Proposed Update to the Taxonomy of Pestiviruses: Eight Additional Species within the Genus Pestivirus, Family Flaviviridae
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
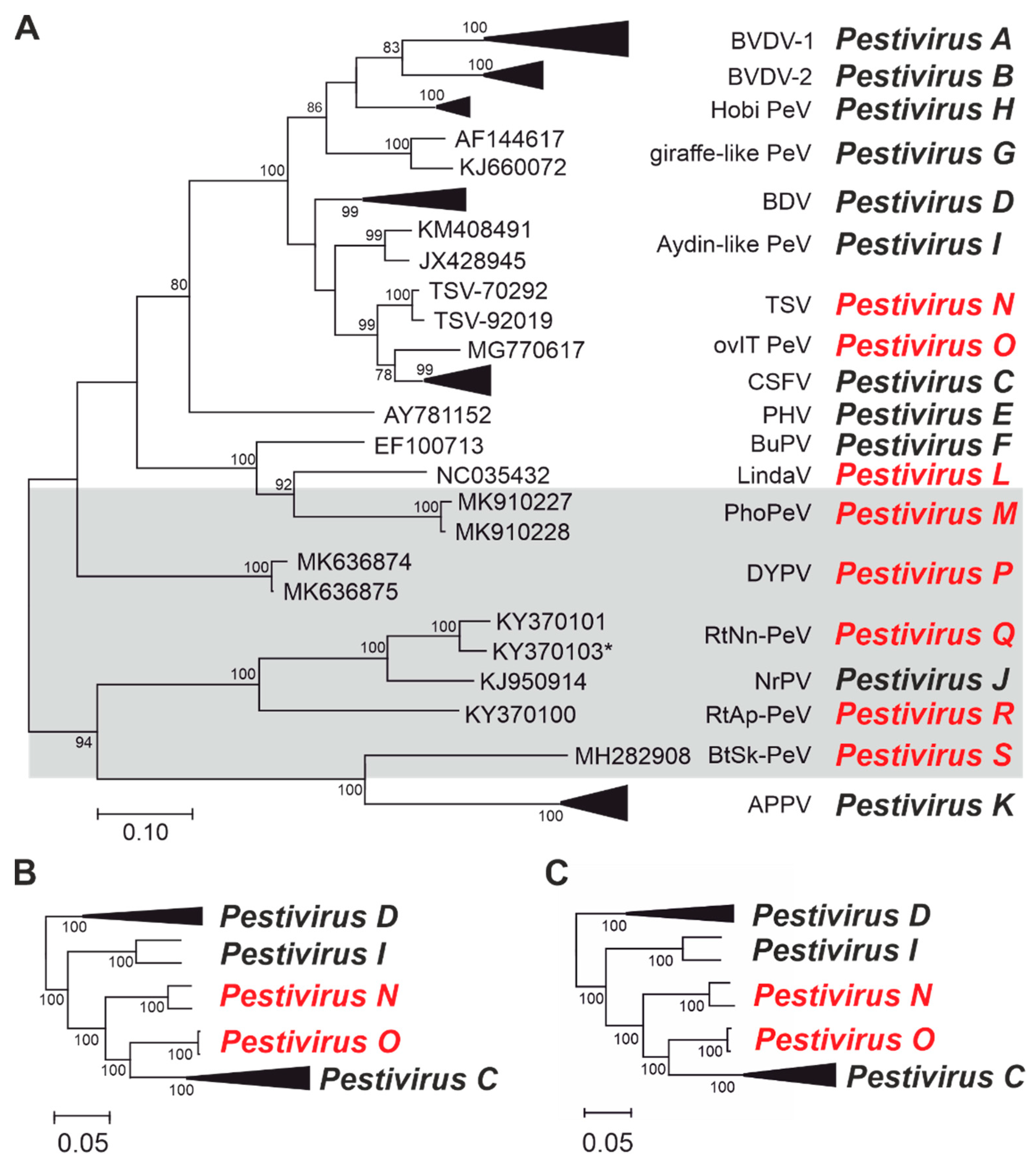
3.1. Update of the Reference Data Set
3.2. Recently Identified Pestiviruses Represent Novel Species
3.2.1. Linda Virus
3.2.2. Phocoena Pestivirus
3.2.3. Pangolin Pestivirus
3.2.4. Bat Pestiviruses
3.2.5. Rodent Pestiviruses
3.2.6. Tunisian Sheep-Like Viruses
3.2.7. Ovine/Italy Pestiviruses
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Simmonds, P.; Becher, P.; Bukh, J.; Gould, E.A.; Meyers, G.; Monath, T.; Muerhoff, S.; Pletnev, A.; Rico-Hesse, R.; Smith, D.B.; et al. ICTV Virus Taxonomy Profile: Flaviviridae. J. Gen. Virol. 2017, 98, 2–3. [Google Scholar] [CrossRef] [PubMed]
- Houe, H. Economic impact of BVDV infection in dairies. Biologicals 2003, 31, 137–143. [Google Scholar] [CrossRef]
- Moennig, V.; Becher, P. Pestivirus control programs: How far have we come and where are we going? Anim. Health Res. Rev. 2015, 16, 83–87. [Google Scholar] [CrossRef] [PubMed]
- Becher, P.; König, M.; Paton, D.J.; Thiel, H.J. Further characterization of Border disease virus isolates: Evidence for the presence of more than three species within the genus Pestivirus. Virology 1995, 209, 200–206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Becher, P.; Orlich, M.; Shannon, A.D.; Horner, G.; Konig, M.; Thiel, H.J. Phylogenetic analysis of pestiviruses from domestic and wild ruminants. J. Gen. Virol. 1997, 78, 1357–1366. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Xia, H.; Wahlberg, N.; Belak, S.; Baule, C. Phylogeny, classification and evolutionary insights into pestiviruses. Virology 2009, 385, 351–357. [Google Scholar] [CrossRef] [Green Version]
- Schirrmeier, H.; Strebelow, G.; Depner, K.; Hoffmann, B.; Beer, M. Genetic and antigenic characterization of an atypical pestivirus isolate, a putative member of a novel pestivirus species. J. Gen. Virol. 2004, 85, 3647–3652. [Google Scholar] [CrossRef]
- Neill, J.D.; Ridpath, J.F.; Fischer, N.; Grundhoff, A.; Postel, A.; Becher, P. Complete genome sequence of pronghorn virus, a pestivirus. Genome Announc. 2014, 2, e00575-14. [Google Scholar] [CrossRef] [Green Version]
- Vilcek, S.; Ridpath, J.F.; Van Campen, H.; Cavender, J.L.; Warg, J. Characterization of a novel pestivirus originating from a pronghorn antelope. Virus Res. 2005, 108, 187–193. [Google Scholar] [CrossRef]
