Bulk Milk Tank Samples Are Suitable to Assess Circulation of Tick-Borne Encephalitis Virus in High Endemic Areas
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. TBEV Detection and Viral Load Quantification
2.3. TBEV Isolation
3. Results
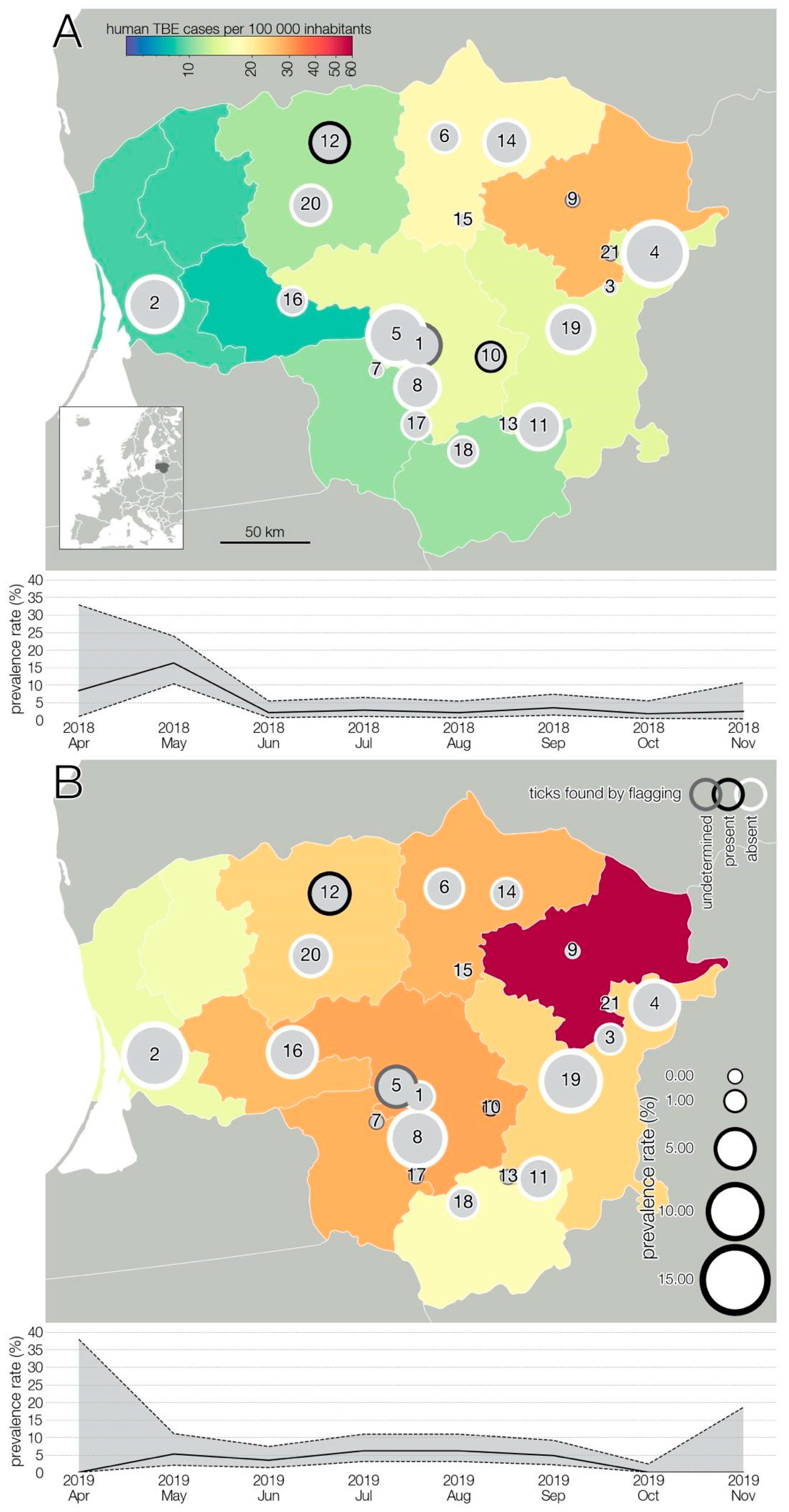
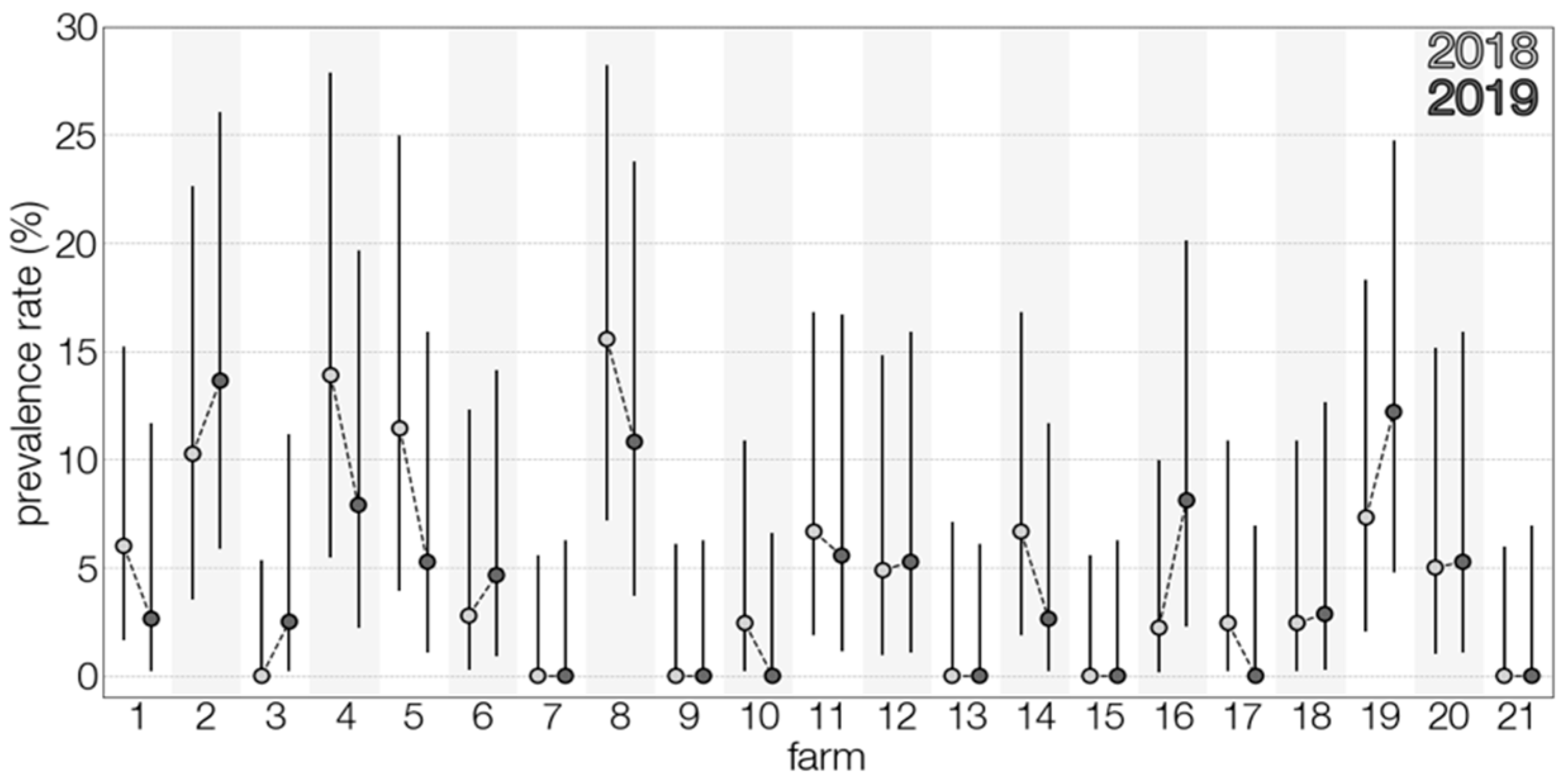
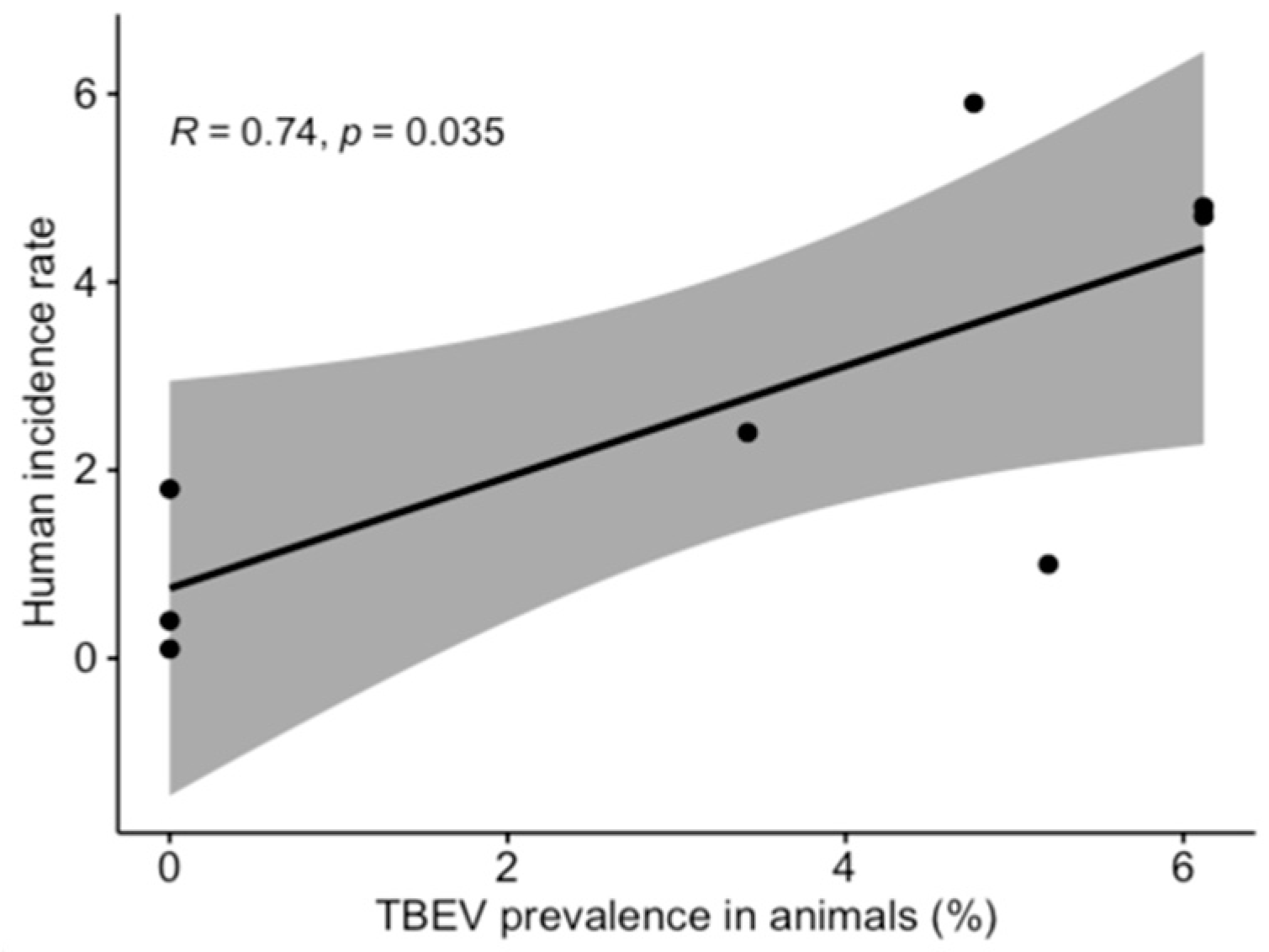
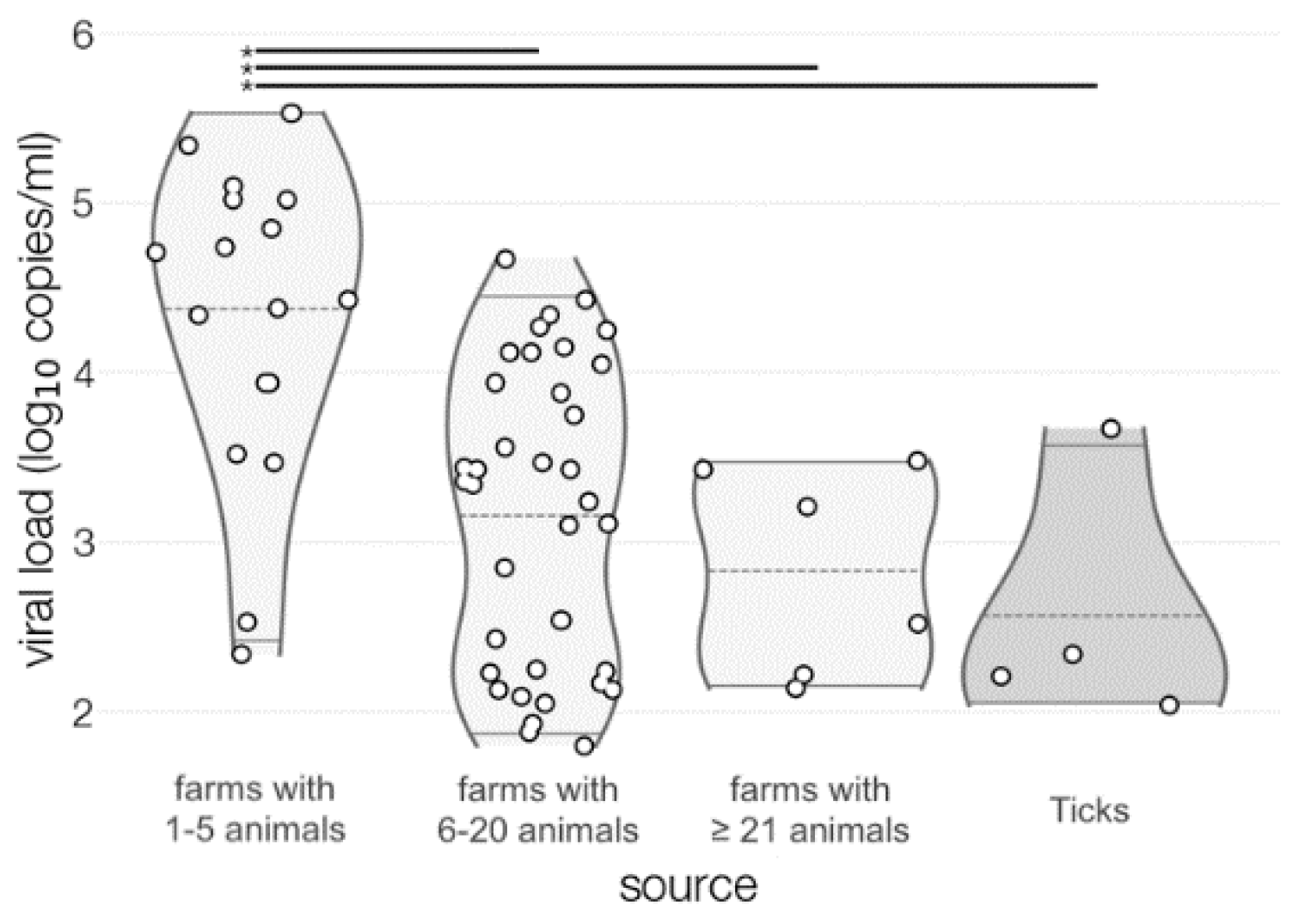
3.1. TBEV Prevalence in Milk
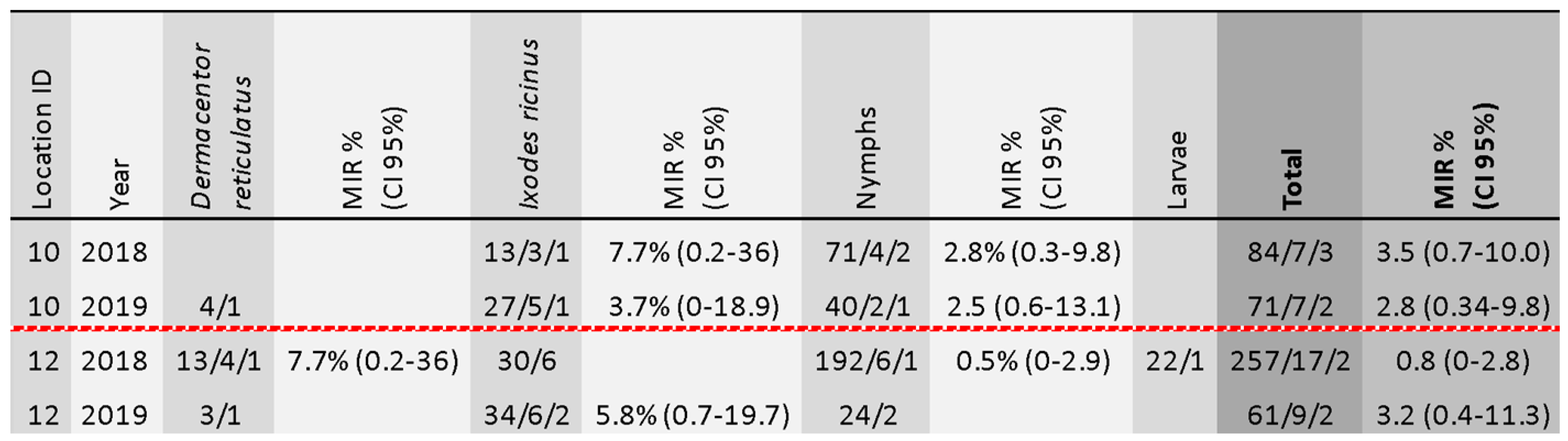
3.2. TBEV Prevalence in Ticks
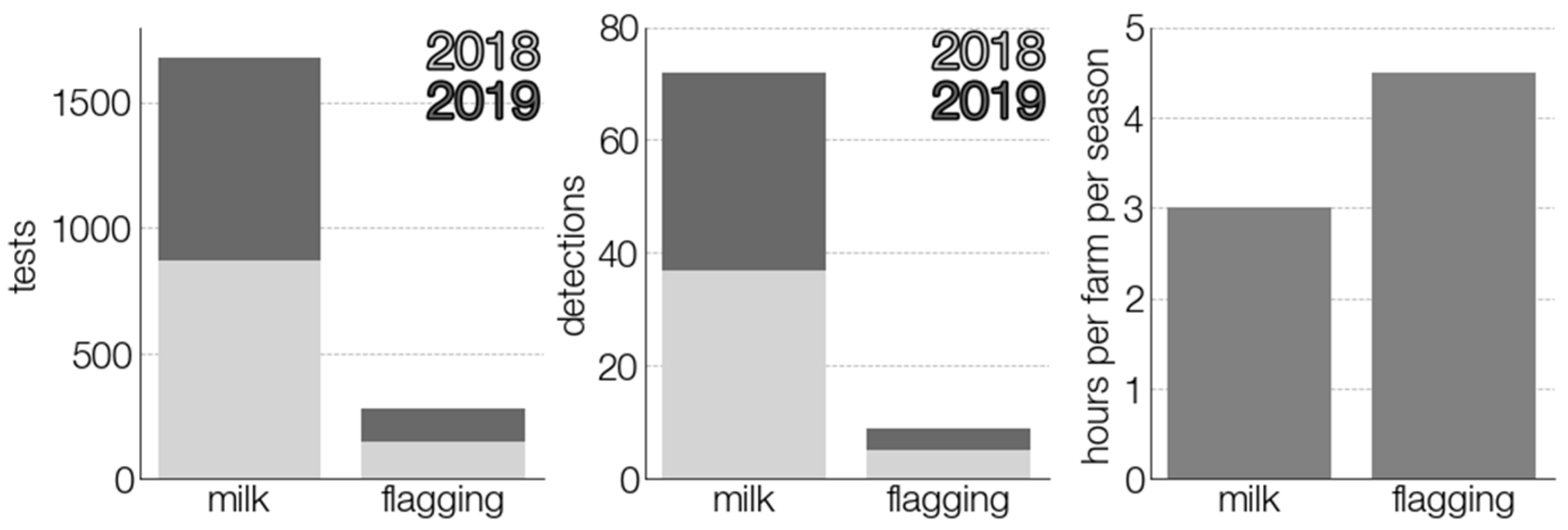
3.3. Comparison of TBEV Detection Methods
3.4. Virus Isolation
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Süss, J. Tick-borne encephalitis 2010: Epidemiology, risk areas, and virus strains in Europe and Asia—An overview. Ticks Tick-Borne Dis. 2011, 2, 2–15. [Google Scholar] [CrossRef]
- Varlacher, J.-F.; Hägglund, S.; Juremalm, M.; Blomqvist, G.; Renström, L.; Zohari, S.; Leijon, M.; Chirico, J. Tick-borne encephalitis: -EN- -FR- Encéphalite transmise par les tiques -ES- Encefalitis transmitida por garrapatas. Rev. Sci. Tech. OIE 2015, 34, 453–466. [Google Scholar] [CrossRef]
- Ilic, M.; Barbic, L.; Bogdanic, M.; Tabain, I.; Savic, V.; Licina, M.L.K.; Kaic, B.; Jungic, A.; Vucelja, M.; Angelov, V.; et al. Tick-borne encephalitis outbreak following raw goat milk consumption in a new micro-location, Croatia, June 2019. Ticks Tick-Borne Dis. 2020, 11, 101513. [Google Scholar] [CrossRef]
