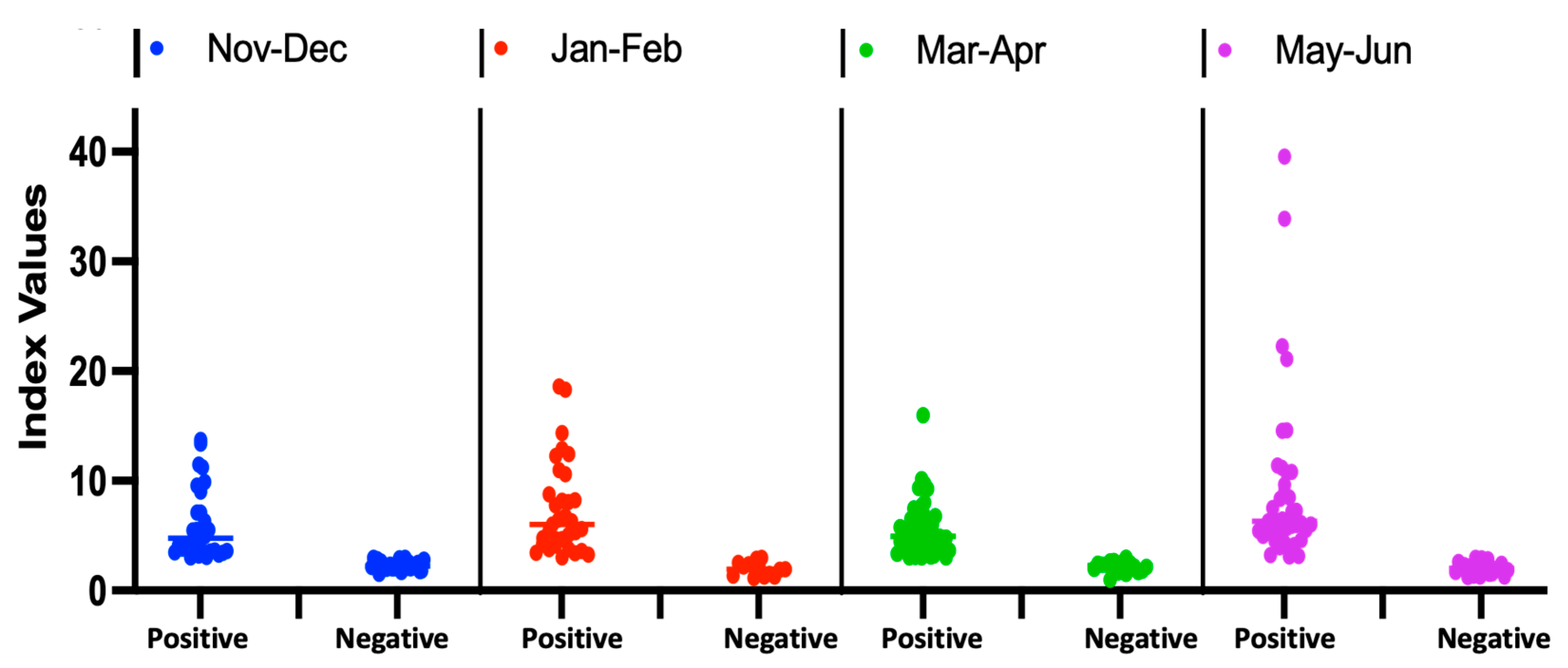
3.3.2. Seroprevalence Trends Overtime
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Hui, D.S.; Azhar, E.I.; Madani, T.A.; Ntoumi, F.; Kock, R.; Dar, O.; Ippolito, G.; Mchugh, T.D.; Memish, Z.A.; Drosten, C. The continuing 2019-nCoV epidemic threat of novel coronaviruses to global health—The latest 2019 novel coronavirus outbreak in Wuhan, China. Int. J. Infect. Dis. 2020, 91, 264–266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Word Health Organisation (WHO). WHO Coronavirus (COVID-19) Dashboard. Available online: https://covid19.who.int (accessed on 24 December 2021).
