HPV-Associated Benign Squamous Cell Papillomas in the Upper Aero-Digestive Tract and Their Malignant Potential
Abstract
1. Introduction
2. Sinonasal Papilloma (SNP)
2.1. Subtypes of SNPs
2.2. Etiology of Sinonasal Papillomas
HPV in Sinonasal Papilloma
2.3. Malignant Transformation
2.3.1. Malignant Transformation and HPV
2.3.2. Malignant Transformation and Genetic Profile
3. Nasopharyngeal Papillomas
4. Oral Squamous Cell Papilloma (OP)
4.1. Multiple OPs and Genetic Syndromes
4.2. HPV and Oral Squamous Cell Papilloma
5. Oropharyngeal Papilloma
HPV Prevalence
6. Laryngeal Squamous Cell Papillomas (RRP)
6.1. HPV6 and HPV11 Are the Major Causative Agents of RRP
6.2. Factors Associated with RRP and Its Outcome
6.2.1. Acquisition of RRP
6.2.2. JO-RRP and HPV11 Are Prognostic Factors of RPP Outcome
6.2.3. Physical State of HPV6 and HPV11, Their Variants and Other Viral Factors in RRP
6.2.4. Risk Factors for Distal Spread
6.2.5. Malignant Transformation
6.3. RRP Can Be Prevented by HPV Vaccinations Targeting HPV6 and 11 (Gardasil)
7. Tracheal Papillomas (TP)
8. Esophageal Squamous Cell Papilloma (ESP)
8.1. Etiology of ESP
HPV and ESP
9. HPV Can Be Acquired at Early Age
9.1. Mother Is the Main Transmitter of Her Offspring
9.2. Perinatal Transmission of Cutaneous HPV into Nasopharynx Is Additionally Possible
9.3. Outcome of Vertically Transmitted HPV Only Partly Known
10. Animal Models to Study SCPs of the Head and Neck
10.1. Rabbit Oral Papillomavirus (ROPV)—A Model to Study the Outcome of Mucosal Papillomas and Their Infectivity
10.2. Latency and Papillomavirus Infections
10.3. Transmission of Papillomaviruses via Blood
11. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- El-Naggar Chan, J.K.; Grandis, J.R.; Takata, T.; Slootweg, P.J. WHO Classification of Head and Neck Tumours, 4th ed.; International Agency for Research on Cancer: Lyon, France, 2017; pp. 28–31, 93–95, 115–116. [Google Scholar]
- Pähler Vor der Holte, A.; Fangk, I.; Glombitza, S.; LWilkens, L.; Welkoborsky, H.J. Prognostic factors and risk factors for development and recurrence of sinonasal papillomas: Potential role of different HPV subtypes. Eur. Arch. Oto-Rhino-Laryngol. 2020, 277, 767–775. [Google Scholar] [CrossRef]
- Abbey, L.M.; Page, D.G.; Sawyer, D.R. The clinical and histopathologic features of a series of 464 oral squamous cell papillomas. Oral Surg. Oral Med. Oral Pathol. 1980, 49, 419–428. [Google Scholar] [CrossRef]
- Knapp, M.J. Oral Disease in 181,338 Consecutive Oral Examinations. J. Am. Dent. Assoc. 1971, 83, 1288–1293. [Google Scholar] [CrossRef]
- Hyams, V.J. Papillomas of the nasal cavity and paranasal sinuses: A clinicopathological study of 315 cases. Ann. Otol. Rhinol. Laryngol. 1971, 80, 192–206. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Siegel, J.; Chouake, R.J.; Geliebter, J.; Zalvan, C.H. Implication and Management of Incidental Oropharyngeal Papillomas—A Retrospective Case Series Review. Ear Nose Throat J. 2019, 3, 546–551. [Google Scholar] [CrossRef] [PubMed]
- Mosca, S.; Manes, G.; Monaco, R.; Bellomo, P.F.; Bottino, V.; Abalzano, A. Squamous papilloma of the esophagus: Long-term follow up. J. Gastroenterol. Hepatol. 2001, 16, 857–861. [Google Scholar] [CrossRef]
- Derkay, C.S.; Bluher, A.E. Update on Recurrent Respiratory Papillomatosis. Otolaryngol. Clin. N. Am. 2019, 52, 669–679. [Google Scholar] [CrossRef] [PubMed]
- Trzcinska, A.; Zhang, W.; Gitman, M.; Westra, W.H. The Prevalence, Anatomic Distribution and Significance of HPV Genotypes in Head and Neck Squamous Papillomas as Detected by Real-Time PCR and Sanger Sequencing. Head Neck Pathol. 2020, 14, 428–434. [Google Scholar] [CrossRef] [PubMed]
