A Gut Reaction to SIV and SHIV Infection: Lower Dysregulation of Mucosal T Cells during Acute Infection Is Associated with Greater Viral Suppression during cART
Abstract
:1. Introduction
2. Materials and Methods
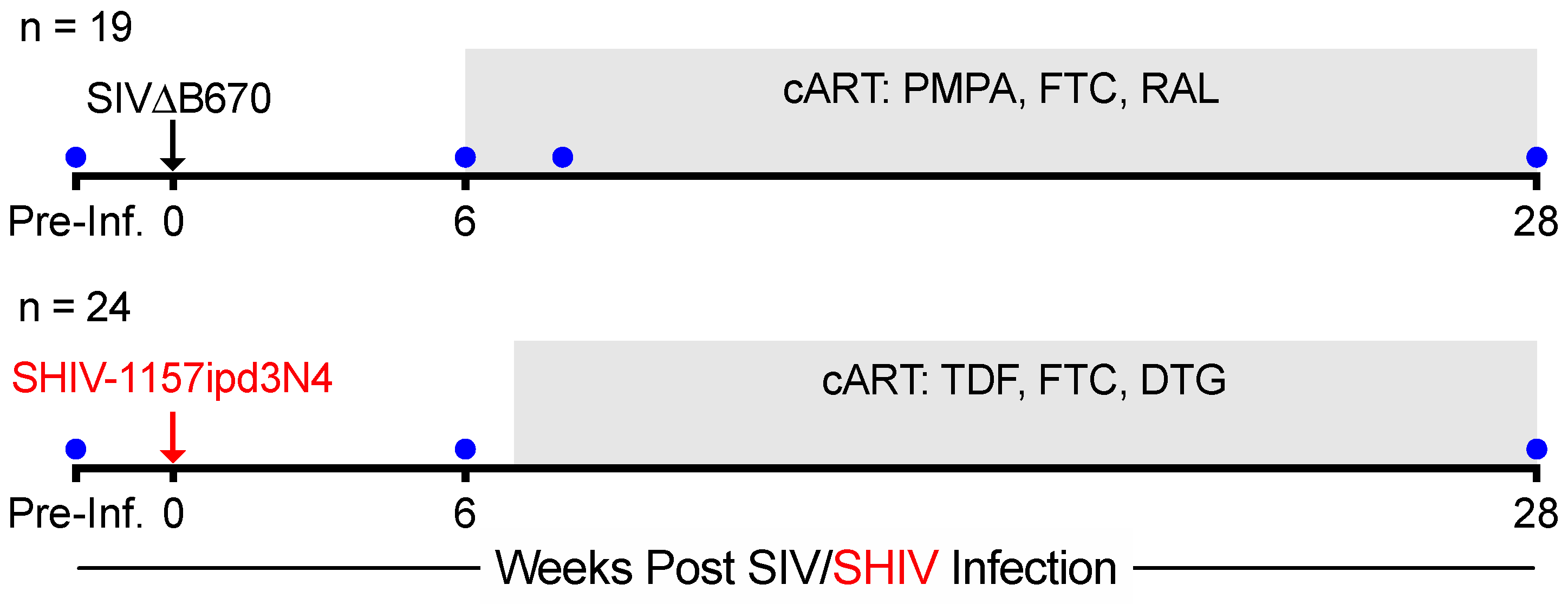
2.1. Animals, Infection, Antiretroviral Therapy, and Specimen Collection
2.2. Plasma Viral Load Quantification
2.3. Intracellular Cytokine Staining
2.4. Blood CD4 and Neutrophil Counts
2.5. Statistical Analyses
3. Results
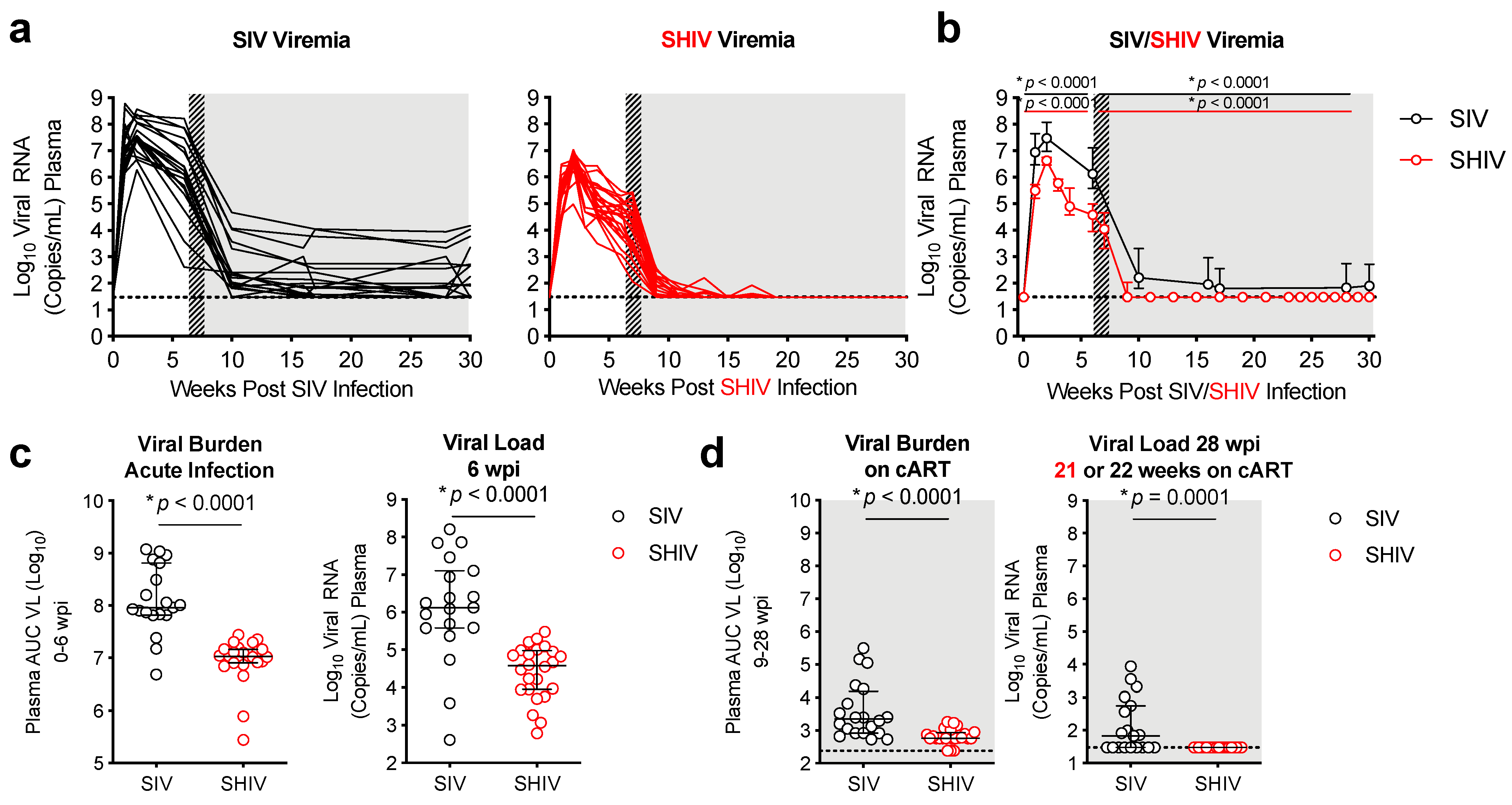
3.1. Lower Viral Burden and Viral Control during cART in SHIV-1157ipd3N4-versus SIVΔB670-Infected Macaques
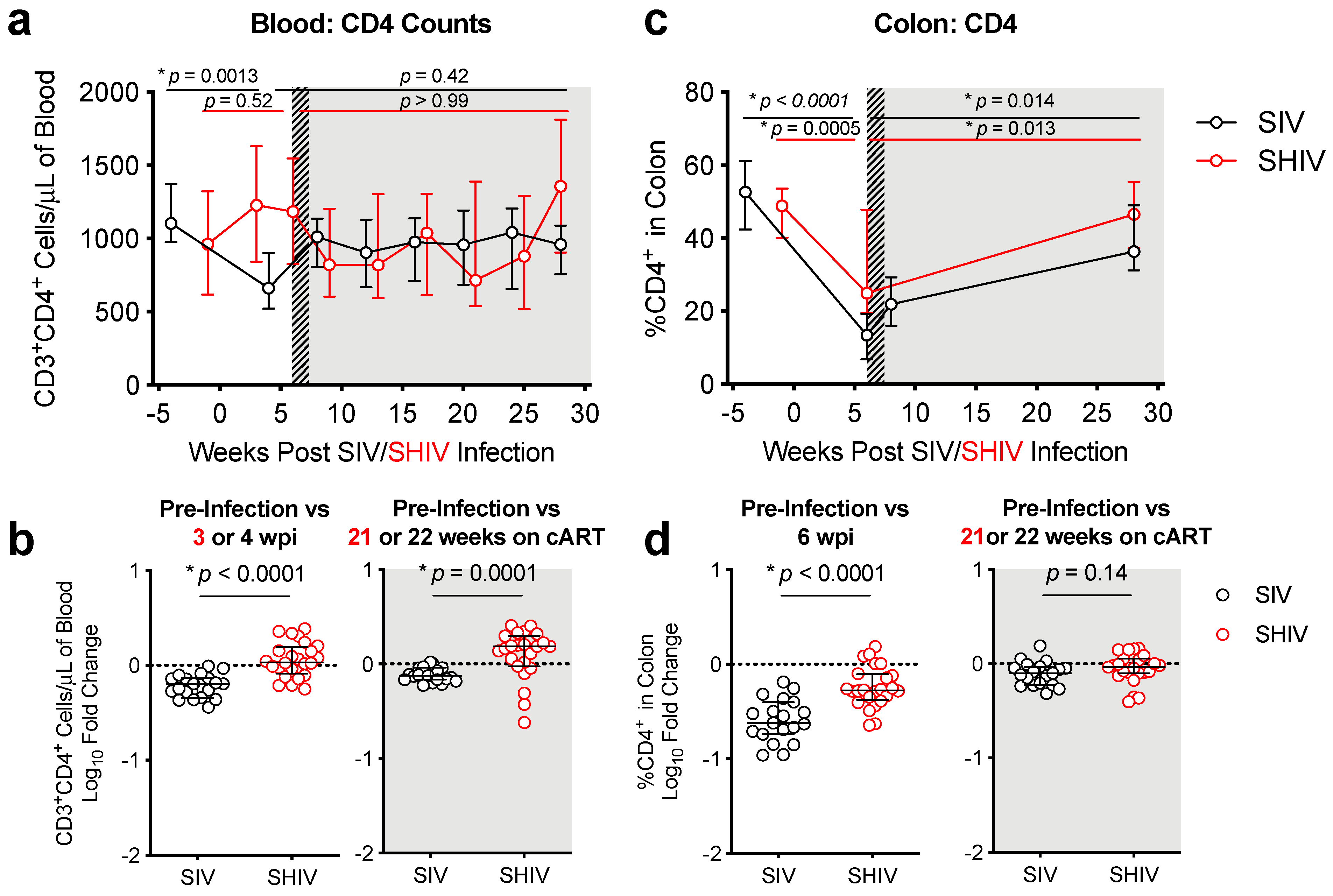
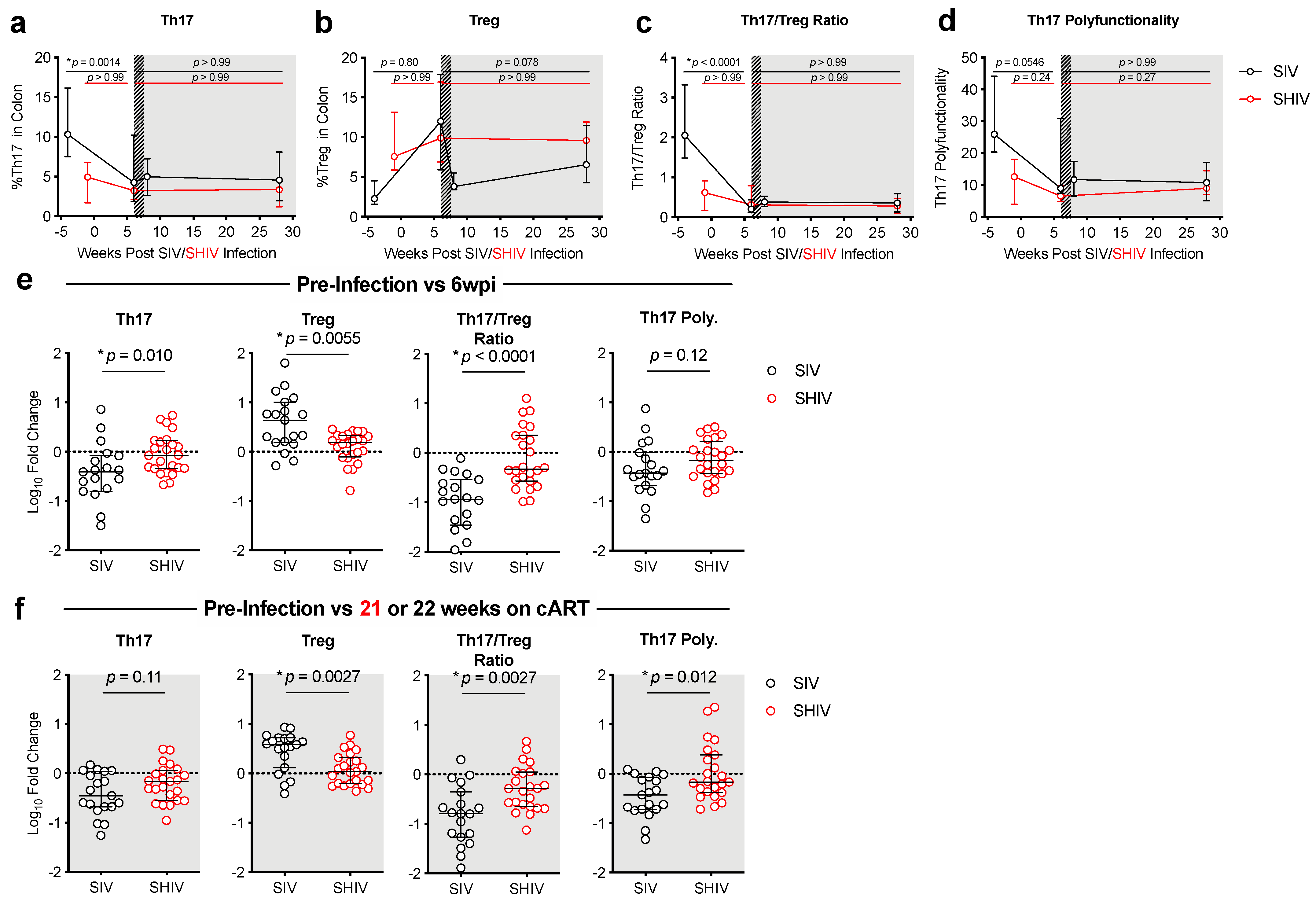
3.2. SHIV-1157ipd3N4 Infected Macaques Exhibited Less Peripheral CD4 Depletion and Lower Gut Immune Dysfunction and Immune Activation Than SIVΔB670-Infected Rhesus Macaques
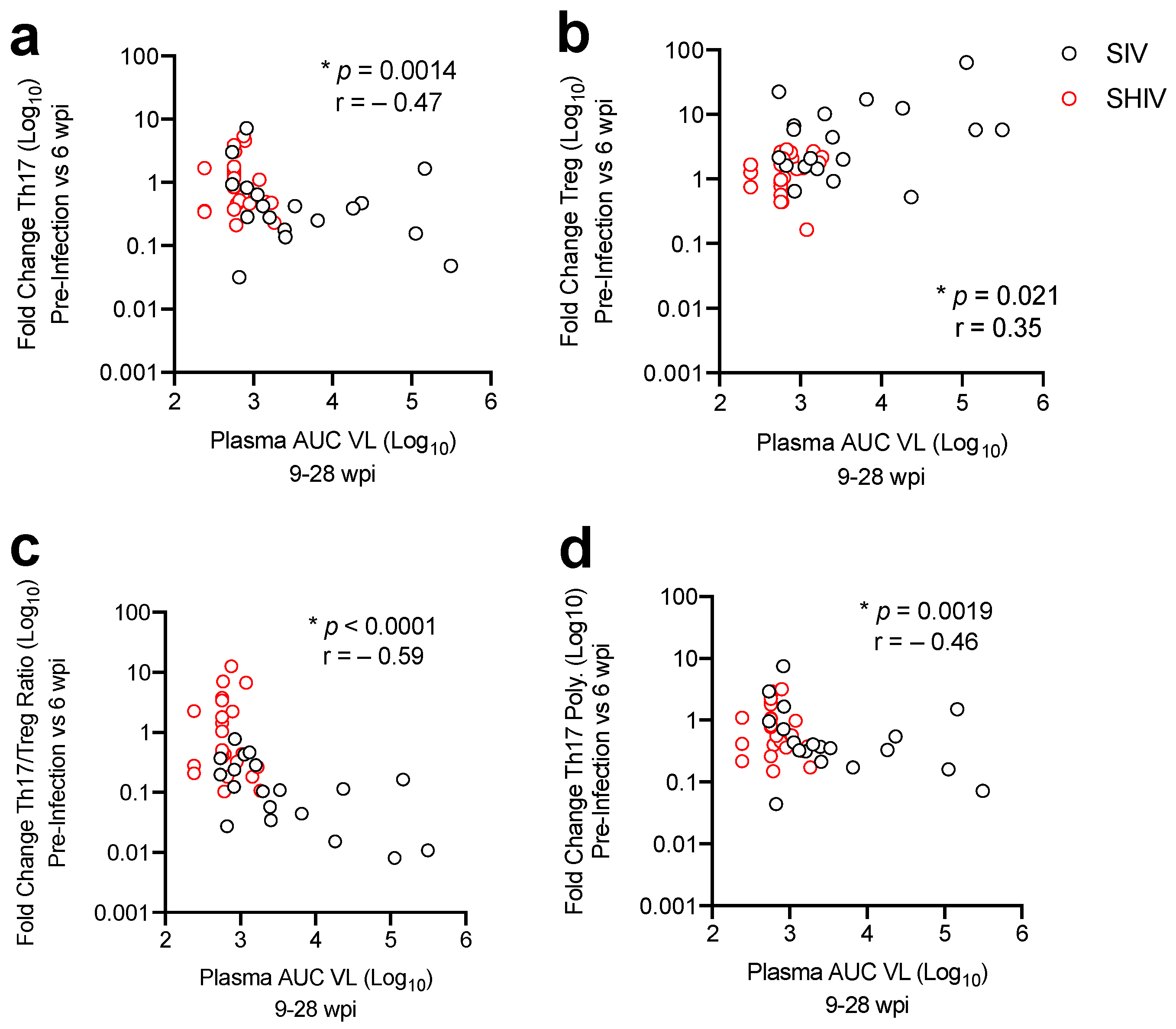
3.3. Lower Acute Gut Immune Dysfunction in SIV/SHIV-Infected Macaques Is Associated with Greater Viral Control by cART
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Evans, D.T.; Silvestri, G. Non-Human Primate Models in AIDS Research. Curr. Opin. HIV AIDS 2013, 8, 255–261. [Google Scholar] [CrossRef] [Green Version]
- Hatziioannou, T.; Evans, D.T. Animal Models for HIV/AIDS Research. Nat. Rev. Microbiol. 2012, 10, 852–867. [Google Scholar] [CrossRef] [PubMed]
- Bar, K.J.; Coronado, E.; Hensley-McBain, T.; O’Connor, M.A.; Osborn, J.M.; Miller, C.; Gott, T.M.; Wangari, S.; Iwayama, N.; Ahrens, C.Y.; et al. Simian-Human Immunodeficiency Virus SHIV.CH505 Infection of Rhesus Macaques Results in Persistent Viral Replication and Induces Intestinal Immunopathology. J. Virol. 2019, 93. [Google Scholar] [CrossRef] [Green Version]