- Wilkinson, E.; Giovanetti, M.; Tegally, H.; San, J.E.; Lessels, R.; Cuadros, D.; Martin, D.P.; Zekri, A.-R.N.; Sangare, A.K.; Ouedraogo, A.-S.; et al. A year of genomic surveillance reveals how the SARS-CoV-2 pandemic unfolded in Africa. Medrxiv 2021, 374, 423–431. [Google Scholar] [CrossRef] [PubMed]
- Sahu, A.K.; Amrithanand, V.T.; Mathew, R.; Aggarwal, P.; Nayer, J.; Bhoi, S. COVID-19 in health care workers–A systematic review and meta-analysis. Am. J. Emerg. Med. 2020, 38, 1727–1731. [Google Scholar] [CrossRef] [PubMed]
- Nelson, S.M.; Ehnert, S.; Gromski, P.S.; Child, T.; Trew, G. SARS-Cov-2 viral and serological screening of staff in 31 European fertility units. Hum. Reprod. Open 2020, 2020, hoaa056. [Google Scholar] [CrossRef] [PubMed]
- Faller, E.; Wyse, A.; Barry, R.; Conlon, K.; Everard, C.; Finnegan, P.; Foran, C.; Herlihy, E.; Kerr, G.; Lapthorne, S. Seroprevalence study of SARS-CoV-2 antibodies in healthcare workers following the first wave of the COVID-19 pandemic in a tertiary-level hospital in the south of Ireland. BMJ Open 2021, 11, e051415. [Google Scholar] [CrossRef] [PubMed]
- Goldblatt, D.; Johnson, M.; Falup-Pecurariu, O.; Ivaskeviciene, I.; Spoulou, V.; Tamm, E.; Wagner, M.; Zar, H.J.; Bleotu, L.; Ivaskevicius, R. Cross-sectional prevalence of SARS-CoV-2 antibodies in healthcare workers in paediatric facilities in eight countries. J. Hosp. Infect. 2021, 110, 60–66. [Google Scholar] [CrossRef] [PubMed]
- Johnson, C.C.; Coleman, C.M.; Sitarik, A.R.; Leon, J.E.; Tibbetts, R.J.; Cook, B.C.; Muma, B.K.; Weinmann, A.J.; Samuel, L.P. SARS-CoV-2 RT-PCR positivity and antibody prevalence among asymptomatic hospital-based health care workers. J. Clin. Virol. 2021, 140, 104794. [Google Scholar] [CrossRef] [PubMed]
- Yazaki, S.; Yoshida, T.; Kojima, Y.; Yagishita, S.; Nakahama, H.; Okinaka, K.; Matsushita, H.; Shiotsuka, M.; Kobayashi, O.; Iwata, S. Difference in SARS-CoV-2 Antibody Status Between Patients with Cancer and Health Care Workers During the COVID-19 Pandemic in Japan. JAMA Oncol. 2021, 7, 1141–1148. [Google Scholar] [CrossRef] [PubMed]
- Scohy, A.; Gruson, D.; Simon, A.; Kabamba-Mukadi, B.; De Greef, J.; Belkhir, L.; Rodriguez-Villalobos, H.; Robert, A.; Yombi, J.C. Seroprevalence of SARS-CoV-2 infection in health care workers of a teaching hospital in Belgium: Self-reported occupational and household risk factors for seropositivity. Diagn. Microbiol. Infect. Dis. 2021, 100, 115414. [Google Scholar] [CrossRef] [PubMed]
- Wilkins, J.T.; Gray, E.L.; Wallia, A.; Hirschhorn, L.R.; Zembower, T.R.; Ho, J.; Kalume, N.; Agbo, O.; Zhu, A.; Rasmussen-Torvik, L.J. Seroprevalence and correlates of SARS-CoV-2 antibodies in health care workers in Chicago. In Open Forum Infectious Diseases; Oxford University Press: New York, NY, USA, 2021; Volume 8, p. ofaa582. [Google Scholar]
- Mukwege, D.; Byabene, A.K.; Akonkwa, E.M.; Dahma, H.; Dauby, N.; Buhendwa, J.-P.C.; Le Coadou, A.; Montesinos, I.; Bruyneel, M.; Cadière, G.-B. High SARS-CoV-2 Seroprevalence in Healthcare Workers in Bukavu, Eastern Democratic Republic of Congo. Am. J. Trop. Med. Hyg. 2021, 104, 1526. [Google Scholar] [CrossRef] [PubMed]
- Olayanju, O.; Bamidele, O.; Edem, F.; Eseile, B.; Amoo, A.; Nwaokenye, J.; Udeh, C.; Oluwole, G.; Odok, G.; Awah, N. SARS-CoV-2 seropositivity in asymptomatic frontline health workers in Ibadan, Nigeria. Am. J. Trop. Med. Hyg. 2021, 104, 91. [Google Scholar] [CrossRef] [PubMed]
- Rusakaniko, S.; Sibanda, E.N.; Mduluza, T.; Tagwireyi, P.; Dhlamini, Z.; Ndhlovu, C.E.; Chandiwana, P.; Chiwambutsa, S.; Lim, R.M.; Scott, F. SARS-CoV-2 Serological testing in frontline health workers in Zimbabwe. PLoS Negl. Trop. Dis. 2021, 15, e0009254. [Google Scholar] [CrossRef] [PubMed]
- Chisale, M.R.O.; Ramazanu, S.; Mwale, S.E.; Kumwenda, P.; Chipeta, M.; Kaminga, A.C.; Nkhata, O.; Nyambalo, B.; Chavura, E.; Mbakaya, B.C. Seroprevalence of anti-SARS-CoV-2 antibodies in Africa: A systematic review and meta-analysis. Rev. Med. Virol. 2021, e2271. [Google Scholar] [CrossRef] [PubMed]
- Sagara, I.; Woodford, J.; Kone, M.; Assadou, M.H.; Katile, A.; Attaher, O.; Zeguime, A.; Doucoure, M.; Higbee, E.; Lane, J. Rapidly increasing SARS-CoV-2 seroprevalence and limited clinical disease in three Malian communities: A prospective cohort study. medRxiv 2021. [CrossRef]
- Nwosu, K.D.; Fokam, J.; Wanda, F.; Mama, L.; Orel, E.; Ray, N.; Meke, J.; Tassegning, A.; Takou, D.; Mimbe, E. SARS-CoV-2 Antibody Seroprevalence and Associated Risk Factors in an Urban District in Cameroon. 2021. Available online: https://ssrn.com/abstract=3812428 (accessed on 15 November 2021).