- Nogueira, R.L.; Küpper, D.S.; Bonfim, C.M.; Aragon, D.C.; Damico, T.A.; Miura, C.S.; Passos, I.M.; Nogueira, M.L.; Rahal, P.; Valera, F.C.P. HPV genotype is a prognosticator for recurrence of respiratory papillomatosis in children. Clin. Otolaryngol. 2021, 46, 181–188. [Google Scholar] [CrossRef]
- Hoesli, R.C.; Wingo, M.L.; Richardson, B.E.; Bastian, R.W. Identification of 11 Different HPV Subtypes in Adult Patients with Recurrent Respiratory Papillomatosis. Otolaryngol. Head Neck Surg. 2020, 163, 785–790. [Google Scholar] [CrossRef] [PubMed]
- Donà, M.G.; Pichi, B.; Rollo, F.; Gheit, T.; Laquintana, V.; Covello, R.; Pescarmona, E.; Spriano, G.; Pellini, R.; Giuliani, M.; et al. Mucosal and cutaneous human papillomaviruses in head and neck squamous cell papillomas. Head Neck 2017, 39, 254–259. [Google Scholar] [CrossRef]
- Kerge, S.; Vuorinen, J.; Hurme, S.; Soukka, T.; Gheit, T.; Tommasino, M.; Syrjänen, S.; Rautava, J. Benign proliferative epithelial lesions of oral mucosa are infrequently associated with α-, β-, or γ human papillomaviruses. Laryngoscope Investig. Otolaryngol. 2018, 4, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Parsons, R.J.; Kidd, J.G. Oral papillomatosis of rabbits: A virus disease. J. Exp. Med. 1943, 77, 233–250. [Google Scholar] [CrossRef] [PubMed]
- Niyibizi, J.; Rodier, C.; Wassef, M.; Trottier, H. Risk factors for the development and severity of juvenile-onset recurrent respiratory papillomatosis: A systematic review. Int. J. Pediatr. Otorhinolaryngol. 2014, 78, 186–197. [Google Scholar] [CrossRef] [PubMed]
- Buchinsky, F.J.; Valentino, W.L.; Ruszkay, N.; Powell, E.; Derkay, C.S.; Seedat, R.Y.; Uloza, V.; Dikkers, F.G.; Tunkel, D.E.; Choi, S.S.; et al. Age at diagnosis, but not HPV type, is strongly associated with clinical course in recurrent respiratory papillomatosis. PLoS ONE 2019, 14, e0216697. [Google Scholar] [CrossRef]
- Buchwald, C.; Franzmann, M.B.; Tos, M. Sinonasal papillomas: A report of 82 cases in Copenhagen County, including a longitu-dinal epidemiological and clinical study. Laryngoscope 1995, 105, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Eavey, R.D. Inverted papilloma of the nose and paranasal sinuses in childhood and adolescence. Laryngoscope 1985, 95, 17–23. [Google Scholar] [CrossRef]
- Limaye, A.P.; Mirani, N.; Kwartler, J.; Raz, S. Inverted schneiderian papilloma of the sinonasal tract in children. Pediatr. Pathol. 1989, 9, 583–590. [Google Scholar] [CrossRef]
- Jayakody, N.; Ward, M.; Wijayasingham, G.; Fowler, D.; Harries, P.; Salib, R. A rare presentation of a paediatric sinonasal inverted papilloma. J. Surg. Case Rep. 2018, 11, rjy321. [Google Scholar] [CrossRef]
- Korbi, A.E.; Jellali, S.; Kolsi, N.; Bouatay, R.; Njim, L.; Berguaoui, E.; Ferjaoui, M.; Harrathi, K.; Koubaa, J. Pediatric naso-sinusal inverted papilloma: Report of a case and literature review. Pan. Afr. Med. J. 2020, 37, 373. [Google Scholar] [CrossRef]
- Vorasubin, N.; Vira, D.; Suh, J.D.; Bhuta, S.; Wang, M.B. Schneiderian papillomas: Comparative review of exophytic, oncocytic, and inverted types. Am. J. Rhinol. Allergy 2013, 27, 287–292. [Google Scholar] [CrossRef]
- Buchwald, C.; Nielsen, L.H.; Nielsen, P.L.; Ahlgren, P.; Tos, M. Inverted papilloma: A follow-up study including primarily unacknowledged cases. Am. J. Otolaryngol. 1989, 10, 273–281. [Google Scholar] [CrossRef]
- Buchwald, C.; Lindeberg, H.; Pedersen, B.L.; Franzmann, M.B. Human papilloma virus and p53 expression in carcinomas associated with sinonasal papillomas: A Danish Epidemiological study 1980–1998. Laryngoscope 2001, 111, 1104–1110. [Google Scholar] [CrossRef]
- Bishop, J.A. OSPs and ESPs and ISPs, Oh My! An Update on Sinonasal (Schneiderian) Papillomas. Head Neck Pathol. 2017, 11, 269–277. [Google Scholar] [CrossRef]
- Wang, M.J.; Noel, J.E. Etiology of sinonasal inverted papilloma: A narrative review. World J. Otorhinolaryngol. Head Neck Surg. 2017, 3, 54–58. [Google Scholar] [CrossRef]
