Focal Epithelial Hyperplasia
Abstract
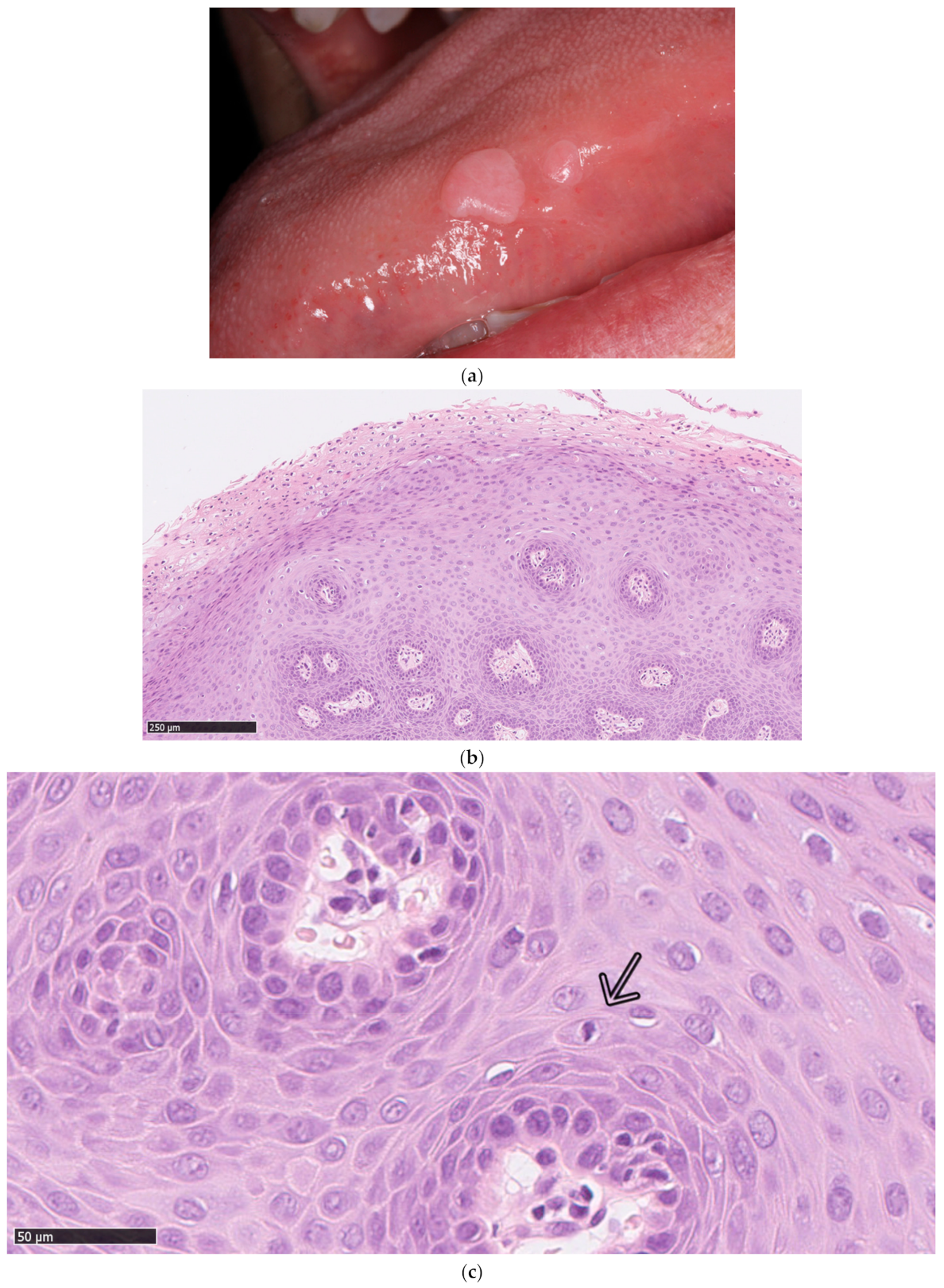
1. Introduction
2. Human Papillomavirus Genotypes in Focal Epithelial Hyperplasia
3. Transmission and Diagnosis
4. Geographical and Ethnical Distribution
5. Comorbidities
6. Prevention and Treatment
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sethi, S.; Ali, A.; Ju, X.; Antonsson, A.; Logan, R.; Jamieson, L. An update on Heck’s disease—A systematic review. J. Public Health 2021, fdaa256. [Google Scholar] [CrossRef]
- Ledesma-Montes, C.; Mendez-Mendoza, A. Unusually high incidence of multifocal epithelial hyperplasia in children of the Nahuatl population of Mexico. Indian J. Dermatol. Venereol. Leprol. 2017, 83, 663–666. [Google Scholar] [CrossRef]
- Syrjänen, S. Oral manifestations of human papillomavirus infections. Eur. J. Oral Sci. 2018, 126, 49–66. [Google Scholar] [CrossRef] [PubMed]
- Bozca, B.; Ozbudak, I.; Alpsoy, E. A case of Heck’s disease with primary intestinal lymphangiectasia treated with imiquimod. Indian J. Dermatol. Venereol. Leprol. 2020, 86, 724–725. [Google Scholar] [PubMed]
- Agnew, C.; Alexander, S.; Prabhu, N. Multifocal Epithelial Hyperplasia. J. Dent. Child 2017, 84, 47–49. [Google Scholar]
- Ledesma-Montes, C.; Vega-Memije, E.; Garcés-Ortíz, M.; Cardiel-Nieves, M.; Juárez-Luna, C. Multifocal epithelial hyperplasia. Report of nine cases. Med. Oral Patol. Oral Cir. Bucal 2005, 10, 394–401. [Google Scholar]
- Schwenger, J.U.; von Buchwald, C.; Lindeberg, H. Oral focal epithelial hyperplasia. Any risk of confusion with oral condylomas? Ugeskr. læger 2002, 164, 4287–4290. [Google Scholar]
- Chompunud Na Ayudhya, C.; Alawi, F.; Akintoye, S.O. Unusual oral multifocal epithelial hyperplasia in an adult African-American lung transplant patient. Transpl. Infect. Dis. 2021, 23, e13497. [Google Scholar] [CrossRef]
- Al Ameer, A.; Kaliyadan, F.; Almarzooq, Y.M.; Imran, M.; Alkhars, H.F. Dermoscopy of focal epithelial hyperplasia. Indian J. Dermatol. 2020, 65, 429–430. [Google Scholar]
- Borborema-Santos, C.M.; De Castro, M.M.; Dos Santos, P.J.B.; Talhari, S.; Astolfi-Filho, S. Oral focal epithelial hyperplasia: Report of five cases. Braz. Dent. J. 2006, 17, 79–82. [Google Scholar] [CrossRef] [PubMed]
- Stoopler, E.T.; Alhendi, F.; Musto, C.J.; Bilodeau, E.A. Multiple oral soft tissue nodules in a Caucasian septuagenarian. Int. J. Dermatol. 2021, 60, e122–e124. [Google Scholar] [CrossRef] [PubMed]
- Brehm, M.A.; Gordon, K.; Firan, M.; Rady, P.; Agim, N. Case Report of Focal Epithelial Hyperplasia (Heck’s Disease) with Polymerase Chain Reaction Detection of Human Papillomavirus 13. Pediatr. Dermatol. 2016, 33, e224–e225. [Google Scholar] [CrossRef]