- Kirkland, P.D.; Frost, M.J.; Finlaison, D.S.; King, K.R.; Ridpath, J.F.; Gu, X. Identification of a novel virus in pigs—Bungowannah virus: A possible new species of pestivirus. Virus Res. 2007, 129, 26–34. [Google Scholar] [CrossRef]
- Becher, P.; Schmeiser, S.; Oguzoglu, T.C.; Postel, A. Complete genome sequence of a novel pestivirus from sheep. J. Virol. 2012, 86, 11412. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Postel, A.; Schmeiser, S.; Oguzoglu, T.C.; Indenbirken, D.; Alawi, M.; Fischer, N.; Grundhoff, A.; Becher, P. Close relationship of ruminant pestiviruses and classical swine fever virus. Emerg. Infect. Dis. 2015, 21, 668–672. [Google Scholar] [CrossRef] [PubMed]
- Sevik, M. Genomic characterization of pestiviruses isolated from bovine, ovine and caprine foetuses in turkey: A potentially new genotype of Pestivirus I species. Transbound. Emerg. Dis. 2020, 8, 417–442. [Google Scholar]
- Hause, B.; Collin, E.A.; Peddireddi, L.; Yuan, F.; Chen, Z.; Hesse, R.A.; Gauger, P.C.; Clement, T.; Fang, Y.; Anderson, G. Discovery of a novel putative atypical porcine pestivirus in pigs in the United States. J. Gen. Virol. 2015, 96, 2994–2998. [Google Scholar] [CrossRef] [PubMed]
- Postel, A.; Hansmann, F.; Baechlein, C.; Fischer, N.; Alawi, M.; Grundhoff, A.; Derking, S.; Tenhundfeld, J.; Pfankuche, V.M.; Herder, V.; et al. Presence of atypical porcine pestivirus (APPV) genomes in newborn piglets correlates with congenital tremor. Sci. Rep. 2016, 6, 27735. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Postel, A.; Meyer, D.; Cagatay, G.N.; Feliziani, F.; De Mia, G.M.; Fischer, N.; Grundhoff, A.; Milicevic, V.; Deng, M.C.; Chang, C.Y.; et al. High abundance and genetic variability of atypical porcine pestivirus in pigs from Europe and Asia. Emerg. Infect. Dis. 2017, 23, 2104–2107. [Google Scholar] [CrossRef] [Green Version]
- Arruda, B.L.; Arruda, P.H.; Magstadt, D.R.; Schwartz, K.J.; Dohlman, T.; Schleining, J.A.; Patterson, A.R.; Visek, C.A.; Victoria, J.G. Identification of a divergent lineage porcine pestivirus in nursing piglets with congenital tremors and reproduction of disease following experimental inoculation. PLoS ONE 2016, 11, e0150104. [Google Scholar] [CrossRef] [Green Version]
- de Groof, A.; Deijs, M.; Guelen, L.; van Grinsven, L.; van Os-Galdos, L.; Vogels, W.; Derks, C.; Cruijsen, T.; Geurts, V.; Vrijenhoek, M.; et al. Atypical porcine pestivirus: A possible cause of congenital tremor type A-II in newborn piglets. Viruses 2016, 8, 271. [Google Scholar] [CrossRef]