- Zhao, R.W.; Guo, Z.Q.; Zhang, R.X. Human papillomavirus infection and the malignant transformation of sinonasal inverted papilloma: A meta-analysis. J. Clin. Virol. 2016, 79, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, C.; Wang, R.; Zhang, J.; Liu, H.; Shi, Q.; Chen, X.; Hou, L.; Ma, H.; Zhong, Q.; et al. Prognostic Factors of Sinonasal Squamous Cell Carcinomas Arising De Novo and From Inverted Papilloma. Am. J. Rhinol. Allergy 2021, 35, 114–121. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Wang, Y. Clinical characteristics of sinonasal inverted papilloma associated with recurrence and malignant transformation. Auris Nasus Larynx 2021, 48, 1105–1108. [Google Scholar] [CrossRef] [PubMed]
- Lilja, M.; Viitasalo, S.; Hytönen, M.; Haapaniemi, A.; Hagström, J.; Mäkitie, A. Sinonasal Oncocytic Papilloma-A Series of 20 Cases with Special Emphasis on Recurrences. Laryngoscope Investig. Otolaryngol. 2019, 4, 567–572. [Google Scholar] [CrossRef]
- Moon, I.J.; Lee, D.Y.; Suh, M.W.; Han, D.H.; Kim, S.T.; Min, Y.G.; Lee, C.H.; Rhee, C.S. Cigarette smoking increases risk of recurrence for sinonasal inverted papilloma. Am. J. Rhinol. Allergy 2010, 24, 325–329. [Google Scholar] [CrossRef]
- Sham, C.L.; Lee, D.L.Y.; van Hasselt, C.A.; Tong, M.C.F. A case-control study of the risk factors associated with sinonasal inverted papilloma. Am. J. Rhinol. Allergy 2010, 24, e37–e40. [Google Scholar] [CrossRef] [PubMed]
- Syrjänen, K.; Syrjänen, S. Detection of human papillomavirus in sinonasal papillomas: Systematic review and meta-analysis. Laryngoscope 2013, 123, 181–192. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.; Rady, P.L.; Sikora, A.G.; Tyring, S.K. The role of human papillomavirus in the pathogenesis of sinonasal inverted papilloma: A narrative review. Rev. Med. Virol. 2021, 31, e2178. [Google Scholar] [CrossRef]
- Forslund, O.; Johansson, H.; Madsen, K.G.; Kofoed, K. The nasal mucosa contains a large spectrum of human papillomavirus types from the Betapapillomavirus and Gammapapillomavirus genera. J. Infect. Dis. 2013, 208, 1335–1341. [Google Scholar] [CrossRef]
- Re, M.; Gioacchini, F.M.; Bajraktari, A.; Tomasetti, M.; Kaleci, S.; Rubini, C.; Bertini, A.; Magliulo, G.; Pasquini, E. Malignant transformation of sinonasal inverted papilloma and related genetic alterations: A systematic review. Eur. Arch. Oto-Rhino-Laryngol. 2017, 274, 2991–3000. [Google Scholar] [CrossRef]
- Katori, H.; Nozawa, A.; Tsukuda, M. Histopathological parameters of recurrence and malignant transformation in sinonasal inverted papilloma. Acta Otolaryngol. 2006, 126, 214–218. [Google Scholar] [CrossRef]
- Klemi, P.J.; Joensuu, H.; Siivonen, L.; Virolainen, E.; Syrjänen, S.; Syrjänen, K. Association of DNA aneuploidy with human papillomavirus-induced malignant transformation of sinonasal transitional papillomas. Otolaryngol. Head Neck Surg. 1989, 100, 563–567. [Google Scholar] [CrossRef]
- Lawson, W.; Schlecht, N.F.; Brandwein-Gensler, M. The role of the human papillomavirus in the pathogenesis of Schneiderian inverted papillomas: An analytic overview of the evidence. Head Neck Pathol. 2008, 2, 49–59. [Google Scholar] [CrossRef]
- Ding, R.; Sun, Q.; Wang, Y. Association between Human Papilloma Virus Infection and Malignant Sinonasal Inverted Papilloma. Laryngoscope 2021, 131, 1200–1205. [Google Scholar] [CrossRef]
- Stepp, W.H.; Zainab Farzal, Z.; Kimple, A.J.; Ebert, C.S., Jr.; Senior, B.A.; Zanation, A.M.; Thorp, B.D. HPV in the malignant transformation of sinonasal inverted papillomas: A meta-analysis. Int. Forum Allergy Rhinol. 2021. [Google Scholar] [CrossRef]