- Caini, S.; Szomor, K.; Ferenczi, E.; Gáspár, Á.S.; Csohán, Á.; Krisztalovics, K.; Molnár, Z.; Horváth, J.K. Tick-borne encephalitis transmitted by unpasteurised cow milk in western Hungary, September to October 2011. Eurosurveillance 2012, 17, 20128. [Google Scholar] [CrossRef]
- Holzmann, H.; Aberle, S.W.; Stiasny, K.; Werner, P.; Mischak, A.; Zainer, B.; Netzer, M.; Koppi, S.; Bechter, E.; Heinz, F.X. Tick-borne Encephalitis from Eating Goat Cheese in a Mountain Region of Austria. Emerg. Infect. Dis. 2009, 15, 1671–1673. [Google Scholar] [CrossRef] [PubMed]
- Hudopisk, N.; Korva, M.; Janet, E.; Simetinger, M.; Grgič-Vitek, M.; Gubensek, J.; Natek, V.; Kraigher, A.; Strle, F.; Avšič-Županc, T. Tick-borne Encephalitis Associated with Consumption of Raw Goat Milk, Slovenia, 2012. Emerg. Infect. Dis. 2013, 19, 806–808. [Google Scholar] [CrossRef]
- Markovinović, L.; Kosanović Ličina, M.L.; Tešić, V.; Vojvodić, D.; Vladušić Lucić, I.; Kniewald, T.; Kutleša, M.; Krajinović, L.C. An outbreak of tick-borne encephalitis associated with raw goat milk and cheese consumption, Croatia, 2015. Infection 2016, 44, 661–665. [Google Scholar] [CrossRef] [PubMed]
- Walter, M.; Vogelgesang, J.R.; Rubel, F.; Brugger, K. Tick-Borne Encephalitis Virus and Its European Distribution in Ticks and En-dothermic Mammals. Microorganisms 2020, 8, 1065. [Google Scholar] [CrossRef] [PubMed]
