Factors Determining Transmission of Persistent Viruses by Bemisia tabaci and Emergence of New Virus–Vector Relationships
Abstract
1. Introduction
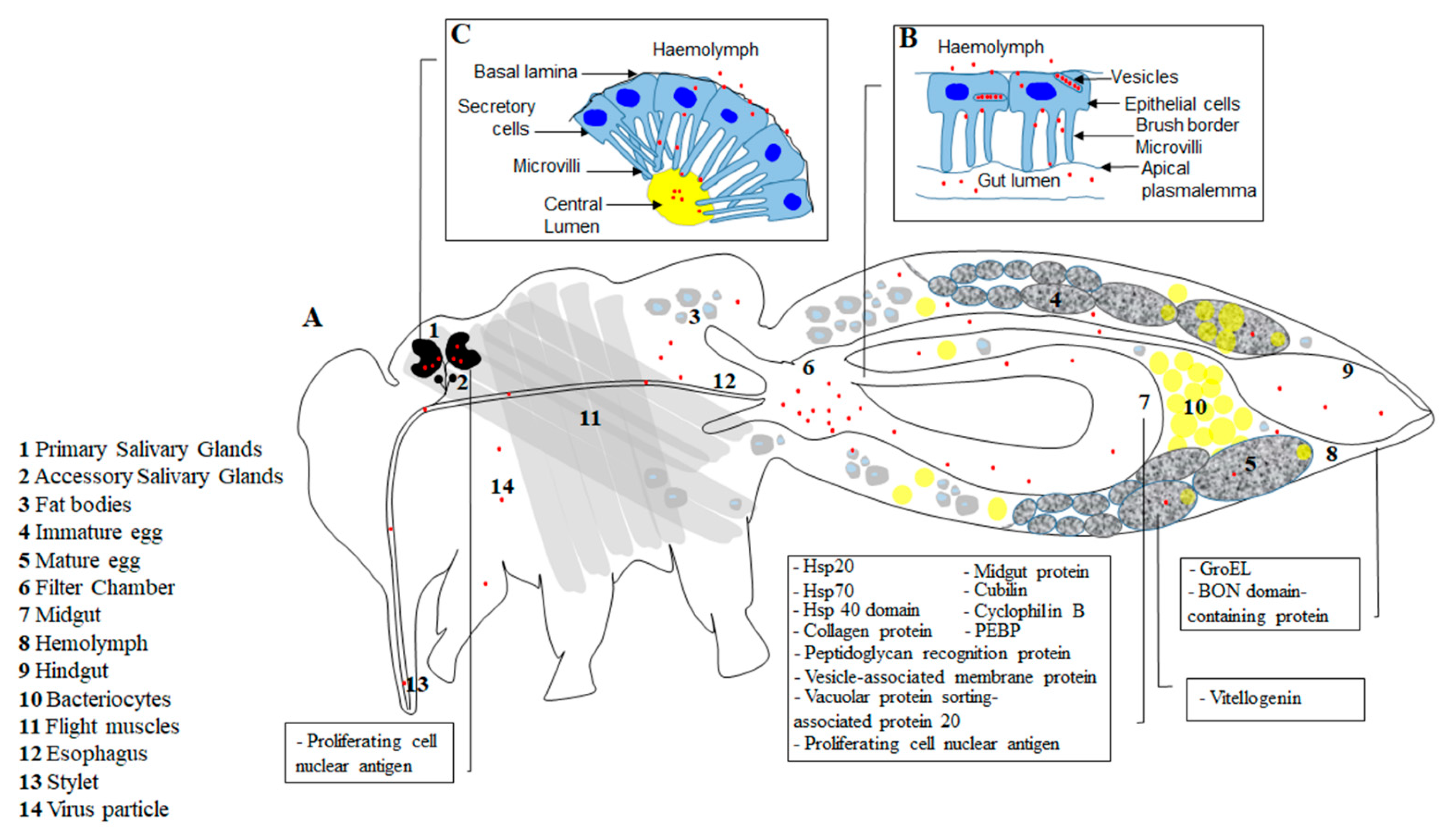
2. Transmission Route of Persistent Viruses
3. Viral Structural Proteins: The Key Determining Whitefly Transmissibility
4. Regulation of Virus Transmission
5. Evolution of New Relationships and Associated Risk
6. Is It MEAM1 Breaking Barriers?
7. Concluding Remarks
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Hogenhout, S.A.; Ammar, E.-D.; Whitfield, A.E.; Redinbaugh, M.G. Insect vector interactions with persistently transmitted viruses. Annu. Rev. Phytopathol. 2008, 46, 327–359. [Google Scholar] [CrossRef]
- Fereres, A.; Raccah, B. Plant Virus Transmission by Insects. In eLS.; John Wiley & Sons, Ltd.: Chichester, UK, 2015; pp. 1–12. [Google Scholar]
- Ng, J.C.K.; Falk, B.W. Virus-vector interactions mediating nonpersistent and semipersistent transmission of plant viruses. Annu. Rev. Phytopathol. 2006, 44, 183–212. [Google Scholar] [CrossRef] [PubMed]
- Gray, S.; Cilia, M.; Ghanim, M. Circulative, “Nonpropagative” virus transmission: An orchestra of virus-, insect-, and plant-derived instruments. Adv. Virus Res. 2014, 89, 141–199. [Google Scholar]
- Whitfield, A.E.; Falk, B.W.; Rotenberg, D. Insect vector-mediated transmission of plant viruses. Virology 2015, 479, 278–289. [Google Scholar] [CrossRef]
- Power, A.G. Insect transmission of plant viruses: A constraint on virus variability. Curr. Opin. Plant. Biol. 2000, 3, 336–340. [Google Scholar] [CrossRef]
- Lefeuvre, P.; Martin, D.P.; Elena, S.F.; Shepherd, D.N.; Roumagnac, P.; Varsani, A. Evolution and ecology of plant viruses. Nat. Rev. Microbiol. 2019, 17, 632–644. [Google Scholar] [CrossRef]
- Martin, R.R.; Keese, P.K.; Young, M.J.; Waterhouse, P.M.; Gerlach, W.L. Evolution and molecular biology of luteoviruses. Annu. Rev. Phytopathol. 1990, 28, 341–363. [Google Scholar] [CrossRef]
- Martin, J.H.; Mound, L.A. An annotated check list of the world’s whiteflies (Insecta: Hemiptera: Aleyrodidae). Zootaxa 2007, 1–84. [Google Scholar] [CrossRef]
- De Barro, P.J.; Liu, S.-S.; Boykin, L.M.; Dinsdale, A.B. Bemisia tabaci: A statement of species status. Annu. Rev. Entomol. 2011, 56, 1–19. [Google Scholar] [CrossRef]
- Fiallo-Olivé, E.; Pan, L.L.; Liu, S.S.; Navas-Castillo, J. Transmission of begomoviruses and other whitefly-borne viruses: Dependence on the vector species. Phytopathology 2020, 110, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Ghanim, M. A review of the mechanisms and components that determine the transmission efficiency of tomato yellow leaf curl virus (Geminiviridae; Begomovirus) by its whitefly vector. Virus Res. 2014, 186, 47–54. [Google Scholar] [CrossRef]
- Rosen, R.; Kanakala, S.; Kliot, A.; Cathrin Pakkianathan, B.; Farich, B.A.; Santana-Magal, N.; Elimelech, M.; Kontsedalov, S.; Lebedev, G.; Cilia, M.; et al. Persistent, circulative transmission of begomoviruses by whitefly vectors. Curr. Opin. Virol. 2015, 15, 1–8. [Google Scholar] [CrossRef]
- Dombrovsky, A.; Reingold, V.; Antignus, Y. Ipomovirus—an atypical genus in the family Potyviridae transmitted by whiteflies. Pest. Manag. Sci. 2014, 70, 1553–1567. [Google Scholar] [CrossRef] [PubMed]
- Desbiez, C.; Verdin, E.; Tepfer, M.; Wipf-Scheibel, C.; Millot, P.; Dafalla, G.; Lecoq, H. Characterization of a new cucurbit-infecting ipomovirus from Sudan. Arch. Virol. 2016, 161, 2913–2915. [Google Scholar] [CrossRef] [PubMed]
- Verbeek, M.; van Bekkum, P.J.; Dullemans, A.M.; van der Vlugt, R.A.A. Torradoviruses are transmitted in a semi-persistent and stylet-borne manner by three whitefly vectors. Virus Res. 2014, 186, 55–60. [Google Scholar] [CrossRef]