- Sahnane, N.; Ottini, G.; Turri-Zanoni, M.; Furlan, D.; Battaglia, P.; Karligkiotis, A.; Albeni, C.; Cerutti, R.; Mura, E.; Chiaravalli, A.M.; et al. Comprehensive analysis of HPV infection, EGFR exon 20 mutations and LINE1 hypomethylation as risk factors for malignant transformation of sinonasal-inverted papilloma to squamous cell carcinoma. Int. J. Cancer 2019, 144, 1313–1320. [Google Scholar] [CrossRef]
- Hongo, T.; Yamamoto, H.; Jiromaru, R.; Nozaki, Y.; Yasumatsu, R.; Hashimoto, K.; Yoneda, R.; Sugii, A.; Taguchi, K.; Masuda, M.; et al. Clinicopathologic Significance of EGFR Mutation and HPV Infection in Sinonasal Squamous Cell Carcinoma. Am. J. Surg. Pathol. 2021, 45, 108–118. [Google Scholar] [CrossRef]
- Brown, N.A.; Plouffe, K.R.; Yilmaz, O.; Weindorf, S.C.; Betz, B.L.; Carey, T.E.; Seethala, R.R.; McHugh, J.B.; Tomlins, S.A.; Udager, A.M. TP53 mutations and CDKN2A mutations/deletions are highly recurrent molecular alterations in the malignant progression of sinonasal papillomas. Mod. Pathol. 2021, 34, 1133–1142. [Google Scholar] [CrossRef]
- Udager, A.M.; McHugh, J.B.; Goudsmit, C.M.; Weigelin, H.C.; Lim, M.S.; Elenitoba-Johnson, K.S.J.; Betz, B.L.; Carey, T.E.; Brown, N.A. Human papillomavirus (HPV) and somatic EGFR mutations are essential, mutually exclusive oncogenic mechanisms for inverted sinonasal papillomas and associated sinonasal squamous cell carcinomas. Ann. Oncol. 2018, 29, 466–471. [Google Scholar] [CrossRef]
- Maglennon, G.A.; McIntosh, P.; Doorbar, J. Persistence of viral DNA in the epithelial basal layer suggests a model for papillomavirus latency following immune regression. Virology 2011, 414, 153–163. [Google Scholar] [CrossRef]
- Axéll, T. A prevalence study of oral mucosal lesions in an adult Swedish population. Odontol. Rev. Suppl. 1976, 36, 1–103. [Google Scholar]
- Greer, R.O.; Goldman, H.M. Oral papillomas. Clinicopathologic evaluation and retrospective examination for dyskeratosis in 110 lesions. Oral Surg. Oral Med. Oral Pathol. 1974, 38, 435–440. [Google Scholar] [CrossRef]
- Syrjänen, S. Oral manifestations of human papillomavirus infections. Eur. J. Oral Sci. 2018, 126 (Suppl. 1), 49–66. [Google Scholar] [CrossRef]
- OMIM Database. Available online: http://www.ncbi.nlm.nih.gov/omim (accessed on 6 April 2021).
- WHIM Syndrome. Available online: https://www.omim.org/entry/193670 (accessed on 6 April 2021).
- Frithiof, L.; Wersäll, J. Virus-like particles in human oral papilloma. Acta Otolaryngol. 1967, 64, 263–266. [Google Scholar] [CrossRef]
- Shaffer, E.L., Jr.; Reimann, B.E.; Gysland, W.B. Oral condyloma acuminatum: A case report with light microscopic and ultrastructural features. J. Oral Pathol. 1980, 9, 163–173. [Google Scholar] [CrossRef]
- Syrjänen, K.; Syrjänen, S. Papillomavirus Infections in Human Pathology, 1st ed.; Wiley: New York, NY, USA, 2000; pp. 1–630. [Google Scholar]
- Bottalico, D.; Chen, Z.; Dunne, A.; Ostoloza, J.; McKinney, S.; Sun, C.; Schlecht, N.F.; Fatahzadeh, M.; Herrero, R.; Schiffman, M.; et al. The oral cavity contains abundant known and novel human papillomaviruses from the Betapapillomavirus and Gammapapillomavirus genera. J. Infect. Dis. 2011, 204, 787–792. [Google Scholar] [CrossRef]
- MacKenzie, M. Essays on Growths in the Larynx with Reports and an Analysis of One Hundred Consecutive Cases Treated by the Author; Lindsay & Blakiston: Philadelphia, PA, USA, 1871. [Google Scholar]
- Kashima, H.K.; Shah, K. Recurrent respiratory papillomatosis: Clinical overview and management principles. Obstet. Gynecol. Clin. N. Am. 1987, 14, 581–588. [Google Scholar] [CrossRef]
- Donne, A.J.; Keltie, K.; Cole, H.; Sims, A.J.; Patrick, H.; Powell, S. Prevalence and management of recurrent respiratory papillomatosis (RRP) in the UK: Cross-sectional study. Clin. Otolaryngol. 2017, 42, 86–91. [Google Scholar] [CrossRef] [PubMed]