- Favre, D.; Lederer, S.; Kanwar, B.; Ma, Z.-M.; Proll, S.; Kasakow, Z.; Mold, J.; Swainson, L.; Barbour, J.D.; Baskin, C.R.; et al. Critical Loss of the Balance between Th17 and T Regulatory Cell Populations in Pathogenic SIV Infection. PLoS Pathog. 2009, 5, e1000295. [Google Scholar] [CrossRef]
- Khowawisetsut, L.; Pattanapanyasat, K.; Onlamoon, N.; Mayne, A.E.; Little, D.M.; Villinger, F.; Ansari, A.A. Relationships between IL-17(+) Subsets, Tregs and PDCs That Distinguish among SIV Infected Elite Controllers, Low, Medium and High Viral Load Rhesus Macaques. PLoS ONE 2013, 8, e61264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amedee, A.M.; Lacour, N.; Gierman, J.L.; Martin, L.N.; Clements, J.E.; Bohm, R.; Harrison, R.M.; Murphey-Corb, M. Genotypic Selection of Simian Immunodeficiency Virus in Macaque Infants Infected Transplacentally. J. Virol. 1995, 69, 7982–7990. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hong, F.F.; Mellors, J.W. Changes in HIV Reservoirs during Long-Term Antiretroviral Therapy. Curr. Opin. HIV AIDS 2015, 10, 43–48. [Google Scholar] [CrossRef] [Green Version]
- Hunt, P.W. Th17, Gut, and HIV: Therapeutic Implications. Curr. Opin. HIV AIDS 2010, 5, 189–193. [Google Scholar] [CrossRef]
- Fuller, D.H.; Rajakumar, P.; Che, J.W.; Narendran, A.; Nyaundi, J.; Michael, H.; Yager, E.J.; Stagnar, C.; Wahlberg, B.; Taber, R.; et al. Therapeutic DNA Vaccine Induces Broad T Cell Responses in the Gut and Sustained Protection from Viral Rebound and AIDS in SIV-Infected Rhesus Macaques. PLoS ONE 2012, 7, e33715. [Google Scholar] [CrossRef] [Green Version]