- Lecoq, H.; Verdin, E.; Tepfer, M.; Wipf-Scheibel, C.; Millot, P.; Dafalla, G.; Desbiez, C. Characterization and occurrence of squash chlorotic leaf spot virus, a tentative new torradovirus infecting cucurbits in Sudan. Arch. Virol. 2016, 161, 1651–1655. [Google Scholar] [CrossRef] [PubMed]
- Wintermantel, W.M.; Hladky, L.L.; Cortez, A.A. Genome sequence, host range, and whitefly transmission of the torradovirus Tomato necrotic dwarf virus. Proc. Acta Hortic. 2018, 1207, 295–301. [Google Scholar] [CrossRef]
- Muniyappa, V. Transmission of Cowpea Mild Mottle Virus by Bemisia tabaci in a Nonpersistent Manner. Plant Dis. 1983, 67, 391. [Google Scholar] [CrossRef]
- Nagata, T.; Alves, D.M.T.; Inoue-Nagata, A.K.; Tian, T.Y.; Kitajima, E.W.; Cardoso, J.E.; De Ávila, A.C. A novel melon flexivirus transmitted by whitefly. Arch. Virol. 2005, 150, 379–387. [Google Scholar] [CrossRef] [PubMed]
- Menzel, W.; Abang, M.M.; Winter, S. Characterization of cucumber vein-clearing virus, a whitefly (Bemisia tabaci G.)-transmitted carlavirus. Arch. Virol. 2011, 156, 2309–2311. [Google Scholar] [CrossRef] [PubMed]
- Thekke-veetil, T.; McCoppin, N.K.; Hobbs, H.A.; Hartman, G.L.; Lambert, K.N.; Lim, H.S.; Domier, L.L. Discovery of a novel member of the carlavirus genus from soybean (Glycine max l. merr.). Pathogens 2021, 10, 223. [Google Scholar] [CrossRef]
- Ghosh, S.; Kanakala, S.; Lebedev, G.; Kontsedalov, S.; Silverman, D.; Alon, T.; Mor, N.; Sela, N.; Luria, N.; Dombrovsky, A.; et al. Transmission of a new polerovirus infecting pepper by the whitefly Bemisia tabaci. J. Virol. 2019, 93, e00488-19. [Google Scholar] [CrossRef]
- Costa, T.M.; Inoue-Nagata, A.K.; Vidal, A.H.; Ribeiro, S.G.; Nagata, T. The recombinant isolate of cucurbit aphid-borne yellows virus from Brazil is a polerovirus transmitted by whiteflies. Plant Pathol. 2020, 69, 1042–1050. [Google Scholar] [CrossRef]
- Pinheiro-Lima, B.; Pereira-Carvalho, R.C.; Alves-Freitas, D.M.T.; Kitajima, E.W.; Vidal, A.H.; Lacorte, C.; Godinho, M.T.; Fontenele, R.S.; Faria, J.C.; Abreu, E.F.M.; et al. Transmission of the bean-associated cytorhabdovirus by the whitefly Bemisia tabaci MEAM1. Viruses 2020, 12, 1028. [Google Scholar] [CrossRef]
- Ghanim, M.; Morin, S.; Czosnek, H. Rate of Tomato yellow leaf curl virus translocation in the circulative transmission pathway of its vector, the whitefly Bemisia tabaci. Phytopathology 2001, 91, 188–196. [Google Scholar] [CrossRef]
- Wang, L.L.; Wei, X.M.; Ye, X.D.; Xu, H.X.; Zhou, X.P.; Liu, S.S.; Wang, X.W. Expression and functional characterisation of a soluble form of Tomato yellow leaf curl virus coat protein. Pest. Manag. Sci. 2014, 70, 1624–1631. [Google Scholar] [CrossRef] [PubMed]
- Hunter, W.B.; Hiebert, E.; Webb, S.E.; Tsai, J.H.; Polston, J.E. Location of geminiviruses in the whitefly Bemisia tabaci (Homoptera: Aleyrodidae). Plant. Dis. 1998, 82, 1147–1151. [Google Scholar] [CrossRef] [PubMed]
- Uchibori, M.; Hirata, A.; Suzuki, M.; Ugaki, M. Tomato yellow leaf curl virus accumulates in vesicle-like structures in descending and ascending midgut epithelial cells of the vector whitefly, Bemisia tabaci, but not in those of nonvector whitefly Trialeurodes vaporariorum. J. Gen. Plant Pathol. 2013, 79, 115–122. [Google Scholar] [CrossRef]
- Zhao, J.; Lei, T.; Zhang, X.J.; Yin, T.Y.; Wang, X.W.; Liu, S.S. A vector whitefly endocytic receptor facilitates the entry of begomoviruses into its midgut cells via binding to virion capsid proteins. PLoS Pathog. 2020, 16, e1009053. [Google Scholar] [CrossRef]
- Morin, S.; Ghanim, M.; Sobol, I.; Czosnek, H. The GroEL protein of the whitefly Bemisia tabaci interacts with the coat protein of transmissible and nontransmissible begomoviruses in the yeast two-hybrid system. Virology 2000, 276, 404–416. [Google Scholar] [CrossRef] [PubMed]