- Méndez-Flores, S.; Esquivel-Pedraza, L.; Hernández-Salazar, A.; Charli-Joseph, Y.; Saeb-Lima, M. Focal epithelial hyperplasia in adult patients with HIV infection: Clearance with topical imiquimod. Skinmed 2016, 14, 395–397. [Google Scholar] [PubMed]
- Waldman, G.H.; Shelton, D.W. Focal epithelial hyperplasia (Heck’s disease) in an adult Caucasian. Oral Surg. Oral Med. Oral Pathol. 1968, 26, 124–127. [Google Scholar] [CrossRef]
- Arbelo-Ramos, N.; Anderson, K.; Joshi, A.; Wieland, C.; Tyring, S.; Rady, P.; Tollefson, M. Heck’s disease occurring after Epstein-Barr virus-associated smooth muscle tumors in an immunosuppressed child. Pediatr. Dermatol. 2021, 38, 257–259. [Google Scholar] [CrossRef]
- Do Vale, D.A.; Ferracini, L.M.A.; Da Silva Rodrigues Bueno, M.V.; Fernandes Souza, A.C.M.; Braz-Silva, P.H.; Ortega, K.L. Unusual presentation of a disseminated oral HPV infection after combined antiretroviral therapy initiation. Rev. Inst. Med. Trop. Sao Paulo 2019, 61, 1–4. [Google Scholar] [CrossRef]
- Rubio Sánchez, P.; Calatayud Lallana, L.M.; Hidalgo Sanz, J.; Vicente González, E. Lesiones Papilomatosas Orales: Hiperplasia epitelial focal. An. Pediatría 2021. [Google Scholar] [CrossRef]
- Jiménez Aguilar, S.M.; Rodríguez, D.L.; Muñoz Estrada, V.F.; Cázarez Salazar, S.G.; Velarde Félix, J.S.; Méndez Martínez, R.S. Great diversity of oncogenic human papillomaviruses is revealed in an outbreak of multifocal epithelial hyperplasia. J. Am. Acad. Dermatol. 2019. [Google Scholar] [CrossRef]
- Garcia-Corona, C.; Vega-Memije, E.; Mosqueda-Taylor, A.; Yamamoto-Furusho, J.K.; Rodríguez-Carreón, A.A.; Ruiz-Morales, J.A.; Salgado, N.; Granados, J. Association of HLA-DR4 (DRB1*0404) with Human papillomavirus infection in patients with focal epithelial hyperplasia. Arch. Dermatol. 2004, 140, 1227–1231. [Google Scholar] [CrossRef]
- González, L.V.; Gaviria, A.M.; Sanclemente, G.; Rady, P.; Tyring, S.K.; Carlos, R.; Correa, L.A.; Sanchez, G.I. Clinical, histopathological and virological findings in patients with focal epithelial hyperplasia from Colombia. Int. J. Dermatol. 2005, 44, 274–279. [Google Scholar] [CrossRef] [PubMed]
- Khanal, S.; Cole, E.T.; Joh, J.; Ghim, S.J.; Jenson, A.B.; Rai, S.N.; Trainor, P.J.; Shumway, B.S. Human papillomavirus detection in histologic samples of multifocal epithelial hyperplasia: A novel demographic presentation. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2015, 120, 733–743. [Google Scholar] [CrossRef] [PubMed]