- Mahnke, C.G.; Fröhlich, O.; Lippert, B.M.; Werner, J.A. Recurrent laryngeal papillomatosis. Retrospective analysis of 95 patients and review of the literature. Otolaryngol. Pol. 1996, 50, 567–578. [Google Scholar] [PubMed]
- Ullman, E.V. On the etiology of the laryngeal papilloma. Acta Otolaryngol. 1923, 5, 317–338. [Google Scholar]
- Hajek, E. Contribution to the etiology of laryngeal papilloma in children. J. Laryngol. Otol. 1956, 70, 166–168. [Google Scholar] [CrossRef]
- Mounts, P.; Shah, K.V.; Kashima, H. Viral etiology of juvenile- and adult-onset squamous papilloma of the larynx. Proc. Natl. Acad. Sci. USA 1982, 79, 5425–5429. [Google Scholar] [CrossRef]
- Byrne, J.C.; Tsao, M.S.; Fraser, R.S.; Howley, P.M. Human papillomavirus-11 DNA in a patient with chronic laryngotracheobronchial papillomatosis and metastatic squamous-cell carcinoma of the lung. N. Engl. J. Med. 1987, 317, 873–878. [Google Scholar] [CrossRef]
- Omland, T.; Lie, K.A.; Akre, H.; Sandlie, L.E.; Jebsen, P.; Sandvik, L.; Nymoen, D.A.; Bzhalava, D.; Dillner, J.; Brøndbo, K. Recurrent respiratory papillomatosis: HPV genotypes and risk of high-grade laryngeal neoplasia. PLoS ONE 2014, 11, e99114. [Google Scholar] [CrossRef]
- Kashima, H.; Shah, F.; Lyles, A.; Glackin, R.; Muhammad, N.; Turner, L.; Van Zandt, S.; Whitt, S.; Shah, K. A comparison of risk factors in juvenile-onset and adult-onset recurrent respiratory papillomatosis. Laryngoscope 1992, 102, 9–13. [Google Scholar] [CrossRef]
- Ivancic, R.; Iqbal, H.; deSilva, B.; Pan, Q.; Matrka, L. Immunological tolerance of low-risk HPV in recurrent respiratory papillomatosis. Clin. Exp. Immunol. 2020, 199, 131–142. [Google Scholar] [CrossRef] [PubMed]
- Bonagura, V.R.; Vambutas, A.; DeVoti, J.A.; Rosenthal, D.W.; Steinberg, B.M.; Abramson, A.L.; Shikowitz, M.J.; Gjertson, D.F.; Reed, E.F. HLA alleles, IFN-gamma responses to HPV-11 E6, and disease severity in patients with recurrent respiratory papillomatosis. Hum. Immunol. 2004, 65, 773–782. [Google Scholar] [CrossRef]
- Bonagura, V.R.; Hatam, L.J.; Rosenthal, D.W.; de Voti, J.A.; Lam, F.; Steinberg, B.M.; Allan, L.; Abramson, A.L. Recurrent respiratory papillomatosis: A complex defect in immune responsiveness to human papillomavirus-6 and -11. APMIS 2010, 118, 455–470. [Google Scholar] [CrossRef] [PubMed]
- Koskimaa, H.M.; Paaso, A.; Welters, M.J.P.; Grénman, S.; Syrjänen, K.; van der Burg, S.H.; Syrjänen, S. The presence of human papillomavirus (HPV) in placenta and/or cord blood might result in Th2 polarization. Eur. J. Clin. Microbiol. Infect. Dis. 2017, 36, 1491–1503. [Google Scholar] [CrossRef] [PubMed]
- Koskimaa, H.M.; Paaso, A.; Welters, M.J.; Grénman, S.; Syrjänen, K.; van der Burg, S.H.; Syrjänen, S. Human papillomavirus 16-specific cell-mediated immunity in children born to mothers with incident cervical intraepithelial neoplasia (CIN) and to those constantly HPV negative. J. Transl. Med. 2015, 13, 370. [Google Scholar] [CrossRef]
- Costa, V.; El-Achkar, V.N.; de Barros, P.P.; León, J.E.; Ribeiro-Silva, A.; Carlos, R.; Pignatari, S.S.N.; Ferreira, S.; Mello, B.P.; Sichero, L.; et al. Role of Epstein-Barr virus in the severity of recurrent respiratory papillomatosis. Laryngoscope 2020, 130, E611–E618. [Google Scholar] [CrossRef]
- do Bonfim, C.M.; Sobrinho, J.S.; Nogueira, R.L.; Kupper, D.S.; Valera, F.C.P.; Nogueira, M.L.; Luisa Lina Villa, L.L.; Rahal, P.; Sichero, L. Differences in Transcriptional Activity of Human Papillomavirus Type 6 Molecular Variants in Recurrent Respiratory Papillomatosis. PLoS ONE 2015, 10, e0132325. [Google Scholar] [CrossRef]
- Ikegami, T.; Hirakawa, H.; Tsukahara, N.; Murakami, A.; Kise, N.; Kiyuna, A.; Kosug, T.; Agena, S.; Kinjyo, H.; Hasegawa, N.; et al. Coordinated Expression of HPV-6 Genes with Predominant E4 and E5 Expression in Laryngeal Papilloma. Microorganisms 2021, 9, 520. [Google Scholar] [CrossRef]