- Tunggal, H.C.; Munson, P.V.; O’Connor, M.A.; Hajari, N.; Dross, S.E.; Bratt, D.; Fuller, J.T.; Bagley, K.; Fuller, D.H. Effects of Therapeutic Vaccination on the Control of SIV in Rhesus Macaques with Variable Responsiveness to Antiretroviral Drugs. PLoS ONE 2021, 16, e0253265. [Google Scholar] [CrossRef]
- O’Connor, M.A.; Munson, P.V.; Tunggal, H.; Hajari, N.; Lewis, T.B.; Bratt, D.; Moats, C.; Smedley, J.; Bagley, K.; Mullins, J.I.; et al. Mucosal T Helper 17 and T Regulatory Cell Homeostasis Correlates with Acute Simian Immunodeficiency Virus Viremia and Responsiveness to Antiretroviral Therapy in Macaques. AIDS Res. Hum. Retrovir. 2019, 35, 295–305. [Google Scholar] [CrossRef]
- Falivene, J.; Ghiglione, Y.; Laufer, N.; Eugenia Socías, M.; Pía Holgado, M.; Julia Ruiz, M.; Maeto, C.; Inés Figueroa, M.; Giavedoni, L.D.; Cahn, P.; et al. Th17 and Th17/Treg Ratio at Early HIV Infection Associate with Protective HIV-Specific CD8(+) T-Cell Responses and Disease Progression. Sci. Rep. 2015, 5, 11511. [Google Scholar] [CrossRef] [Green Version]
- Ponte, R.; Mehraj, V.; Ghali, P.; Couëdel-Courteille, A.; Cheynier, R.; Routy, J.-P. Reversing Gut Damage in HIV Infection: Using Non-Human Primate Models to Instruct Clinical Research. EBioMedicine 2016, 4, 40–49. [Google Scholar] [CrossRef]
- Baskin, G.B.; Murphey-Corb, M.; Watson, E.A.; Martin, L.N. Necropsy Findings in Rhesus Monkeys Experimentally Infected with Cultured Simian Immunodeficiency Virus (SIV)/Delta. Vet. Pathol. 1988, 25, 456–467. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murphey-Corb, M.; Martin, L.N.; Rangan, S.R.; Baskin, G.B.; Gormus, B.J.; Wolf, R.H.; Andes, W.A.; West, M.; Montelaro, R.C. Isolation of an HTLV-III-Related Retrovirus from Macaques with Simian AIDS and Its Possible Origin in Asymptomatic Mangabeys. Nat. Cell Biol. 1986, 321, 435–437. [Google Scholar] [CrossRef] [PubMed]
- Song, R.J.; Chenine, A.-L.; Rasmussen, R.A.; Ruprecht, C.R.; Mirshahidi, S.; Grisson, R.D.; Xu, W.; Whitney, J.B.; Goins, L.M.; Ong, H.; et al. Molecularly Cloned SHIV-1157ipd3N4: A Highly Replication- Competent, Mucosally Transmissible R5 Simian-Human Immunodeficiency Virus Encoding HIV Clade C Env. J. Virol. 2006, 80, 8729–8738. [Google Scholar] [CrossRef] [Green Version]
- Humbert, M.; Rasmussen, R.A.; Song, R.; Ong, H.; Sharma, P.; Chenine, A.L.; Kramer, V.G.; Siddappa, N.B.; Xu, W.; Else, J.G.; et al. SHIV-1157i and Passaged Progeny Viruses Encoding R5 HIV-1 Clade C Env Cause AIDS in Rhesus Monkeys. Retrovirology 2008, 5, 94. [Google Scholar] [CrossRef] [Green Version]
- Smedley, J.; Macalister, R.; Wangari, S.; Gathuka, M.; Ahrens, J.; Iwayama, N.; May, D.; Bratt, D.; O’Connor, M.; Munson, P.; et al. Laparoscopic Technique for Serial Collection of Para-Colonic, Left Colic, and Inferior Mesenteric Lymph Nodes in Macaques. PLoS ONE 2016, 11, e0157535. [Google Scholar] [CrossRef]
- Hofmann-Lehmann, R.; Swenerton, R.K.; Liska, V.; Leutenegger, C.M.; Lutz, H.; McClure, H.M.; Ruprecht, R.M. Sensitive and Robust One-Tube Real-Time Reverse Transcriptase-Polymerase Chain Reaction to Quantify SIV RNA Load: Comparison of One- versus Two-Enzyme Systems. AIDS Res. Hum. Retrovir. 2000, 16, 1247–1257. [Google Scholar] [CrossRef] [PubMed]