- Caldeira, S.; Dong, W.; Tomakidi, P.; Paradiso, A.; Tommasino, M. Human papillomavirus type 32 does not display in Vitro transforming properties. Virology 2002, 301, 157–164. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Conde-Ferráez, L.; Ek-Hernández, G.E.; Canché-Pech, J.R.; Gómez-Carballo, J.G.; Kantún-Moreno, N.E.; González-Losa, M.d.R. Genomic characterization of human papillomavirus type 13, associated to multifocal epithelial hyperplasia, in a Mayan community. Infect. Genet. Evol. 2021, 91, 104595. [Google Scholar] [CrossRef] [PubMed]
- Lorenzi, A.; Rautava, J.; Kero, K.; Syrjänen, K.; Longatto-Filho, A.; Grenman, S.; Syrjänen, S. Physical state and copy numbers of HPV16 in oral asymptomatic infections that persisted or cleared during the 6-year follow-up. J. Gen. Virol. 2017, 98, 681–689. [Google Scholar] [CrossRef]
- Jamieson, L.M.; Garvey, G.; Hedges, J.; Leane, C.; Hill, I.; Brown, A.; Ju, X.; Sethi, S.; Roder, D.; Logan, R.M.; et al. Cohort profile: Indigenous human papillomavirus and oropharyngeal squamous cell carcinoma study—A prospective longitudinal cohort. BMJ Open 2021, 11, e046928. [Google Scholar] [CrossRef] [PubMed]
- Biryukov, J.; Meyers, C. Superinfection Exclusion between Two High-Risk Human Papillomavirus Types during a Coinfection. J. Virol. 2018, 92, e01993-17. [Google Scholar] [CrossRef]
- Vermeulen, C.F.W.; Jordanova, E.S.; Szuhai, K.; Kolkman-Uljee, S.; Vrede, M.A.; Peters, A.A.W.; Schuuring, E.; Fleuren, G.J. Physical status of multiple human papillomavirus genotypes in flow-sorted cervical cancer cells. Cancer Genet. Cytogenet. 2007, 175, 132–137. [Google Scholar] [CrossRef]
- Lopez-Villanueva, M.E.; Conde-Ferráez, L.; Ayora-Talavera, G.; Cerón-Espinosa, J.D.; González-Losa, M.d.R. Human papillomavirus 13 in a Mexican Mayan community with multifocal epithelial hyperplasia: Could saliva be involved in household transmission? Eur. J. Dermatol. 2011, 21, 396–400. [Google Scholar] [CrossRef]
- Puriene, A.; Rimkevicius, A.; Gaigalas, M. Focal Epithelial Hyperplasia: Case Report. Stomatologija 2011, 13, 102–106. [Google Scholar]
- Said, A.K.; Leao, J.C.; Fedele, S.; Porter, S.R. Focal epithelial hyperplasia—An update. J. Oral Pathol. Med. 2013, 42, 435–442. [Google Scholar] [CrossRef]
- Ledesma-Montes, C.; Garcés-Ortíz, M.; Hernández-Guerrero, J.C. Clinicopathological and Immunocytochemical Study of Multifocal Epithelial Hyperplasia. J. Oral Maxillofac. Surg. 2007, 65, 2211–2217. [Google Scholar] [CrossRef] [PubMed]
- Camacho-Aguilar, S.; Ramírez-Amador, V.; Rosendo-Chalma, P.; Guido-Jiménez, M.; García-Carrancá, A.; Anaya-Saavedra, G. Human papillomavirus load in benign HPV-associated oral lesions from HIV/AIDS individuals. Oral Dis. 2018, 24, 210–214. [Google Scholar] [CrossRef]