- Ohnishi, J.; Kitamura, T.; Terami, F.; Honda, K.I. A selective barrier in the midgut epithelial cell membrane of the nonvector whitefly Trialeurodes vaporariorum to Tomato yellow leaf curl virus uptake. J. Gen. Plant Pathol. 2009, 75, 131–139. [Google Scholar] [CrossRef]
- Pan, L.-L.; Cui, X.-Y.; Chen, Q.-F.; Wang, X.-W.; Liu, S.-S. Cotton leaf curl disease: Which whitefly is the vector? Phytopathology 2018, 108, 1172–1183. [Google Scholar] [CrossRef] [PubMed]
- Medina, V.; Pinner, M.S.; Bedford, I.D.; Achon, M.A.; Gemeno, C.; Markham, P.G. Immunolocalization of Tomato yellow leaf curl sardinia virus in natural host plants and its vector Bemisia tabaci. J. Plant Pathol. 2006, 88, 299–308. [Google Scholar]
- Rosell, R.C.; Torres-Jerez, I.; Brown, J.K. Tracing the geminivirus-whitefly transmission pathway by polymerase chain reaction in whitefly extracts, saliva, hemolymph, and honeydew. Phytopathology 1999, 89, 239–246. [Google Scholar] [CrossRef]
- Chi, Y.; Pan, L.L.; Bouvaine, S.; Fan, Y.Y.; Liu, Y.Q.; Liu, S.S.; Seal, S.; Wang, X.W. Differential transmission of Sri Lankan cassava mosaic virus by three cryptic species of the whitefly Bemisia tabaci complex. Virology 2020, 540, 141–149. [Google Scholar] [CrossRef]
- Guo, T.; Zhao, J.; Pan, L.L.; Geng, L.; Lei, T.; Wang, X.W.; Liu, S.S. The level of midgut penetration of two begomoviruses affects their acquisition and transmission by two species of Bemisia tabaci. Virology 2018, 515, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Xia, W.Q.; Liang, Y.; Chi, Y.; Pan, L.L.; Zhao, J.; Liu, S.S.; Wang, X.W. Intracellular trafficking of begomoviruses in the midgut cells of their insect vector. PLoS Pathog. 2018, 14, e1006866. [Google Scholar] [CrossRef]
- Pan, L.L.; Chen, Q.F.; Zhao, J.J.; Guo, T.; Wang, X.W.; Hariton-Shalev, A.; Czosnek, H.; Liu, S.S. Clathrin-mediated endocytosis is involved in Tomato yellow leaf curl virus transport across the midgut barrier of its whitefly vector. Virology 2017, 502, 152–159. [Google Scholar] [CrossRef]
- Pakkianathan, B.C.; Kontsedalov, S.; Lebedev, G.; Mahadav, A.; Zeidan, M.; Czosnek, H.; Ghanim, M. Replication of Tomato Yellow Leaf Curl Virus in Its Whitefly Vector, Bemisia tabaci. J. Virol. 2015, 89, 9791–9803. [Google Scholar] [CrossRef]
- Ohnesorge, S.; Bejarano, E.R. Begomovirus coat protein interacts with a small heat-shock protein of its transmission vector (Bemisia tabaci). Insect Mol. Biol. 2009, 18, 693–703. [Google Scholar] [CrossRef]
- Götz, M.; Popovski, S.; Kollenberg, M.; Gorovits, R.; Brown, J.K.; Cicero, J.M.; Czosnek, H.; Winter, S.; Ghanim, M. Implication of Bemisia tabaci heat shock protein 70 in Begomovirus-whitefly interactions. J. Virol. 2012, 86, 13241–13252. [Google Scholar] [CrossRef]
- Kanakala, S.; Ghanim, M. Implication of the whitefly Bemisia tabaci cyclophilin B protein in the transmission of tomato yellow leaf curl virus. Front. Plant Sci. 2016, 7, 1702. [Google Scholar] [CrossRef]
- Wei, J.; He, Y.Z.; Guo, Q.; Guo, T.; Liu, Y.Q.; Zhou, X.P.; Liu, S.S.; Wang, X.W. Vector development and vitellogenin determine the transovarial transmission of begomoviruses. Proc. Natl. Acad. Sci. USA 2017, 114, 6746–6751. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.Z.; Shi, M.; Huang, Y.C.; Wang, X.W.; Stanley, D.; Chen, X.X. A peptidoglycan recognition protein acts in whitefly (Bemisia tabaci) immunity and involves in Begomovirus acquisition. Sci. Rep. 2016, 6, 1–11. [Google Scholar]