- Sichero, L.; Ferreira, S.; López, R.V.M.; Mello, B.P.; Costa, V.; El-Achkar, V.N.R.; Carlos, R.; Ribeiro-Silva, A.; Pignatari, S.; Kaminagakura, E.; et al. Prevalence of human papillomavirus 6 and 11 variants in recurrent respiratory papillomatosis. J. Med. Virol. 2021, 93, 3835–3840. [Google Scholar] [CrossRef]
- Ure, A.; Forslund, O. Lack of methylation in the upstream region of human papillomavirus type 6 from aerodigestive tract papillomas. J. Virol. 2012, 86, 13790–13794. [Google Scholar] [CrossRef][Green Version]
- Deng, Z.; Ikegami, T.; Kiyuna, A.; Zhang, C.; Zhang, T.; Matayoshi, S.; Uehara, T.; Maeda, H.; Suzuki, M.; Ganaha, A. Methylation of CpG sites in the upstream regulatory region, physical status and mRNA expression of HPV-6 in adult-onset laryngeal papilloma. Oncotarget 2017, 8, 85368–85377. [Google Scholar] [CrossRef] [PubMed]
- Major, T.; Szarka, K.; Sziklai, I.; Gergely, L.; Czeglédy, J. The characteristics of human papillomavirus DNA in head and neck cancers and papillomas. J. Clin. Pathol. 2005, 58, 51–55. [Google Scholar] [CrossRef]
- Reidy, P.M.; Dedo, H.H.; Rabah, R.; Field, J.B.; Robert HMathog, R.H.; Gregoire, L.; Lancaster, W.D. Integration of human papillomavirus type 11 in recurrent respiratory papilloma-associated cancer. Laryngoscope 2004, 114, 1906–1909. [Google Scholar] [CrossRef] [PubMed]
- Doorbar, J. Model systems of human papillomavirus-associated disease. J. Pathol. 2016, 238, 166–179. [Google Scholar] [CrossRef]
- Fortes, H.R.; von Ranke, F.M.; Escuissato, D.L.; Neto, C.A.A.; Zanetti, G.; Hochhegger, B.; Souza, C.A.; Marchiori, E. Recurrent respiratory papillomatosis: A state-of-the-art review. Respir. Med. 2017, 126, 116–121. [Google Scholar] [CrossRef]
- Labedz, G.; Scatolini, M.L.; Ruvinsky, S.; Rodriguez, H.A. Factors related to extralaryngeal spread in juvenile recurrent respiratory papillomatosis. Laryngoscope 2020, 131, 1652–1656. [Google Scholar] [CrossRef]
- Gélinas, J.-F.; John Manoukian, J.; Côté, A. Lung involvement in juvenile onset recurrent respiratory papillomatosis: A systematic review of the literature. Int. J. Pediatr. Otorhinolaryngol. 2008, 72, 433–452. [Google Scholar] [CrossRef]
- Mitsumoto, G.L.; Bernardi, F.D.C.; Paes, J.F.; Villa, L.L.; Mello, B.; Pozzan, G. Juvenile-onset recurrent respiratory papillomatosis with pulmonary involvement and carcinomatous transformation. Autops. Case Rep. 2018, 30, e2018035. [Google Scholar] [CrossRef]
- Egawa, N.; Doorbar, J. The low-risk papillomaviruses. Virus Res. 2017, 2, 119–127. [Google Scholar] [CrossRef]
- Karatayli-Ozgursoy, S.; Bishop, J.A.; Hille, A.; Akst, L.; Best, S.R. Risk Factors for Dysplasia in Recurrent Respiratory Papillomatosis in an Adult and Pediatric Population. Ann. Otol. Rhinol. Laryngol. 2016, 125, 235–241. [Google Scholar] [CrossRef]
- Huebbers, C.U.; Preuss, S.F.; Kolligs, J.; Vent, J.; Stenner, M.; Wieland, U.; Silling, S.; Drebber, U.; Speel, E.J.; Klussmann, J.P. Integration of HPV6 and downregulation of AKR1C3 expression mark malignant transformation in a patient with juvenile-onset laryngeal papillomatosis. PLoS ONE 2013, 8, e57207. [Google Scholar] [CrossRef] [PubMed]
- Australian Paediatric Surveillance Unit (APSU). Annual Surveillance Report 2019. Commun. Dis. Intell. 2020, 44. [Google Scholar] [CrossRef]
- Novakovic, D.; Cheng, A.T.L.; Zurynski, Y.; Booy, R.; Walker, P.J.; Berkowitz, R.; Harrison, H.; Black, R.; Perry, C.; Vijayasekaran, S.; et al. A prospective study of the incidence of juvenile-onset recurrent respiratory papillomatosis after implementation of a national HPV vaccination program. J. Infect. Dis. 2018, 217, 208–212. [Google Scholar] [CrossRef]