- Erber, W.; Schmitt, H.-J. Self-reported tick-borne encephalitis (TBE) vaccination coverage in Europe: Results from a cross-sectional study. Ticks Tick-Borne Dis. 2018, 9, 768–777. [Google Scholar] [CrossRef] [PubMed]
- Süss, J. Tick-borne encephalitis in Europe and beyond—The epidemiological situation as of 2007. Eurosurveillance 2008, 13, 18916. [Google Scholar] [CrossRef] [PubMed]
- Daniel, M.; Kříž, B.; Danielová, V.; Valter, J.; Kott, I. Correlation between meteorological factors and tick-borne encephalitis inci-dence in the Czech Republic. Parasitol. Res. 2008, 103, 97–107. [Google Scholar] [CrossRef]
- Randolph, S.E. Tick-borne encephalitis incidence in Central and Eastern Europe: Consequences of political transition. Microbes Infect. 2008, 10, 209–216. [Google Scholar] [CrossRef]
- Randolph, S.E. Collective on behalf of the EDEN-TBD sub-project team Human activities predominate in determining changing incidence of tick-borne encephalitis in Europe. Eurosurveillance 2010, 15, 19606–19631. [Google Scholar] [CrossRef] [PubMed]
- Süss, J.; Schrader, C.; Falk, U.; Wohanka, N. Tick-borne encephalitis (TBE) in Germany—Epidemiological data, development of risk areas and virus prevalence in field-collected ticks and in ticks removed from humans. Int. J. Med Microbiol. Suppl. 2004, 293, 69–79. [Google Scholar] [CrossRef]