- Rana, V.S.; Popli, S.; Saurav, G.K.; Raina, H.S.; Chaubey, R.; Ramamurthy, V.V.; Rajagopal, R. A Bemisia tabaci midgut protein interacts with begomoviruses and plays a role in virus transmission. Cell. Microbiol. 2016, 18, 663–678. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Chi, Y.; Zhang, X.J.; Wang, X.W.; Liu, S.S. Implication of whitefly vesicle associated membrane protein-associated protein B in the transmission of Tomato yellow leaf curl virus. Virology 2019, 535, 210–217. [Google Scholar] [CrossRef] [PubMed]
- Chi, Y.; Pan, L.L.; Liu, S.S.; Mansoor, S.; Wang, X.W. Implication of the whitefly protein vps twenty associated 1 (Vta1) in the transmission of cotton leaf curl multan virus. Microorganisms 2021, 9, 304. [Google Scholar] [CrossRef]
- Saurav, G.K.; Rana, V.S.; Popli, S.; Daimei, G.; Rajagopal, R. A thioredoxin-like protein of Bemisia tabaci interacts with coat protein of begomoviruses. Virus Genes 2019, 55, 356–367. [Google Scholar] [CrossRef]
- Rana, V.S.; Popli, S.; Saurav, G.K.; Raina, H.S.; Jamwal, R.; Chaubey, R.; Ramamurthy, V.V.; Natarajan, K.; Rajagopal, R. Implication of the whitefly, Bemisia tabaci, collagen protein in begomoviruses acquisition and transmission. Phytopathology 2019, 109, 1481–1493. [Google Scholar] [CrossRef]
- Zhao, J.; Guo, T.; Lei, T.; Zhu, J.C.; Wang, F.; Wang, X.W.; Liu, S.S. Proteomic Analyses of Whitefly-Begomovirus Interactions Reveal the Inhibitory Role of Tumorous Imaginal Discs in Viral Retention. Front. Immunol. 2020, 11, 1596. [Google Scholar] [CrossRef]
- Wang, S.; Guo, H.; Zhu-Salzman, K.; Ge, F.; Sun, Y. PEBP regulates balance between apoptosis and autophagy, enabling coexistence of arbovirus and insect vector. bioRxiv 2021. [Google Scholar] [CrossRef]
- Gottlieb, Y.; Zchori-Fein, E.; Mozes-Daube, N.; Kontsedalov, S.; Skaljac, M.; Brumin, M.; Sobol, I.; Czosnek, H.; Vavre, F.; Fleury, F. The transmission efficiency of tomato yellow leaf curl virus by the whitefly Bemisia tabaci is correlated with the presence of a specific symbiotic bacterium species. J. Virol. 2010, 84, 9310–9317. [Google Scholar] [CrossRef] [PubMed]
- Rana, V.S.; Singh, S.T.; Priya, N.G.; Kumar, J.; Rajagopal, R. Arsenophonus GroEL interacts with CLCuV and is localized in midgut and salivary gland of whitefly B. tabaci. PLoS ONE 2012, 7, e42168. [Google Scholar] [CrossRef]
- Morin, S.; Ghanim, M.; Zeidan, M.; Czosnek, H.; Verbeek, M.; van den Heuvel, J.F.J.M. A GroEL Homologue from Endosymbiotic Bacteria of the Whitefly Bemisia tabaci is implicated in the Circulative Transmission of Tomato Yellow Leaf Curl Virus. Virology 1999, 256, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Lei, T.; Zhao, J.; Wang, H.L.; Liu, Y.Q.; Liu, S.S. Impact of a novel Rickettsia symbiont on the life history and virus transmission capacity of its host whitefly (Bemisia tabaci). Insect Sci. 2021, 28, 377–391. [Google Scholar] [CrossRef] [PubMed]
- He, Y.Z.; Wang, Y.M.; Yin, T.Y.; Fiallo-Olivé, E.; Liu, Y.Q.; Hanley-Bowdoin, L.; Wang, X.W. A plant DNA virus replicates in the salivary glands of its insect vector via recruitment of host DNA synthesis machinery. Proc. Natl. Acad. Sci. USA 2020, 117, 16928–16937. [Google Scholar] [CrossRef]
- Ghosh, S.; Bello, V.H.; Ghanim, M. Transmission parameters of pepper whitefly-borne vein yellows virus (PeWBVYV) by Bemisia tabaci and identification of an insect protein with a putative role in polerovirus transmission. Virology 2021, 560, 54–65. [Google Scholar] [CrossRef]
- Geng, L.; Qian, L.X.; Shao, R.X.; Liu, Y.Q.; Liu, S.S.; Wang, X.W. Transcriptome profiling of whitefly guts in response to Tomato yellow leaf curl virus infection. Virol. J. 2018, 15, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Pan, L.; Chen, Q.; Guo, T.; Wang, X.; Li, P.; Wang, X.; Liu, S. Differential efficiency of a begomovirus to cross the midgut of different species of whiteflies results in variation of virus transmission by the vectors. Sci. China Life Sci. 2018, 61, 1254–1265. [Google Scholar] [CrossRef]