- Becher, P.; Moennig, V.; Tautz, N. Bovine viral diarrhea, border disease, and classical swine fever viruses (Flaviviridae). In Encyclopedia of Virology (Fourth Edition); Bamford, D.H., Zuckerman, M., Eds.; Academic Press Amsterdam: Oxford, Cambridge, 2021; Volume 2, pp. 153–164. [Google Scholar]
- Firth, C.; Bhat, M.; Firth, M.A.; Williams, S.H.; Frye, M.J.; Simmonds, P.; Conte, J.M.; Ng, J.; Garcia, J.; Bhuva, N.P.; et al. Detection of zoonotic pathogens and characterization of novel viruses carried by commensal Rattus norvegicus in New York City. mBio 2014, 5, e01933-14. [Google Scholar] [CrossRef] [Green Version]
- Gao, W.H.; Lin, X.D.; Chen, Y.M.; Xie, C.G.; Tan, Z.Z.; Zhou, J.J.; Chen, S.; Holmes, E.C.; Zhang, Y.Z. Newly identified viral genomes in pangolins with fatal disease. Virus Evol. 2020, 6, veaa020. [Google Scholar] [CrossRef]
- Jo, W.K.; van Elk, C.; van de Bildt, M.; van Run, P.; Petry, M.; Jesse, S.T.; Jung, K.; Ludlow, M.; Kuiken, T.; Osterhaus, A. An evolutionary divergent pestivirus lacking the Npro gene systemically infects a whale species. Emerg. Microbes Infect. 2019, 8, 1383–1392. [Google Scholar] [CrossRef] [Green Version]
- Wu, Z.; Liu, B.; Du, J.; Zhang, J.; Lu, L.; Zhu, G.; Han, Y.; Su, H.; Yang, L.; Zhang, S.; et al. Discovery of diverse rodent and bat pestiviruses with distinct genomic and phylogenetic characteristics in several Chinese provinces. Front. Microbiol. 2018, 9, 2562. [Google Scholar] [CrossRef] [Green Version]
- Wu, Z.; Lu, L.; Du, J.; Yang, L.; Ren, X.; Liu, B.; Jiang, J.; Yang, J.; Dong, J.; Sun, L.; et al. Comparative analysis of rodent and small mammal viromes to better understand the wildlife origin of emerging infectious diseases. Microbiome 2018, 6, 178. [Google Scholar] [CrossRef]
- Wu, Z.; Ren, X.; Yang, L.; Hu, Y.; Yang, J.; He, G.; Zhang, J.; Dong, J.; Sun, L.; Du, J.; et al. Virome analysis for identification of novel mammalian viruses in bat species from Chinese provinces. J. Virol. 2012, 86, 10999–11012. [Google Scholar] [CrossRef] [Green Version]
- Smith, D.B.; Meyers, G.; Bukh, J.; Gould, E.A.; Monath, T.; Scott Muerhoff, A.; Pletnev, A.; Rico-Hesse, R.; Stapleton, J.T.; Simmonds, P.; et al. Proposed revision to the taxonomy of the genus Pestivirus, family Flaviviridae. J. Gen. Virol. 2017, 98, 2106–2112. [Google Scholar] [CrossRef] [PubMed]
- ICTV Report. Flaviviridae, Genus: Pestivirus. Available online: https://talk.ictvonline.org/ictv-reports/ictv_online_report/positive-sense-rna-viruses/w/flaviviridae/361/genus-pestivirus (accessed on 2 January 2021).