- Stefanoff, P.; Pfeffer, M.; Hellenbrand, W.; Rogalska, J.; Rühe, F.; Makówka, A.; Michalik, J.; Wodecka, B.; Rymaszewska, A.; Kiewra, D. Virus Detection in Questing Ticks is not a Sen-sitive Indicator for Risk Assessment of Tick-Borne Encephalitis in Humans: TBEV Detection in Ticks to Assess TBE Risk. Zo-Onoses Public Health 2013, 60, 215–226. [Google Scholar] [CrossRef] [PubMed]
- Blomqvist, G.; Näslund, K.; Svensson, L.; Beck, C.; Valarcher, J.F. Mapping geographical areas at risk for tick-borne encephalitis (TBE) by analysing bulk tank milk from Swedish dairy cattle herds for the presence of TBE virus–specific antibodies. Acta Veter- Scand. 2021, 63, 1–9. [Google Scholar] [CrossRef]
- Wallenhammar, A.; Lindqvist, R.; Asghar, N.; Gunaltay, S.; Fredlund, H.; Davidsson, Å.; Andersson, S.; Överby, A.K.; Johansson, M. Revealing new tick-borne encephali-tis virus foci by screening antibodies in sheep milk. Parasites Vectors 2020, 13, 185. [Google Scholar] [CrossRef] [PubMed]
- Paulsen, K.M.; Stuen, S.; das Neves, C.G.; Suhel, F.; Gurung, D.; Soleng, A.; Stiasny, K.; Vikse, R.; Andreassen, Å.K.; Granquist, E.G. Tick-borne encephalitis virus in cows and unpas-teurized cow milk from Norway. Zoonoses Public Health 2019, 66, 216–222. [Google Scholar] [CrossRef] [PubMed]
- Cisak, E.; Wójcik-Fatla, A.; Zając, V.; Sroka, J.; Buczek, A.; Dutkiewicz, J. Prevalence of tick-borne encephalitis virus (TBEV) in samples of raw milk taken randomly from cows, goats and sheep in eastern Poland. Ann. Agric. Environ. Med. 2010, 17, 283–286. [Google Scholar]