- Hunter, B.R.; Dbeibo, L.; Weaver, C.S.; Beeler, C.; Saysana, M.; Zimmerman, M.K.; Weaver, L. Seroprevalence of severe acute respiratory coronavirus virus 2 (SARS-CoV-2) antibodies among healthcare workers with differing levels of coronavirus disease 2019 (COVID-19) patient exposure. Infect. Control Hosp. Epidemiol. 2020, 41, 1441–1442. [Google Scholar] [CrossRef] [PubMed]
- Statista Africa: COVID-19 Delta Variant Cases 2021|Statista. Available online: https://www.statista.com/statistics/1249798/number-of-sars-cov-2-delta-variant-cases-in-africa-by-country/ (accessed on 15 November 2021).
- Minic, R.; Zivkovic, I. Optimization, Validation and Standardization of ELISA. In Norovirus; IntechOpen: London, UK, 2020. [Google Scholar]
- Woodford, J.; Sagara, I.; Kwan, J.; Zeguime, A.; Zaidi, I.; Attaher, O.; Kone, M.; Doritchamou, J.Y.A.; Renn, J.P.; Maiga, M. SARS-CoV-2 Seroassay Optimization and Performance in a Population with High Background Reactivity in Mali. 2021. Available online: https://ssrn.com/abstract=3802506 (accessed on 14 November 2021).
- World Health Organization. Only 1 in 4 African Health Workers Fully Vaccinated AGAINST COVID-19. Available online: https://www.afro.who.int/news/only-1-4-african-health-workers-fully-vaccinated-against-covid-19 (accessed on 5 December 2021).
- Mali-Rural Population. Available online: https://www.indexmundi.com/facts/mali/indicator/SP.RUR.TOTL.ZS (accessed on 14 November 2021).
- Finlay, B.B.; Amato, K.R.; Azad, M.; Blaser, M.J.; Bosch, T.C.G.; Chu, H.; Dominguez-Bello, M.G.; Ehrlich, S.D.; Elinav, E.; Geva-Zatorsky, N. The hygiene hypothesis, the COVID pandemic, and consequences for the human microbiome. Proc. Natl. Acad. Sci. USA 2021, 118, e2010217118. [Google Scholar] [CrossRef] [PubMed]
- Lechien, J.R.; Chiesa-Estomba, C.M.; De Siati, D.R.; Horoi, M.; Le Bon, S.D.; Rodriguez, A.; Dequanter, D.; Blecic, S.; El Afia, F.; Distinguin, L. Olfactory and gustatory dysfunctions as a clinical presentation of mild-to-moderate forms of the coronavirus disease (COVID-19): A multicenter European study. Eur. Arch. Oto-Rhino-Laryngol. 2020, 277, 2251–2261. [Google Scholar] [CrossRef] [PubMed]
- Matta, J.; Wiernik, E.; Robineau, O.; Carrat, F.; Touvier, M.; Severi, G.; de Lamballerie, X.; Blanché, H.; Deleuze, J.-F.; Gouraud, C. Association of Self-reported COVID-19 Infection and SARS-CoV-2 Serology Test Results with Persistent Physical Symptoms Among French Adults During the COVID-19 Pandemic. JAMA Intern. Med. 2021. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | All | Seronegative | Seropositive |
|---|---|---|---|
| n = 238 | n = 91 | n = 147 | |
| Age (years), mean (+/−SD) | 33 (9) | 34 (9) | 33 (9) |
| Female, n (%) | 98 (41.2) | 35 (35.7) | 63 (64.3) |
| Male, n (%) | 140 (58.8) | 56 (40.0) | 84 (60.0) |
| Profession n (%) | |||
| Physicians | 45 (18.9) | 19 (42.2) | 26 (57.8) |