- Hariton Shalev, A.; Sobol, I.; Ghanim, M.; Liu, S.-S.; Czosnek, H. The whitefly Bemisia tabaci knottin-1 gene is implicated in regulating the quantity of Tomato yellow leaf curl virus ingested and transmitted by the insect. Viruses 2016, 8, 205. [Google Scholar] [CrossRef]
- Wang, L.L.; Wang, X.R.; Wei, X.M.; Huang, H.; Wu, J.X.; Chen, X.X.; Liu, S.S.; Wang, X.W. The autophagy pathway participates in resistance to tomato yellow leaf curl virus infection in whiteflies. Autophagy 2016, 12, 1560–1574. [Google Scholar] [CrossRef]
- Wang, X.-R.; Wang, C.; Ban, F.-X.; Ghanim, M.; Pan, L.-L.; Qian, L.-X.; Liu, Y.-Q.; Wang, X.-W.; Liu, S.-S. Apoptosis in a Whitefly Vector Activated by a Begomovirus Enhances Viral Transmission. mSystems 2020, 5, e00433-20. [Google Scholar] [CrossRef]
- Kliot, A.; Cilia, M.; Czosnek, H.; Ghanim, M. Implication of the bacterial endosymbiont Rickettsia spp. in interactions of the whitefly Bemisia tabaci with Tomato yellow leaf curl virus. J. Virol. 2014, 88, 5652–5660. [Google Scholar] [CrossRef] [PubMed]
- Bello, V.H.; Watanabe, L.F.M.; Santos, B.R.; Marubayashi, J.M.; Yuki, V.A.; De Marchi, B.R.; Pavan, M.A.; Krause-Sakate, R. Evidence for increased efficiency of virus transmission by populations of Mediterranean species of Bemisia tabaci with high Hamiltonella prevalence. Phytoparasitica 2019, 47, 293–300. [Google Scholar] [CrossRef]
- Kliot, A.; Johnson, R.S.; MacCoss, M.J.; Kontsedalov, S.; Lebedev, G.; Czosnek, H.; Heck, M.; Ghanim, M. A proteomic approach reveals possible molecular mechanisms and roles for endosymbiotic bacteria in begomovirus transmission by whiteflies. Gigascience 2020, 9, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Cicero, J.M.; Brown, J.K. Functional anatomy of whitefly organs associated with Squash leaf curl virus (Geminiviridae: Begomovirus) transmission by the B biotype of Bemisia tabaci (Hemiptera: Aleyrodidae). Ann. Entomol. Soc. Am. 2011, 104, 261–279. [Google Scholar] [CrossRef]
- Kliot, A.; Ghanim, M. The role of bacterial chaperones in the circulative transmission of plant viruses by insect vectors. Viruses 2013, 5, 1516–1535. [Google Scholar] [CrossRef] [PubMed]
- Luan, J.; Li, J.; Wang, Y.; Li, F.; Bao, Y.; Zhang, C.; Liu, S.; Wang, X. Global Analysis of the Transcriptional Response of Whitefly to Tomato Yellow Leaf Curl China Virus Reveals the Relationship of Coevolved Adaptations. J. Virol. 2011, 85, 3330–3340. [Google Scholar] [CrossRef]
- Ghanim, M.; Rosell, R.C.; Campbell, L.R.; Czosnek, H.; Brown, J.K.; Ullman, D.E. Digestive, salivary, and reproductive organs of Bemisia tabaci (Gennadius) (Hemiptera: Aleyrodidae) B type. J. Morphol. 2001, 248, 22–40. [Google Scholar] [CrossRef]
- Caciagli, P.; Medina Piles, V.; Marian, D.; Vecchiati, M.; Masenga, V.; Mason, G.; Falcioni, T.; Noris, E. Virion Stability Is Important for the Circulative Transmission of Tomato Yellow Leaf Curl Sardinia Virus by Bemisia tabaci, but Virion Access to Salivary Glands Does Not Guarantee Transmissibility. J. Virol. 2009, 83, 5784–5795. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Zhao, J.-J.; Zhang, T.; Li, F.-F.; Ghanim, M.; Zhou, X.-P.; Ye, G.-Y.; Liu, S.-S.; Wang, X.-W. Specific Cells in the Primary Salivary Glands of the Whitefly Bemisia tabaci Control Retention and Transmission of Begomoviruses. J. Virol. 2014, 88, 13460–13468. [Google Scholar] [CrossRef] [PubMed]
- Höfer, P.; Bedford, I.D.; Markham, P.G.; Jeske, H.; Frischmuth, T. Coat protein gene replacement results in whitefly transmission of an insect nontransmissible geminivirus isolate. Virology 1997, 236, 288–295. [Google Scholar] [CrossRef]
- Briddon, R.W.; Pinner, M.S.; Stanley, J.; Markham, P.G. Geminivirus coat protein gene replacement alters insect specificity. Virology 1990, 177, 85–94. [Google Scholar] [CrossRef]