- Deng, R.; Brock, K.V. Molecular cloning and nucleotide sequence of a pestivirus genome, noncytopathic bovine viral diarrhea virus strain SD-1. Virology 1992, 191, 867–869. [Google Scholar] [CrossRef]
- Collett, M.S.; Larson, R.; Gold, C.; Strick, D.; Anderson, D.K.; Purchio, A.F. Molecular cloning and nucleotide sequence of the pestivirus bovine viral diarrhea virus. Virology 1988, 165, 191–199. [Google Scholar] [CrossRef]
- Ridpath, J.F.; Bolin, S.R. The genomic sequence of a virulent bovine viral diarrhea virus (BVDV) from the type 2 genotype: Detection of a large genomic insertion in a noncytopathic BVDV. Virology 1995, 212, 39–46. [Google Scholar] [CrossRef]
- Ruggli, N.; Tratschin, J.D.; Mittelholzer, C.; Hofmann, M.A. Nucleotide sequence of classical swine fever virus strain Alfort/187 and transcription of infectious RNA from stably cloned full-length cDNA. J. Virol. 1996, 70, 3478–3487. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Becher, P.; Shannon, A.D.; Tautz, N.; Thiel, H.J. Molecular characterization of Border disease virus, a pestivirus from sheep. Virology 1994, 198, 542–551. [Google Scholar] [CrossRef]
- Becher, P.; Fischer, N.; Grundhoff, A.; Stalder, H.; Schweizer, M.; Postel, A. Complete genome sequence of bovine pestivirus strain PG-2, a second member of the tentative pestivirus species giraffe. Genome Announc. 2014, 2, e00376-14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Becher, P.; Orlich, M.; Kosmidou, A.; König, M.; Baroth, M.; Thiel, H.J. Genetic diversity of pestiviruses: Identification of novel groups and implications for classification. Virology 1999, 262, 64–71. [Google Scholar] [CrossRef] [Green Version]
- Liu, L.; Kampa, J.; Belak, S.; Baule, C. Virus recovery and full-length sequence analysis of atypical bovine pestivirus TH/04_Khonkaen. Vet. Microbiol. 2009, 138, 62–68. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Sameroff, S.; Tokarz, R.; Charles, R.A.; Jain, K.; Oleynik, A.; Che, X.; Georges, K.; Carrington, C.V.; Lipkin, W.I.; Oura, C. Viral diversity of tick species parasitizing cattle and dogs in Trinidad and Tobago. Sci. Rep. 2019, 9, 10421. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ciulli, S.; Purpari, G.; Agnello, S.; Di Marco, P.; Di Bella, S.; Volpe, E.; Mira, F.; de Aguiar Saldanha Pinheiro, A.C.; Vullo, S.; Guercio, A. Evidence for Tunisian-like pestiviruses presence in small ruminants in Italy since 2007. Transbound. Emerg. Dis. 2017, 64, 1243–1253. [Google Scholar] [CrossRef] [PubMed]
- Meyer, D.; Postel, A.; Wiedemann, A.; Cagatay, G.N.; Becher, P. Comparatative analysis of Tunisian sheep-like pestivirus, Bungowannah virus and Border disease virus infection in the porcine host. Viruses 2021, in press. [Google Scholar]
- Lamp, B.; Schwarz, L.; Hogler, S.; Riedel, C.; Sinn, L.; Rebel-Bauder, B.; Weissenbock, H.; Ladinig, A.; Rumenapf, T. Novel pestivirus species in pigs, Austria, 2015. Emerg. Infect. Dis. 2017, 23, 1176–1179. [Google Scholar] [CrossRef]
- Sozzi, E.; Lavazza, A.; Gaffuri, A.; Bencetti, F.C.; Prosperi, A.; Lelli, D.; Chiapponi, C.; Moreno, A. Isolation and full-length sequence analysis of a pestivirus from aborted lamb fetuses in Italy. Viruses 2019, 11, 744. [Google Scholar] [CrossRef] [Green Version]