- Cline, A.N.; Bess, J.W.; Piatak, M.; Lifson, J.D. Highly Sensitive SIV Plasma Viral Load Assay: Practical Considerations, Realistic Performance Expectations, and Application to Reverse Engineering of Vaccines for AIDS. J. Med. Primatol. 2005, 34, 303–312. [Google Scholar] [CrossRef]
- Ho, O.; Larsen, K.; Polacino, P.; Li, Y.; Anderson, D.; Song, R.; Ruprecht, R.M.; Hu, S.-L. Pathogenic Infection of Macaca Nemestrina with a CCR5-Tropic Subtype-C Simian-Human Immunodeficiency Virus. Retrovirology 2009, 6, 65. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Serrano-Villar, S.; Sainz, T.; Lee, S.A.; Hunt, P.W.; Sinclair, E.; Shacklett, B.L.; Ferre, A.L.; Hayes, T.L.; Somsouk, M.; Hsue, P.Y.; et al. HIV-Infected Individuals with Low CD4/CD8 Ratio despite Effective Antiretroviral Therapy Exhibit Altered T Cell Subsets, Heightened CD8+ T Cell Activation, and Increased Risk of Non-AIDS Morbidity and Mortality. PLoS Pathog. 2014, 10, e1004078. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buggert, M.; Frederiksen, J.; Noyan, K.; Svärd, J.; Barqasho, B.; Sönnerborg, A.; Lund, O.; Nowak, P.; Karlsson, A.C. Multiparametric Bioinformatics Distinguish the CD4/CD8 Ratio as a Suitable Laboratory Predictor of Combined T Cell Pathogenesis in HIV Infection. J. Immunol. 2014, 192, 2099–2108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hensley-McBain, T.; Klatt, N.R. The Dual Role of Neutrophils in HIV Infection. Curr. HIV/AIDS Rep. 2018, 15, 1–10. [Google Scholar] [CrossRef]
- Grimm, D.U.S. Labs Using a Record Number of Monkeys. Science 2018, 362, 630. [Google Scholar] [CrossRef]