- Schwaiger, M.; Cassinotti, P. Development of a quantitative real-time RT-PCR assay with internal control for the laboratory detection of tick borne encephalitis virus (TBEV) RNA. J. Clin. Virol. 2003, 27, 136–145. [Google Scholar] [CrossRef]
- Süss, J.; Béziat, P.; Ramelow, C.; Kahl, O. Tick-borne encephalitis virus (TBEV)-specific RT-PCR for characterization of natural foci of TBE and for other applications. Zentralblatt für Bakteriologie 1997, 286, 125–138. [Google Scholar] [CrossRef]
- Van Tongeren, H.A. Encephalitis in Austria. IV. Excretion of virus by milk of the experimentally infected goat. Arch Gesamte Virusforsch 1955, 6, 158–162. [Google Scholar] [PubMed]
- Gritsun, T.S.; Lashkevich, V.A.; Gould, E.A. Tick-borne encephalitis. Antivir. Res. 2003, 57, 129–146. [Google Scholar] [CrossRef]
- Balogh, Z.; Egyed, L.; Ferenczi, E.; Bán, E.; Szomor, K.N.; Takács, M.; Berencsi, G. Experimental Infection of Goats with Tick-Borne Enceph-alitis Virus and the Possibilities to Prevent Virus Transmission by Raw Goat Milk. Intervirology 2012, 55, 194–200. [Google Scholar] [CrossRef] [PubMed]
- Sidorenko, M.; Radzijevskaja, J.; Mickevičius, S.; Bratčikovienė, N.; Paulauskas, A. Prevalence of tick-borne encephalitis virus in questing Dermacentor reticulatus and Ixodes ricinus ticks in Lithuania. Ticks Tick-Borne Diseases 2021, 12, 101594. [Google Scholar] [CrossRef] [PubMed]