- Höhnle, M.; Höfer, P.; Bedford, I.D.; Briddon, R.W.; Markham, P.G.; Frischmuth, T. Exchange of three amino acids in the coat protein results in efficient whitefly transmission of a nontransmissible Abutilon mosaic virus isolate. Virology 2001, 290, 164–171. [Google Scholar] [CrossRef] [PubMed]
- Noris, E.; Vaira, A.M.; Caciagli, P.; Masenga, V.; Gronenborn, B.; Accotto, G.P. Amino Acids in the Capsid Protein of Tomato Yellow Leaf Curl Virus That Are Crucial for Systemic Infection, Particle Formation, and Insect Transmission. J. Virol. 1998, 72, 10050–10057. [Google Scholar] [CrossRef]
- Kheyr-Pour, A.; Bananej, K.; Dafalla, G.A.; Caciagli, P.; Noris, E.; Ahoonmanesh, A.; Lecoq, H.; Gronenborn, B. Watermelon chlorotic stunt virus from the Sudan and Iran: Sequence comparisons and identification of a whitefly-transmission determinant. Phytopathology 2000, 90, 629–635. [Google Scholar] [CrossRef] [PubMed]
- Pan, L.L.; Chi, Y.; Liu, C.; Fan, Y.Y.; Liu, S.S. Mutations in the coat protein of a begomovirus result in altered transmission by different species of whitefly vectors. Virus Evol. 2020, 6, veaa014. [Google Scholar] [CrossRef]
- Sinisterra, X.H.; McKenzie, C.L.; Hunter, W.B.; Powell, C.A.; Shatters, R.G. Differential transcriptional activity of plant-pathogenic begomoviruses in their whitefly vector (Bemisia tabaci, Gennadius: Hemiptera Aleyrodidae). J. Gen. Virol. 2005, 86, 1525–1532. [Google Scholar] [CrossRef] [PubMed]
- Lacatus, G.; Sunter, G. The Arabidopsis PEAPOD2 transcription factor interacts with geminivirus AL2 protein and the coat protein promoter. Virology 2009, 392, 196–202. [Google Scholar] [CrossRef]
- Lacatus, G.; Sunter, G. Functional analysis of bipartite begomovirus coat protein promoter sequences. Virology 2008, 376, 79–89. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wang, Y.M.; He, Y.Z.; Ye, X.T.; He, W.Z.; Liu, S.S.; Wang, X.W. Whitefly HES1 binds to the intergenic region of Tomato yellow leaf curl China virus and promotes viral gene transcription. Virology 2020, 542, 54–62. [Google Scholar] [CrossRef]
- Kunik, T.; Palanichelvam, K.; Czosnek, H.; Citovsky, V.; Gafni, Y. Nuclear import of the capsid protein of tomato yellow leaf curl virus (TYLCV) in plant and insect cells. Plant J. 1998, 13, 393–399. [Google Scholar] [CrossRef]
- Rojas, M.R.; Jiang, H.; Salati, R.; Xoconostle-Cázares, B.; Sudarshana, M.R.; Lucas, W.J.; Gilbertson, R.L. Functional analysis of proteins involved in movement of the monopartite begomovirus, Tomato yellow leaf curl virus. Virology 2001, 291, 110–125. [Google Scholar] [CrossRef]
- Priyadarshini, C.G.P.; Ambika, M.V.; Tippeswamy, R.; Savithri, H.S. Functional characterization of coat protein and V2 involved in cell to cell movement of cotton leaf curl Kokhran Virus-Dabawali. PLoS ONE 2011, 6, e26929. [Google Scholar] [CrossRef]
- Gafni, Y. Tomato yellow leaf curl virus, the intracellular dynamics of a plant DNA virus. Mol. Plant Pathol. 2003, 4, 9–15. [Google Scholar] [CrossRef]
- Li, R.; Weldegergis, B.T.; Li, J.; Jung, C.; Qu, J.; Sun, Y.; Qian, H.; Tee, C.; Van Loon, J.J.A.; Dicke, M.; et al. Virulence factors of geminivirus interact with MYC2 to subvert plant resistance and promote vector performance. Plant Cell 2014, 26, 4991–5008. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Liu, C.; Deng, W.-H.; Yao, D.M.; Pan, L.L.; Li, Y.Q.; Liu, Y.Q.; Liang, Y.; Zhou, X.P.; Wang, X.W. Plant begomoviruses subvert ubiquitination to suppress plant defenses against insect vectors. PLoS Pathog. 2019, 15, e1007607. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, D.K.; Chen, W.; Zheng, Y.; Kaur, N.; Wintermantel, W.M.; Simmons, A.M.; Fei, Z.; Ling, K.S. Comparative transcriptome analysis reveals networks of genes activated in the whitefly, Bemisia tabaci when fed on tomato plants infected with Tomato yellow leaf curl virus. Virology 2018, 513, 52–64. [Google Scholar] [CrossRef] [PubMed]