- Kiesler, A.; Seitz, K.; Schwarz, L.; Buczolich, K.; Petznek, H.; Sassu, E.; Durlinger, S.; Hogler, S.; Klang, A.; Riedel, C.; et al. Clinical and serological evaluation of LINDA virus infections in post-weaning piglets. Viruses 2019, 11, 975. [Google Scholar] [CrossRef] [Green Version]
- Cagatay, G.N.; Meyer, D.; Wendt, M.; Becher, P.; Postel, A. Characterization of the humoral immune response induced after infection with atypical porcine pestivirus (APPV). Viruses 2019, 11, 880. [Google Scholar] [CrossRef]
- Thabti, F.; Fronzaroli, L.; Dlissi, E.; Guibert, J.M.; Hammami, S.; Pepin, M.; Russo, P. Experimental model of Border disease virus infection in lambs: Comparative pathogenicity of pestiviruses isolated in France and Tunisia. Vet. Res. 2002, 33, 35–45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thabti, F.; Letellier, C.; Hammami, S.; Pepin, M.; Ribiere, M.; Mesplede, A.; Kerkhofs, P.; Russo, P. Detection of a novel Border disease virus subgroup in Tunisian sheep. Arch. Virol. 2005, 150, 215–229. [Google Scholar] [CrossRef] [PubMed]
- Dubois, E.; Russo, P.; Prigent, M.; Thiery, R. Genetic characterization of ovine pestiviruses isolated in France, between 1985 and 2006. Vet. Microbiol. 2008, 130, 69–79. [Google Scholar] [CrossRef] [Green Version]
- Martin, C.; Duquesne, V.; Adam, G.; Belleau, E.; Gauthier, D.; Champion, J.L.; Saegerman, C.; Thiery, R.; Dubois, E. Pestiviruses infections at the wild and domestic ruminants interface in the French Southern Alps. Vet. Microbiol. 2015, 175, 341–348. [Google Scholar] [CrossRef] [PubMed]
- Hurtado, A.; Garcia-Perez, A.L.; Aduriz, G.; Juste, R.A. Genetic diversity of ruminant pestiviruses from Spain. Virus Res. 2003, 92, 67–73. [Google Scholar] [CrossRef]
- Becher, P.; Tautz, N. RNA recombination in pestiviruses: Cellular RNA sequences in viral genomes highlight the role of host factors for viral persistence and lethal disease. RNA Biol. 2011, 8, 216–224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Casciari, C.; Sozzi, E.; Bazzucchi, M.; Moreno Martin, A.M.; Gaffuri, A.; Giammarioli, M.; Lavazza, A.; De Mia, G.M. Serological relationship between a novel ovine pestivirus and classical swine fever virus. Transbound. Emerg. Dis. 2020, 67, 1406–1410. [Google Scholar] [CrossRef]
- Bohorquez, J.A.; Sozzi, E.; Wang, M.; Alberch, M.; Abad, X.; Gaffuri, A.; Lelli, D.; Rosell, R.; Perez, L.J.; Moreno, A.; et al. The new emerging ovine pestivirus can infect pigs and confers strong protection against classical swine fever virus. Transbound. Emerg. Dis. 2021. In press. [Google Scholar] [CrossRef]
| Species | Virus Name | Abbreviation 1 | Host Species | Reference Isolate | Reference Sequence | ||
|---|---|---|---|---|---|---|---|
| GenBank | Length [b] | Reference | |||||
| Pestivirus A | bovine viral diarrhea virus-1 | BVDV-1 | Bos sp., Ovis spp., Capra spp., Artiodactyla | SD-1 NADL (cp) | M96751 M31182 | 12,308 12,573 | [28,29] |
| Pestivirus B | bovine viral diarrhea virus-2 | BVDV-2 | Bos sp., Ovis spp., Capra spp., Artiodactyla | XJ-04 890 | FJ527854 U18059 | 12,284 12,513 | Gen-Bank, [30] |