- Rieille, N.; Bressanelli, S.; Freire, C.C.M.; Arcioni, S.; Gern, L.; Péter, O.; Voordouw, M.J. Prevalence and phylogenetic analysis of tick-borne en-cephalitis virus (TBEV) in field-collected ticks (Ixodes ricinus) in southern Switzerland. Parasit Vectors 2014, 7, 443. [Google Scholar] [CrossRef] [PubMed]
- Lommano, E.; Burri, C.; Maeder, G.; Guerne, M.; Bastic, V.; Patalas, E.; Gern, L. Prevalence and Genotyping of Tick-Borne Encephalitis Virus in QuestingIxodes ricinusTicks in a New Endemic Area in Western Switzerland. J. Med. Èntomol. 2012, 49, 156–164. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Randolph, S.E.; Asokliene, L.; Avsic-Zupanc, T.; Bormane, A.; Burri, C.; Gern, L.; Golovljova, I.; Hubalek, Z.; Knap, N.; Kondrusik, M.; et al. Variable spikes in tick-borne encephalitis in-cidence in 2006 independent of variable tick abundance but related to weather. Parasites Vectors 2008, 1, 44. [Google Scholar] [CrossRef]
- Bormane, A.; Lucenko, I.; Duks, A.; Mavtchoutko, V.; Ranka, R.; Salmina, K.; Baumanis, V. Vectors of tick-borne diseases and epidemiologi-cal situation in latvia in 1993–2002. Int. J. Med Microbiol. Suppl. 2004, 293, 36–47. [Google Scholar]
- Oehme, R.; Hartelt, K.; Backe, H.; Brockmann, S.; Kimmig, P. Foci of tick-borne diseases in Southwest Germany. Int. J. Med Microbiol. 2002, 291, 22–29. [Google Scholar] [CrossRef]