- Jamieson, L.M.; Antonsson, A.; Garvey, G.; Ju, X.; Smith, M.; Logan, R.M.; Johnson, N.W.; Hedges, J.; Sethi, S.; Dunbar, T.; et al. Prevalence of oral human papillomavirus infection among Australian indigenous adults. JAMA Netw. Open 2020, 3, e204951. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, J.T.; Allen, C.T.; Dodge, J.T.; Van Doorslaer, K.; McBride, A.A.; Pavletic, S.Z.; Mays, J.W. HPV32-related Heck’s disease in a chronic graft-versus-host disease patient with long-term successful KTP laser treatment: A rare case report. Clin. Case Rep. 2021, 9, e04253. [Google Scholar] [CrossRef] [PubMed]
- Borghelli, R.F.; Stirparo, M.A.; Paroni, H.C.; Barros, R.E.; Dominguez, F.V. Focal epithelial hyperplasia. Report of five new cases from Argentina. Oral Surg. Oral Med. Oral Pathol. 1975, 40, 107–112. [Google Scholar] [CrossRef]
- Axéll, T.; Hammarström, L.; Larsson, A. Focal epithelial hyperplasia in Sweden. Acta Odontol. Scand. 1981, 39, 201–208. [Google Scholar] [CrossRef]
- Clausen, F.P.; Møgeltoft, M.; Roed-Petersen, B.; Pindborg, J.J. Focal epithelial hyperplasia of the oral mucosa in a south-west Greenlandic population. Scand. J. Dent. Res. 1970, 78, 287–294. [Google Scholar] [CrossRef]
- Mealey, B.L.; Hallmon, W.W.; Waldrop, T.C. Occurrence and Resolution of Focal Epithelial Hyperplasia in Two Siblings With Leukocyte Adhesion Deficiency. J. Periodontol. 1993, 64, 149–152. [Google Scholar] [CrossRef] [PubMed]
- Ryndock, E.J.; Biryukov, J.; Meyers, C. Replication of Human Papillomavirus in Culture. Methods Mol. Biol. 2015, 1249, 39–52. [Google Scholar] [CrossRef] [PubMed]
- Ocadiz-Delgado, R.; Marroquin-Chavira, A.; Hernandez-Mote, R.; Valencia, C.; Manjarrez-Zavala, M.E.; Covarrubias, L.; Gariglio, P. Induction of focal epithelial hyperplasia in tongue of young bk6-E6/E7 HPV16 transgenic mice. Transgenic Res. 2009, 18, 513–527. [Google Scholar] [CrossRef]
- Brown, D.R.; Kjaer, S.K.; Sigurdsson, K.; Iversen, O.E.; Mauricio, H.A.; Wheeler, C.M.; Perez, G.; Koutsky, L.A.; Tay, E.H.; Garcia, P.; et al. The impact of quadrivalent human papillomavirus (HPV.; Types 6, 11, 16, and 18) L1 virus-like particle vaccine on infection and disease due to oncogenic nonvaccine HPV types in generally HPV-naive women aged 16–26 years. J. Infect. Dis. 2009, 199, 926–935. [Google Scholar] [CrossRef] [PubMed]
- Handisurya, A.; Schellenbacher, C.; Haitel, A.; Senger, T.; Kirnbauer, R. Human papillomavirus vaccination induces neutralising antibodies in oral mucosal fluids. Br. J. Cancer 2016, 114, 409–416. [Google Scholar] [CrossRef] [PubMed]