| Pestivirus C | classical swine fever virus | CSFV | Sus scrofa | Alfort/187 | NC038912 | 12,298 | [31] |
| Pestivirus D | border disease virus | BDV | Ovis spp., Capra spp., Artiodactyla | X818 | NC003679 | 12,333 | [32] |
| Pestivirus E | pronghorn antelope pestivirus | PHV | Antilocapra americana | NC024018 | 12,273 | [8] | |
| Pestivirus F | porcine pestivirus | BuPV | Sus scrofa | Bungowannah | NC023176 | 12,656 | [10] |
| Pestivirus G | giraffe pestivirus | - | Bos taurus, Giraffa camelo-pardalis | PG-2 H138 (cp) | KJ660072 NC003678 | 12,264 12,602 | [33,34] |
| Pestivirus H | HoBi-like pestivirus | HoBi | Bos sp. | Th/04_ KhonKaen | NC012812 | 12,337 | [35] |
| Pestivirus I | Aydin-like pestivirus | - | Ovis spp., Capra spp., Bos taurus | 04-TR | NC018713 | 12,292 | [11] |
| Pestivirus J | rat pestivirus | NrPV | Rattus norvegicus | NYC-D23 | NC025677 | 12,983 2 | [20] |
| Pestivirus K | atypical porcine pestivirus | APPV | Sus scrofa | 515 | NC038964 | 11,276 2 | [14] |
| Species | GenBank Acc. No. of Virus Sequences Used |
|---|---|
| Pestivirus A | JN400273, AF526381, KX987157, M96751, AB078950, LT631725, KF896608, KP313732, KX577637, KP941591, JQ799141, KC757383, LC089876, KC853441 |
| Pestivirus B | LC006970, KT875169, FJ527854, GQ888686, KX096718, KJ000672, AB567658, AF002227, KT832818, HQ258810, JF714967 |
| Pestivirus C | X87939, AY646427, KF669877, J04358, KC851953, AF407339, KJ619377, FJ529205, GQ923951, KU504339, KP233070, KM362426 |
| Pestivirus D | AF037405, AB897785, U70263, KJ463422, KC963426, KF918753, GU270877, KF925348, AF144618 |
| Pestivirus E | AY781152 |
| Pestivirus F | EF100713 |
| Pestivirus G | AF144617, KJ660072 |
| Pestivirus H | FJ040215, KC297709, JX469119, JX985409, AB871953, KC788748, HQ231763, JQ612704 |
| Pestivirus I | KM408491, JX428945 |
| Pestivirus J | KJ950914 |
| Pestivirus K | KU041639, KX77872, KR011347, KX929062, KU194229, LT594521, MN099169, MH885413, MH499646, MH307700, KY475593, MH499642, MH493896, MK216752 |
| Proposed Species | Virus Name | Abbreviation 1 | Host Species | Isolates | Available Sequences | Ref. | ||
|---|---|---|---|---|---|---|---|---|
| GenBank | Region | Length [b] | ||||||
| Pestivirus L | Linda virus | LindaV | Sus scrofa | Austria1 | NC035432 | genome | 12,614 | [40] |
| Pestivirus M | Phocoena pestivirus | PhoPeV | Phocoena phocoena | PhoPeV-1 | MK910227 | genome | 11,880 | [22] |
| PhoPeV-2 | MK910228 | genome | 11,880 | [22] | ||||
| PhoPeV-3 | MK910229 | genome | 12,060 | [22] | ||||
| Pestivirus N | Tunisian sheep-like pestivirus | TSV | Capra aegagrus hircus | 70292/2007/EN | MZ664273 | cds | 12,268 | [39] |
| Ovis gmelini aries | 92019/2007/AG | MZ664274 | genome | 12,286 | [39] | |||
| Pestivirus O | ovine/IT pestivirus | ovIT PeV | Ovis gmelini aries | 338710-2/2017 | MK618725 | partial | 11,143 | [41] |
| 338710-3/2017 | MK618726 | cds | 12,173 | [41] | ||||
| 1756/2017 | MG770617 | cds | 12,173 | [41] | ||||
| Pestivirus P | pangolin pestivirus | DYPV | Amblyomma javanense | DYAJ1 | MK636874 | genome | 12,443 | [21] |
| Manis javanica | DYCS | MK636875 | genome | 12,446 | [21] | |||