- Hoenigl, M.; Chaillon, A.; Moore, D.J.; Morris, S.R.; Mehta, S.R.; Gianella, S.; Amico, K.R.; Little, S.J. Rapid HIV Viral Load Suppression in Those Initiating Antiretroviral Therapy at First Visit after HIV Diagnosis. Sci. Rep. 2016, 6, 32947. [Google Scholar] [CrossRef] [Green Version]
- Pahar, B.; Kuebler, D.; Rasmussen, T.; Wang, X.; Srivastav, S.K.; Das, A.; Veazey, R.S. Quantification of Viral RNA and DNA Positive Cells in Tissues From Simian Immunodeficiency Virus/Simian Human Immunodeficiency Virus Infected Controller and Progressor Rhesus Macaques. Front. Microbiol. 2019, 10, 2933. [Google Scholar] [CrossRef]
- Mannioui, A.; Bourry, O.; Sellier, P.; Delache, B.; Brochard, P.; Andrieu, T.; Vaslin, B.; Karlsson, I.; Roques, P.; Le Grand, R. Dynamics of Viral Replication in Blood and Lymphoid Tissues during SIVmac251 Infection of Macaques. Retrovirology 2009, 6, 106. [Google Scholar] [CrossRef] [Green Version]
- Wong, J.K.; Yukl, S.A. Tissue Reservoirs of HIV. Curr. Opin. HIV AIDS 2016, 11, 362–370. [Google Scholar] [CrossRef]
- Del Prete, G.Q.; Lifson, J.D. Considerations in the Development of Nonhuman Primate Models of Combination Antiretroviral Therapy for Studies of AIDS Virus Suppression, Residual Virus, and Curative Strategies. Curr. Opin. HIV AIDS 2013, 8, 262–272. [Google Scholar] [CrossRef] [Green Version]
- Del Prete, G.Q.; Shoemaker, R.; Oswald, K.; Lara, A.; Trubey, C.M.; Fast, R.; Schneider, D.K.; Kiser, R.; Coalter, V.; Wiles, A.; et al. Effect of Suberoylanilide Hydroxamic Acid (SAHA) Administration on the Residual Virus Pool in a Model of Combination Antiretroviral Therapy-Mediated Suppression in SIVmac239-Infected Indian Rhesus Macaques. Antimicrob. Agents Chemother 2014, 58, 6790–6806. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Podany, A.T.; Scarsi, K.K.; Fletcher, C.V. Comparative Clinical Pharmacokinetics and Pharmacodynamics of HIV-1 Integrase Strand Transfer Inhibitors. Clin. Pharmacokinet. 2017, 56, 25–40. [Google Scholar] [CrossRef] [PubMed]
- Del Prete, G.Q.; Smedley, J.; Macallister, R.; Jones, G.S.; Li, B.; Hattersley, J.; Zheng, J.; Piatak, M.; Keele, B.F.; Hesselgesser, J.; et al. Short Communication: Comparative Evaluation of Coformulated Injectable Combination Antiretroviral Therapy Regimens in Simian Immunodeficiency Virus-Infected Rhesus Macaques. AIDS Res. Hum. Retrovir. 2016, 32, 163–168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crakes, K.R.; Jiang, G. Gut Microbiome Alterations During HIV/SIV Infection: Implications for HIV Cure. Front. Microbiol. 2019, 10, 1104. [Google Scholar] [CrossRef] [PubMed]
- Younas, M.; Psomas, C.; Reynes, J.; Corbeau, P. Immune Activation in the Course of HIV-1 Infection: Causes, Phenotypes and Persistence under Therapy. HIV Med. 2016, 17, 89–105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schuetz, A.; Deleage, C.; Sereti, I.; Rerknimitr, R.; Phanuphak, N.; Phuang-Ngern, Y.; Estes, J.D.; Sandler, N.G.; Sukhumvittaya, S.; Marovich, M.; et al. Initiation of ART during Early Acute HIV Infection Preserves Mucosal Th17 Function and Reverses HIV-Related Immune Activation. PLoS Pathog. 2014, 10, e1004543. [Google Scholar] [CrossRef] [Green Version]
- November 25, C.S.H. govDate Last Updated; 2020 Global Statistics. Available online: https://www.hiv.gov/hiv-basics/overview/data-and-trends/global-statistics (accessed on 12 August 2021).