- Ding, T.B.; Li, J.; Chen, E.H.; Niu, J.Z.; Chu, D. Transcriptome Profiling of the Whitefly Bemisia tabaci MED in Response to Single Infection of Tomato yellow leaf curl virus, Tomato chlorosis virus, and Their Co-infection. Front. Physiol. 2019, 10, 302. [Google Scholar] [CrossRef]
- Li, M.; Zhao, J.; Su, Y.L. Transcriptome analysis of gene expression profiles of tomato yellow leaf curl virus-infected whiteflies over different viral acquisition access periods. Insects 2020, 11, 297. [Google Scholar] [CrossRef] [PubMed]
- Raja, P.; Sanville, B.C.; Buchmann, R.C.; Bisaro, D.M. Viral Genome Methylation as an Epigenetic Defense against Geminiviruses. J. Virol. 2008, 82, 8997–9007. [Google Scholar] [CrossRef]
- Rodríguez-Negrete, E.A.; Carrillo-Tripp, J.; Rivera-Bustamante, R.F. RNA Silencing against Geminivirus: Complementary Action of Posttranscriptional Gene Silencing and Transcriptional Gene Silencing in Host Recovery. J. Virol. 2009, 83, 1332–1340. [Google Scholar] [CrossRef]
- Buchmann, R.C.; Asad, S.; Wolf, J.N.; Mohannath, G.; Bisaro, D.M. Geminivirus AL2 and L2 Proteins Suppress Transcriptional Gene Silencing and Cause Genome-Wide Reductions in Cytosine Methylation. J. Virol. 2009, 83, 5005–5013. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Díaz, E.; Rivero, A.; Chandre, F.; Corces, V.G. Insights into the epigenomic landscape of the human malaria vector Anopheles gambiae. Front. Genet. 2014, 5, 277. [Google Scholar]
- Ruiz, J.L.; Yerbanga, R.S.; Lefèvre, T.; Ouedraogo, J.B.; Corces, V.G.; Gómez-Díaz, E. Chromatin changes in Anopheles gambiae induced by Plasmodium falciparum infection. Epigenetics Chromatin 2019, 12, 1–18. [Google Scholar] [CrossRef]
- Dai, T.M.; Lü, Z.C.; Wang, Y.S.; Liu, W.X.; Hong, X.Y.; Wan, F.H. Molecular characterizations of DNA methyltransferase 3 and its roles in temperature tolerance in the whitefly, Bemisia tabaci Mediterranean. Insect Mol. Biol. 2018, 27, 123–132. [Google Scholar] [CrossRef]
- Dai, T.M.; Lü, Z.C.; Liu, W.X.; Wan, F.H.; Hong, X.Y. The homology gene BtDnmt1 is essential for temperature tolerance in invasive Bemisia tabaci mediterranean cryptic species. Sci. Rep. 2017, 7, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Gray, S.; Gildow, F.E. Luteovirus-aphid interactions. Annu. Rev. Phytopathol. 2003, 41, 539–566. [Google Scholar] [CrossRef] [PubMed]
- Gildow, F.E. Evidence for receptor-mediated endocytosis regulating Luteovirus acquisition by aphids. Phytopathology 1993, 83, 270. [Google Scholar] [CrossRef]
- Brault, V.; Périgon, S.; Reinbold, C.; Erdinger, M.; Scheidecker, D.; Herrbach, E.; Richards, K.; Ziegler-Graff, V. The polerovirus minor capsid protein determines vector specificity and intestinal tropism in the aphid. J. Virol. 2005, 79, 9685–9693. [Google Scholar] [CrossRef]
- Bello, V.H.; Ghosh, S.; Krause-Sakate, R.; Ghanim, M. Competitive Interactions Between Whitefly and Aphid Transmitted Poleroviruses within the Plant Host and the Insect Vectors. Phytopathology 2020. [Google Scholar] [CrossRef] [PubMed]
- DeBlasio, S.L.; Wilson, J.R.; Tamborindeguy, C.; Johnson, R.S.; Pinheiro, P.V.; MacCoss, M.J.; Gray, S.M.; Heck, M. Affinity Purification–Mass Spectrometry Identifies a Novel Interaction between a Polerovirus and a Conserved Innate Immunity Aphid Protein that Regulates Transmission Efficiency. J. Proteome Res. 2021, 20, 3365–3387. [Google Scholar] [CrossRef]
- Whitfield, A.E.; Huot, O.B.; Martin, K.M.; Kondo, H.; Dietzgen, R.G. Plant rhabdoviruses—Their origins and vector interactions. Curr. Opin. Virol. 2018, 33, 198–207. [Google Scholar] [CrossRef] [PubMed]