| Pestivirus Q | rodent pestivirus | RtNn-PeV | Niviventer niviventer | HuB2014 | KY370101 | cds | 13,220 | [24] |
| Pestivirus R | rodent pestivirus | RtAp-PeV | Apodemus peninsulae | JL2014 | KY370100 | cds | 12,768 | [24] |
| n.a. | rodent pestivirus | RtNe-PeV | Niviventer excelsior | SC2014 | KY370099 | partial | 11,644 | [24] |
| n.a. | rodent pestivirus | RtAd-PeV | Apodemus draco | SAX2015 | KY370102 | partial | 11,551 | [24] |
| n.a. | rodent pestivirus | RtNn-PeV | Niviventer niviventer | SAX2015 | KY370103 | partial | 11,435 | [24] |
| Pestivirus S | bat pestivirus | BtSk-PeV | Scotophilus kuhlii | 1/GX2017 | MH282908 | cds | 11,921 | [23] |
| n.a. | bat pestivirus | 3/GX2017 | MH282910 | partial | 7266 | [23] | ||
| n.a. | bat pestivirus | 4/GX2017 | MH282911 | partial | 7132 | [23] | ||
| Species | Virus Name | p-Distances | ||
|---|---|---|---|---|
| CDS | Polyprotein | NS5B3312–3837 | ||
| Pestivirus A | BVDV-1 | <0.22 | <0.15 | <0.11 |
| Pestivirus B | BVDV-2 | <0.17 | <0.11 | <0.10 |
| Pestivirus C | CSFV | <0.19 | <0.13 | <0.07 |
| Pestivirus D | BDV | <0.24 | <0.15 | <0.13 |
| Pestivirus H | HoBi | <0.10 | <0.07 | <0.05 |
| Pestivirus K | APPV | <0.20 | <0.10 | <0.07 |
| Species Compared | Minimum p-Distances | |||
|---|---|---|---|---|
| CDS | Polyprotein | NS5B3312–3837 | ||
| Pestivirus A | Pestivirus B | >0.30 | >0.24 | >0.17 |
| Pestivirus C | Pestivirus D | >0.29 | >0.21 | >0.14 |
| Pestivirus C | Pestivirus I | >0.28 | >0.20 | >0.14 |
| Pestivirus D | Pestivirus I | >0.28 | >0.19 | >0.14 |
| Pestivirus L | Pestivirus F | 0.35 | 0.31 | 0.22 |
| Pestivirus M | Pestivirus F, Pestivirus L | >0.37 | >0.36 | >0.22 |
| Pestivirus N | Pestivirus C | >0.24 | >0.16 | >0.09 |
| Pestivirus N | Pestivirus I | >0.26 | >0.18 | >0.13 |
| Pestivirus N | Pestivirus O | 0.23 | >0.14 | 0.11 |
| Pestivirus O | Pestivirus C | >0.22 | >0.14 | >0.11 |
| Pestivirus P | Pestivirus F, Pestivirus M | >0.41 | >0.47 | >0.33 |
| Pestivirus Q | Pestivirus R | 0.40 | 0.42 | 0.33 |
| Pestivirus Q | Pestivirus J | 0.30 | 0.22 | 0.17 |
| Pestivirus R | Pestivirus Q | 0.40 | 0.42 | 0.33 |
| Pestivirus R | Pestivirus J | 0.40 | 0.42 | 0.31 |
| Pestivirus S | Pestivirus K | >0.40 | >0.40 | >0.29 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Postel, A.; Smith, D.B.; Becher, P. Proposed Update to the Taxonomy of Pestiviruses: Eight Additional Species within the Genus Pestivirus, Family Flaviviridae. Viruses 2021, 13, 1542. https://doi.org/10.3390/v13081542
Postel A, Smith DB, Becher P. Proposed Update to the Taxonomy of Pestiviruses: Eight Additional Species within the Genus Pestivirus, Family Flaviviridae. Viruses. 2021; 13(8):1542. https://doi.org/10.3390/v13081542
Chicago/Turabian StylePostel, Alexander, Donald B. Smith, and Paul Becher. 2021. "Proposed Update to the Taxonomy of Pestiviruses: Eight Additional Species within the Genus Pestivirus, Family Flaviviridae" Viruses 13, no. 8: 1542. https://doi.org/10.3390/v13081542
APA StylePostel, A., Smith, D. B., & Becher, P. (2021). Proposed Update to the Taxonomy of Pestiviruses: Eight Additional Species within the Genus Pestivirus, Family Flaviviridae. Viruses, 13(8), 1542. https://doi.org/10.3390/v13081542