- Park, L.S.; Tate, J.P.; Sigel, K.; Brown, S.T.; Crothers, K.; Gibert, C.; Goetz, M.B.; Rimland, D.; Rodriguez-Barradas, M.C.; Bedimo, R.J.; et al. Association of Viral Suppression With Lower AIDS-Defining and Non-AIDS-Defining Cancer Incidence in HIV-Infected Veterans: A Prospective Cohort Study. Ann. Intern. Med. 2018, 169, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Martin, G.E.; Gossez, M.; Williams, J.P.; Stöhr, W.; Meyerowitz, J.; Leitman, E.M.; Goulder, P.; Porter, K.; Fidler, S.; Frater, J.; et al. Post-Treatment Control or Treated Controllers? Viral Remission in Treated and Untreated Primary HIV Infection. AIDS 2017, 31, 477–484. [Google Scholar] [CrossRef] [PubMed]
- Namazi, G.; Fajnzylber, J.M.; Aga, E.; Bosch, R.J.; Acosta, E.P.; Sharaf, R.; Hartogensis, W.; Jacobson, J.M.; Connick, E.; Volberding, P.; et al. The Control of HIV After Antiretroviral Medication Pause (CHAMP) Study: Posttreatment Controllers Identified From 14 Clinical Studies. J. Infect. Dis. 2018, 218, 1954–1963. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
O’Connor, M.A.; Munson, P.V.; Dross, S.E.; Tunggal, H.C.; Lewis, T.B.; Osborn, J.; Peterson, C.W.; Huang, M.-L.W.; Moats, C.; Smedley, J.; et al. A Gut Reaction to SIV and SHIV Infection: Lower Dysregulation of Mucosal T Cells during Acute Infection Is Associated with Greater Viral Suppression during cART. Viruses 2021, 13, 1609. https://doi.org/10.3390/v13081609
O’Connor MA, Munson PV, Dross SE, Tunggal HC, Lewis TB, Osborn J, Peterson CW, Huang M-LW, Moats C, Smedley J, et al. A Gut Reaction to SIV and SHIV Infection: Lower Dysregulation of Mucosal T Cells during Acute Infection Is Associated with Greater Viral Suppression during cART. Viruses. 2021; 13(8):1609. https://doi.org/10.3390/v13081609
Chicago/Turabian StyleO’Connor, Megan A., Paul V. Munson, Sandra E. Dross, Hillary C. Tunggal, Thomas B. Lewis, Jessica Osborn, Christopher W. Peterson, Meei-Li W. Huang, Cassandra Moats, Jeremy Smedley, and et al. 2021. "A Gut Reaction to SIV and SHIV Infection: Lower Dysregulation of Mucosal T Cells during Acute Infection Is Associated with Greater Viral Suppression during cART" Viruses 13, no. 8: 1609. https://doi.org/10.3390/v13081609
APA StyleO’Connor, M. A., Munson, P. V., Dross, S. E., Tunggal, H. C., Lewis, T. B., Osborn, J., Peterson, C. W., Huang, M.-L. W., Moats, C., Smedley, J., Jerome, K. R., Kiem, H.-P., Bagley, K. C., Mullins, J. I., & Fuller, D. H. (2021). A Gut Reaction to SIV and SHIV Infection: Lower Dysregulation of Mucosal T Cells during Acute Infection Is Associated with Greater Viral Suppression during cART. Viruses, 13(8), 1609. https://doi.org/10.3390/v13081609