- Xue, Q.; Wang, J. Recurrent respiratory papillomatosis arising in trachea not affecting larynx. Intern. Med. 2010, 49, 1649–1651. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lam, C.-W.; Talbot, A.B.; Yeh, K.-T.; Lin, S.-C.; Hsieh, C.-E.; Fang, H.-Y. Human papillomavirus and squamous cell carcinoma in a solitary tracheal papilloma. Ann. Thorac. Surg. 2004, 77, 2201–2202. [Google Scholar] [CrossRef]
- Shukuya, T.; Hirano, S.; Sugiyama, H.; Kobayashi, N.; Kudo, K. Squamous papillomas in the trachea and main bronchi found in a Cowden’s disease patient. Intern. Med. 2006, 45, 987–988. [Google Scholar] [CrossRef][Green Version]
- Han, J.; Xia, W.M.; Ai, T.; Lu, Z.R.; Yang, Y.J.; Wang, L.; Fan, Y.H. Findings of electric bronchoscopy in 67 children with recurrent wheezing. Chin. J. Contemp. Pediatr. 2010, 12, 447–449. [Google Scholar]
- Shibuya, H.; Kutomi, T.; Kujime, T.K.; Hara, K.; Hisada, T. An adult case of multiple squamous papillomas of the trachea associated with human papilloma virus type 6. Intern. Med. 2008, 47, 1535–1538. [Google Scholar] [CrossRef]
- Harris, K.; Chalhoubet, M. Tracheal papillomatosis: What do we know so far? Chronic Respir. Dis. 2011, 8, 233–235. [Google Scholar] [CrossRef]
- Abramson, A.L.; Nouri, M.; Mullooly, V.; Fisch, G.; Steinberg, B.M. Latent Human Papillomavirus infection is comparable in the larynx and trachea. J. Med. Virol. 2004, 72, 473–477. [Google Scholar] [CrossRef]
- Patterson, E.J. Multiple papillomata of the esophagus report of a case. Transact. Am. Brochosc. Soc. 1928, 5, 1169–1172. [Google Scholar]
- Adler, R.H.; Carberry, D.M.; Ross, C.A. Papilloma of the esophagus association with hiatal hernia. J. Thorac. Surg. 1959, 37, 625–635. [Google Scholar] [CrossRef]
- Pantham, G.; Ganesan, S.; Einstadter, D.; Jin, G.; Weinberg, A.; Fass, R. Assessment of the incidence of squamous cell papilloma of the esophagus and the presence of high-risk human papilloma virus. Dis. Esophagus 2017, 30, 1–5. [Google Scholar] [CrossRef]
- Mavilia, M.G.; Wu, G.Y. Esophageal squamous papilloma: A case series and literature review. J. Dig. Dis. 2018, 19, 254–256. [Google Scholar] [CrossRef] [PubMed]
- Wong, M.W.; Bair, M.J.; Shih, S.C.; Chu, C.-H.; Wang, H.-Y.; Wang, T.-E.; Chang, C.-W.; Chen, M.-J. Using typical endoscopic features to diagnose esophageal squamous papilloma. World J. Gastroenterol. 2016, 22, 2349–2356. [Google Scholar] [CrossRef] [PubMed]
- Brinson, R.R.; Schuman, B.M.; Mills, L.R.; Thigpen, S.; Freedman, S. Multiple squamous papillomas of the esophagus associated with Goltz syndrome. Am. J. Gastroenterol. 1987, 82, 1177–1179. [Google Scholar] [PubMed]
- Umemura, K.; Takagi, S.; Ishigaki, Y.; Iwabuchi, M.; Kuroki, S.; Kinouchi, Y.; Shimosegawa, T. Gastrointestinal polyposis with esophageal polyposis is useful for early diagnosis of Cowden’s disease. World J. Gastroenterol. 2008, 14, 5755–5759. [Google Scholar] [CrossRef] [PubMed]
- Syrjänen, K.; Syrjänen, S. Detection of human papillomavirus in esophageal papillomas: Systematic review and meta-analysis. APMIS 2012, 121, 363–374. [Google Scholar] [CrossRef] [PubMed]
- Tiftikçi, A.; Kutsal, E.; Altıok, E.; İnce, U.; Çicek, B.; Saruç, M.; Türkel, N.; Ersoy, Ö.; Yenmiş, G.; Tözün, N. Analyzing esophageal squamous cell papillomas for the presence of human papilloma virus. Turk. J. Gastroenterol. 2017, 28, 176–178. [Google Scholar] [CrossRef]
- Kunzmann, A.T.; Graham, S.; McShane, C.M.; Doyle, J.; Tommasino, T.; Johnston, B.; Jamison, J.; James, J.A.; McManus, D.; Anderson, L.A. The prevalence of viral agents in esophageal adenocarcinoma and Barrett’s esophagus: A systematic review. Eur. J. Gastroenterol. Hepatol. 2017, 29, 817–825. [Google Scholar] [CrossRef]
- Tou, A.M.; Al-Nimr, A.O. Esophageal Squamous Papilloma in Children: A Single-center Case Series. J. Pediatr. Gastroenterol. Nutr. 2021, 72, 690–692. [Google Scholar] [CrossRef]