- Gäumann, R.; Mühlemann, K.; Strasser, M.; Beuret, C.M. High-Throughput Procedure for Tick Surveys of Tick-Borne Encephalitis Virus and Its Application in a National Surveillance Study in Switzerland. Appl. Environ. Microbiol. 2010, 76, 4241–4249. [Google Scholar] [CrossRef]
- Imhoff, M.; Hagedorn, P.; Schulze, Y.; Hellenbrand, W.; Pfeffer, M.; Niedrig, M. Review: Sentinels of tick-borne encephalitis risk. Ticks Tick-Borne Dis. 2015, 6, 592–600. [Google Scholar] [CrossRef]
- Piesman, J.; Eisen, L. Prevention of Tick-Borne Diseases. Annu. Rev. Èntomol. 2008, 53, 323–343. [Google Scholar] [CrossRef]
- Pautienius, A.; Armonaite, A.; Simkute, E.; Zagrabskaite, R.; Buitkuviene, J.; Alpizar-Jara, R.; Grigas, J.; Zakiene, I.; Zienius, D.; Salomskas, A.; et al. Cross-Sectional Study on the Prevalence and Factors Influencing Occurrence of Tick-Borne Encephalitis in Horses in Lithuania. Pathog 2021, 10, 140. [Google Scholar] [CrossRef]
- Belova, O.A.; Burenkova, L.A.; Karganova, G.G. Different tick-borne encephalitis virus (TBEV) prevalences in unfed versus par-tially engorged ixodid ticks—Evidence of virus replication and changes in tick behavior. Ticks Tick-Borne Dis. 2012, 3, 240–246. [Google Scholar] [CrossRef]
- Updated Guidelines for Evaluating Public Health Surveillance Systems; Recommendations from the Guidelines Working Group. Centers for Disease Control and Prevention. v. 50, no. RR-13; 2001. Available online: https://stacks.cdc.gov/view/cdc/13376 (accessed on 1 September 2021).