- Weinberg, A.; Song, L.Y.; Saah, A.; Brown, M.; Moscicki, A.B.; Meyer, W.A.; Bryan, J.; Levin, M.J. Humoral, mucosal, and cell-mediated immunity against vaccine and nonvaccine genotypes after administration of quadrivalent human papillomavirus vaccine to HIV-infected children. J. Infect. Dis. 2012, 206, 1309–1318. [Google Scholar] [CrossRef] [PubMed]
- Nguyen-Huu, N.; Thilly, N.; Derrough, T.; Sdona, E.; Claudot, F.; Pulcini, C.; Agrinier, N.; HPV Policy Working Group. Human papillomavirus vaccination coverage, policies and practical implementation across Europe. Vaccine 2020, 38, 1315–1331. [Google Scholar] [CrossRef]
- Du, J.; Ährlund-Richter, A.; Näsman, A.; Dalianis, T. Human papilloma virus (HPV) prevalence upon HPV vaccination in Swedish youth: A review based on our findings 2008–2018, and perspectives on cancer prevention. Arch. Gynecol. Obstet. 2021, 303, 329–335. [Google Scholar] [CrossRef]
- Chaturvedi, A.K.; Graubard, B.I.; Broutian, T.; Pickard, R.K.L.; Tong, Z.Y.; Xiao, W.; Kahle, L.; Gillison, M.L. Effect of prophylactic human papillomavirus (HPV) vaccination on oral HPV infections among young adults in the United States. J. Clin. Oncol. 2018, 36, 262–267. [Google Scholar] [CrossRef]
- Hirth, J.M.; Chang, M.; Resto, V.A.; Guo, F.; Berenson, A.B. Prevalence of oral human papillomavirus by vaccination status among young adults (18–30 years old). Vaccine 2017, 35, 3446–3451. [Google Scholar] [CrossRef]
- Nallanchakrava, S.; Sreebala, N.; LNU, B.; Sindgi, F. Laser Excision of Focal Epithelial Hyperplasia (Heck’s Disease): A Rare Case Report. Int. J. Clin. Pediatr. Dent. 2018, 11, 526–528. [Google Scholar] [CrossRef]
- Lorduy, M.C.; Ricardo, J.H.; Arenas, Y.H.; Carmona, W.M. Use of trichloroacetic acid for management of oral lesions caused by human papillomavirus. Gen. Dent. 2018, 66, 47–49. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bendtsen, S.K.; Jakobsen, K.K.; Carlander, A.-L.F.; Grønhøj, C.; von Buchwald, C. Focal Epithelial Hyperplasia. Viruses 2021, 13, 1529. https://doi.org/10.3390/v13081529
Bendtsen SK, Jakobsen KK, Carlander A-LF, Grønhøj C, von Buchwald C. Focal Epithelial Hyperplasia. Viruses. 2021; 13(8):1529. https://doi.org/10.3390/v13081529
Chicago/Turabian StyleBendtsen, Simone Kloch, Kathrine Kronberg Jakobsen, Amanda-Louise Fenger Carlander, Christian Grønhøj, and Christian von Buchwald. 2021. "Focal Epithelial Hyperplasia" Viruses 13, no. 8: 1529. https://doi.org/10.3390/v13081529
APA StyleBendtsen, S. K., Jakobsen, K. K., Carlander, A.-L. F., Grønhøj, C., & von Buchwald, C. (2021). Focal Epithelial Hyperplasia. Viruses, 13(8), 1529. https://doi.org/10.3390/v13081529