| Physician assistant | 4 (1.7) | 2 (50.0) | 2 (50.0) |
| Nurse | 54 (22.7) | 17 (31.5) | 37 (68.5) |
| Environmental service | 15 (6.3) | 7 (46.7) | 8 (53.3) |
| Transportation technician | 18 (7.1) | 9 (50.0) | 9 (50.0) |
| Other | 94 (39.1) | 31 (33.3) | 63 (67.0) |
| Practice Setting n (%) | |||
| Hospital setting | 229 (96.2) | 91 (39.7) | 138 (60.3) |
| Outpatient setting | 12 (5.0) | 2 (16.7) | 10 (83.3) |
| Community setting | 5 (2.1) | 0 (0.0) | 5 (100.0) |
| Symptoms past 3 months n (%) | |||
| Fever | 66 (28.2) | 22 (33.3) | 44 (66.7) |
| Congestion | 55 (8.4) | 17 (30.9) | 38 (69.1) |
| Sore throat | 21 (8.8) | 6 (28.6) | 15 (71.4) |
| Dry cough | 29 (12.2) | 12 (41.4) | 17 (58.6) |
| Headache | 79 (33.2) | 24 (30.4) | 55 (69.6) |
| Tiredness | 72 (30.3) | 24 (33.3) | 48 (66.7) |
| Pain | 67 (28.2) | 25 (37.3) | 42 (62.7) |
| Comorbidities n (%) | |||
| Obesity | 18 (7.6) | 4 (22.2) | 14 (77.8) |
| Diabetes | 3 (1.3) | 1 (33.3) | 2 (66.7) |
| High blood pressure | 4 (1.7) | 3 (75.0) | 1 (25.0) |
| Asthma | 4 (1.7) | 1 (25.0) | 3 (75.0) |
| Smoking | 17 (7.1) | 8 (47.1) | 9 (52.9) |
| Exposure and PPE Use n (%) | All | Seronegative | Seropositive |
|---|---|---|---|
| n = 238 | n = 91 | n = 147 | |
| Higher exposure risk | 234 (99.2) | 87 (37.2) | 147 (62.8) |
| Lower exposure risk | 4 (1.7) | 4 (100.0) | 0 (0.0) |
| PPE use | |||
| As recommended | 199 (83.6) | 74 (37.2) | 125 (62.8) |
| Most of the time | 31 (13.0) | 10 (32.3) | 21 (67.7) |
| Rarely | 4 (1.7) | 3 (75.0) | 1 (25.0) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Somboro, A.M.; Cissoko, Y.; Camara, I.; Kodio, O.; Tolofoudie, M.; Dembele, E.; Togo, A.C.G.; Ba, D.M.; Sarro, Y.d.S.; Baya, B.; et al. High SARS-CoV-2 Seroprevalence among Healthcare Workers in Bamako, Mali. Viruses 2022, 14, 102. https://doi.org/10.3390/v14010102
Somboro AM, Cissoko Y, Camara I, Kodio O, Tolofoudie M, Dembele E, Togo ACG, Ba DM, Sarro YdS, Baya B, et al. High SARS-CoV-2 Seroprevalence among Healthcare Workers in Bamako, Mali. Viruses. 2022; 14(1):102. https://doi.org/10.3390/v14010102
Chicago/Turabian StyleSomboro, Anou M., Yacouba Cissoko, Issiaka Camara, Ousmane Kodio, Mohamed Tolofoudie, Etienne Dembele, Antieme C. G. Togo, Djibril M. Ba, Yeya dit Sadio Sarro, Bocar Baya, and et al. 2022. "High SARS-CoV-2 Seroprevalence among Healthcare Workers in Bamako, Mali" Viruses 14, no. 1: 102. https://doi.org/10.3390/v14010102
APA StyleSomboro, A. M., Cissoko, Y., Camara, I., Kodio, O., Tolofoudie, M., Dembele, E., Togo, A. C. G., Ba, D. M., Sarro, Y. d. S., Baya, B., Samake, S., Diallo, I. B., Kumar, A., Traore, M., Kone, B., Kone, A., Diarra, B., Dabitao, D. K., Wague, M., ... Dao, S. (2022). High SARS-CoV-2 Seroprevalence among Healthcare Workers in Bamako, Mali. Viruses, 14(1), 102. https://doi.org/10.3390/v14010102