- Radzišauskienė, D.; Žagminas, K.; Ašoklienė, L.; Jasionis, A.; Mameniškienė, R.; Ambrozaitis, A.; Jančorienė, L.; Jatužis, D.; Petraitytė, I.; Mockienė, E. Epidemiological patterns of tick-borne encephalitis in Lithuania and clinical features in adults in the light of the high incidence in recent years: A retro-spective study. Eur. J. Neurol. 2018, 25, 268–274. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, M.; Nicolas, G.; Cinardi, G.; Van Boeckel, T.P.; Vanwambeke, S.; Wint, G.R.W.; Robinson, T.P. Global distribution data for cattle, buffaloes, horses, sheep, goats, pigs, chickens and ducks in 2010. Sci. Data 2018, 5, 180227. [Google Scholar] [CrossRef]
- Brockmann, S.; Oehme, R.; Buckenmaier, T.; Beer, M.; Jeffery-Smith, A.; Spannenkrebs, M.; Haag-Milz, S.; Wagner-Wiening, C.; Schlegel, C.; Fritz, J.; et al. A cluster of two human cases of tick-borne encephalitis (TBE) transmitted by unpasteurised goat milk and cheese in Germany, May 2016. Eurosurveillance 2018, 23. [Google Scholar] [CrossRef] [PubMed]
- Klaus, C.; Hoffmann, B.; Moog, U.; Schau, U.; Beer, M.; Süss, J. Can goats be used as sentinels for tick-borne encephalitis (TBE) in nonendemic areas? Experimental studies and epizootiological observations. Berl. Munch. Tierarztl. Wochenschr. 2010, 123, 441–445. [Google Scholar] [PubMed]
- Klaus, C.; Beer, M.; Saier, R.; Schau, U.; Moog, U.; Hoffmann, B.; Diller, R.; Süss, J. Goats and sheep as sentinels for tick-borne encephalitis (TBE) virus—Epidemiological studies in areas endemic and non-endemic for TBE virus in Germany. Ticks Tick-Borne Dis. 2012, 3, 27–37. [Google Scholar] [CrossRef]
- Rizzoli, A.; Neteler, M.; Rosa, R.; Versini, W.; Cristofolini, A.; Bregoli, M.; Buckley, A.; Gould, E.A. Early detection of tick-borne encephalitis virus spatial distribution and activity in the province of Trento, northern Italy. Geospat. Heal. 2007, 1, 169. [Google Scholar] [CrossRef] [PubMed]
- Skarphedinsson, S.; Jensen, P.M.; Kristiansen, K. Survey of Tickborne Infections in Denmark. Emerg. Infect. Dis. 2005, 11, 1055–1061. [Google Scholar] [CrossRef] [PubMed]
- Wurm, R.; Dobler, G.; Peters, M.; Kiessig, S.T. Serological Investigations of Red Foxes (Vulpes vulpes L.) for Determination of the Spread of Tick-borne Encephalitis in Northrhine-Westphalia. J. Vet. Med. Ser. B 2000, 47, 503–509. [Google Scholar] [CrossRef] [PubMed]
- Burri, C.; Korva, M.; Bastic, V.; Knap, N.; Avsic-Zupanc, T.; Gern, L. Serological evidence of tick-borne encephalitis virus infection in rodents captured at four sites in Switzerland. J. Med. Èntomol. 2012, 49, 436–439. [Google Scholar] [CrossRef]
- Lindhe, K.E.; Meldgaard, D.S.; Jensen, P.M.; A Houser, G.; Berendt, M. Prevalence of tick-borne encephalitis virus antibodies in dogs from Denmark. Acta Vet. Scand. 2009, 51, 56. [Google Scholar] [CrossRef]
- Klaus, C.; Ziegler, U.; Kalthoff, D.; Hoffmann, B.; Beer, M. Tick-borne encephalitis virus (TBEV)—Findings on cross reactivity and longevity of TBEV antibodies in animal sera. BMC Vet. Res. 2014, 10, 78. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pautienius, A.; Dudas, G.; Simkute, E.; Grigas, J.; Zakiene, I.; Paulauskas, A.; Armonaite, A.; Zienius, D.; Slyzius, E.; Stankevicius, A. Bulk Milk Tank Samples Are Suitable to Assess Circulation of Tick-Borne Encephalitis Virus in High Endemic Areas. Viruses 2021, 13, 1772. https://doi.org/10.3390/v13091772
Pautienius A, Dudas G, Simkute E, Grigas J, Zakiene I, Paulauskas A, Armonaite A, Zienius D, Slyzius E, Stankevicius A. Bulk Milk Tank Samples Are Suitable to Assess Circulation of Tick-Borne Encephalitis Virus in High Endemic Areas. Viruses. 2021; 13(9):1772. https://doi.org/10.3390/v13091772
Chicago/Turabian StylePautienius, Arnoldas, Gytis Dudas, Evelina Simkute, Juozas Grigas, Indre Zakiene, Algimantas Paulauskas, Austeja Armonaite, Dainius Zienius, Evaldas Slyzius, and Arunas Stankevicius. 2021. "Bulk Milk Tank Samples Are Suitable to Assess Circulation of Tick-Borne Encephalitis Virus in High Endemic Areas" Viruses 13, no. 9: 1772. https://doi.org/10.3390/v13091772
APA StylePautienius, A., Dudas, G., Simkute, E., Grigas, J., Zakiene, I., Paulauskas, A., Armonaite, A., Zienius, D., Slyzius, E., & Stankevicius, A. (2021). Bulk Milk Tank Samples Are Suitable to Assess Circulation of Tick-Borne Encephalitis Virus in High Endemic Areas. Viruses, 13(9), 1772. https://doi.org/10.3390/v13091772