- Horowitz, A.R.; Ghanim, M.; Roditakis, E.; Nauen, R.; Ishaaya, I. Insecticide resistance and its management in Bemisia tabaci species. J. Pest. Sci. (2004) 2020, 93, 893–910. [Google Scholar] [CrossRef]
| Genus | Family | Genetic Material | Mode of Transmission | No. of Species Transmitted by Whitefly | References |
|---|---|---|---|---|---|
| Begomovirus | Geminiviridae | ssDNA | Circulative, non-propagative | 424 a | |
| Crinivirus | Closteroviridae | (+)ve ssRNA | Semi-persistent | 14 a | |
| Ipomovirus | Potyviridae | (+)ve ssRNA | Semi-persistent | 7 | [14,15] |
| Torradovirus | Secoviridae | (+)ve ssRNA | Semi-persistent | 5 | [16,17,18] |
| Carlavirus | Betaflexiviridae | (+)ve ssRNA | Non-persistent | 4 | [19,20,21,22] |
| Polerovirus | Luteoviridae | (+)ve ssRNA | Circulative, non-propagative | 2 | [23,24] |
| Cytorhabdovirus | Rhabdoviridae | (–)ve ssRNA | Unknown | 1 | [25] |
| Total viruses | 457 | ||||
| Virus Protein | Whitefly Protein | Localized Organelle of Interaction | Suggested Site of Interaction | Suggested Role | References |
|---|---|---|---|---|---|
| CP | Heat shock proteins Hsp20, Hsp70 | Midgut | Inhibits virus inside whitefly | [41,42] | |
| CP | Cyclophilin B | Midgut, salivary gland, ovary | Midgut, ovary, salivary gland | Aids virus to suppress whitefly immune response | [43] |
| CP | Cubilin | Midgut | Midgut | Receptor for entry into midgut cells | [30] |
| CP | Vitellogenin | Ovary | Hemolymph, Ovary | Viral entry into ovary cells for transovarial transmission | [44] |
| CP | Peptidoglycan recognition protein | Midgut | Whitefly immunity against virus | [45] | |
| CP | Midgut protein (transcription activator MBF2 protein domain) | Midgut | Midgut | Aids translocation of virus across midgut | [46] |
| CP | Vesicle-associated membrane protein-associated protein B | Midgut | Midgut | Inhibits virus translocation across midgut | [47] |
| CP | Vacuolar protein sorting-associated protein 20 | Midgut | Aids virus translocation across midgut | [48] | |
| CP | Thioredoxin like protein | [49] | |||
| Collagen protein | Midgut | Midgut | Aids adhesion and entry to the midgut epithelial cells | [50] | |
| Tumerous imaginal disc protein (Hsp 40 domain) | Midgut | Inhibits virus inside whitefly | [51] | ||
| CP | Phosphatidylethonolamine-binding protein (PEBP) | Immune homeostasis by regulation of autophagy and apoptosis | [52] | ||
| CP | GroEL * (Hamiltonella, Arsenophonus) | Hemolymph | Protects virions within hemolymph | [53,54,55] | |
| CP | BON domain containing protein (Rickettsia) * | Hemolymph | Protects virions within hemolymph | [56] | |
| Rep | Proliferating cell nuclear antigen | Salivary gland, midgut | Aids viral replication | [57] | |
| CP (polerovirus) | C1QBP | [58] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghosh, S.; Ghanim, M. Factors Determining Transmission of Persistent Viruses by Bemisia tabaci and Emergence of New Virus–Vector Relationships. Viruses 2021, 13, 1808. https://doi.org/10.3390/v13091808
Ghosh S, Ghanim M. Factors Determining Transmission of Persistent Viruses by Bemisia tabaci and Emergence of New Virus–Vector Relationships. Viruses. 2021; 13(9):1808. https://doi.org/10.3390/v13091808
Chicago/Turabian StyleGhosh, Saptarshi, and Murad Ghanim. 2021. "Factors Determining Transmission of Persistent Viruses by Bemisia tabaci and Emergence of New Virus–Vector Relationships" Viruses 13, no. 9: 1808. https://doi.org/10.3390/v13091808
APA StyleGhosh, S., & Ghanim, M. (2021). Factors Determining Transmission of Persistent Viruses by Bemisia tabaci and Emergence of New Virus–Vector Relationships. Viruses, 13(9), 1808. https://doi.org/10.3390/v13091808