- Rombaldi, R.L.; Serafini, E.P.; Mandelli, J.; Zimmermann, E.; Losquiavo, K.P. Perinatal transmission of human papillomavirus DNA. Virol. J. 2009, 6, 83. [Google Scholar] [CrossRef]
- Syrjänen, S. Current concepts on human papillomavirus infections in children. APMIS 2010, 118, 494–509. [Google Scholar] [CrossRef]
- Mammas, J.N.; Dalianis, T.; Doukas, S.G.; Zaravinos, A.; Achtsidis, V.; Thiagarajan, P.; Theodoridou, M.; Spandidos, D.A. Paediatric virology and human papillomaviruses: An update. Exp. Ther. Med. 2019, 17, 4337–4343. [Google Scholar] [CrossRef] [PubMed]
- Skoczyński, M.; Goździcka-Józefiak, A.; Kwaśniewska, A. Co-occurrence of human papillomavirus (HPV) in newborns and their parents. BMC Infect. Dis. 2019, 19, 930. [Google Scholar] [CrossRef] [PubMed]
- Sarkola, M.E.; Grénman, S.E.; Rintala, M.A.; Syrjänen, K.J.; Syrjänen, S.M. Human papillomavirus in the placenta and umbilical cord blood. Acta Obstet. Gynecol. Scand. 2008, 87, 1181–1188. [Google Scholar] [CrossRef]
- Koskimaa, H.M.; Waterboer, T.; Pawlita, M.; Grénman, S.; Syrjänen, K.; Syrjänen, S. Human papillomavirus genotypes present in the oral mucosa of newborns and their concordance with maternal cervical human papillomavirus genotypes. J. Pediatr. 2012, 160, 837–843. [Google Scholar] [CrossRef] [PubMed]
- Leixeira, L.O.; Amaral, S.C.; Finger-Jardim, F.; Pousada da Hora, V.; Gonçalves, C.V.; Soares, M.A.; Barral de Martinez, A.M. Frequency of Human Papillomavirus in the placenta, in the colostrum and in the umbilical cord blood. Rev. Bras. Ginecol. Obstet. 2015, 37, 203–207. [Google Scholar] [CrossRef]
- Trottier, H.; Mayrand, M.-H.; Coutlée, F.; Monnier, P.; Laporte, L.; JNiyibizi, J.; Ana-Maria Carceller, A.-M.; Fraser, W.D.; Brassard, P.; JLacroix, J.; et al. Human papillomavirus (HPV) perinatal transmission and risk of HPV persistence among children: Design, methods and preliminary results of the HERITAGE study. Papillomavirus Res. 2016, 2, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Smith, E.M.; Ritchie, J.M.; Yankowitz, J.; Swarnavel, S.; Wang, D.; Haugen, T.H.; Turek, L.P. Human papillomavirus prevalence and types in newborns and parents: Concordance and modes of transmission. Sex. Transm. Dis. 2004, 31, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Merckx, M.; Liesbeth, W.V.; Arbyn, M.; Meys, J.; Weyers, S.; Temmerman, M.; Vanden Broeck, D. Transmission of carcinogenic human papillomavirus types from mother to child: A meta-analysis of published studies. Eur. J. Cancer Prev. 2013, 22, 277–285. [Google Scholar] [CrossRef] [PubMed]
- Zouridis, A.; Kalampokas, T.; Panoulis, K.; Salakos, N.; Deligeoroglou, E. Intrauterine HPV transmission: A systematic review of the literature. Arch. Gynecol. Obstet. 2018, 298, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Chatzistamatiou, K.; Sotiriadis, A.; Agorastos, T. Effect of mode of delivery on vertical human papillomavirus transmission—A meta-analysis. J. Obstet. Gynaecol. 2016, 36, 10–14. [Google Scholar] [CrossRef] [PubMed]
- Syrjänen, S.; Rintala, M.; Sarkola, M.; Willberg, J.; Rautava, J.; Koskimaa, H.; Paaso, A.; Syrjänen, K.; Grénman, S.; Louvanto, K. Oral Human Papillomavirus Infection in Children during the First 6 Years of Life, Finland. Emerg. Infect. Dis. 2021, 27, 759–766. [Google Scholar] [CrossRef]
- Louvanto, K.; Roger, M.; Faucher, M.C.; Syrjänen, K.; Grenman, S.; Syrjänen, S. HLA-G and vertical mother-to-child transmission of human papillomavirus infection. Hum. Immunol. 2018, 79, 471–476. [Google Scholar] [CrossRef] [PubMed]
- Dassi, L.; Annunziata, C.; Botti, C.; Micillo, A.; Cerasuolo, A.; Starita, N.; Buonaguro, F.M.; Tornesello, M.L. Detection of Human Papillomaviruses in the Nasopharynx of Breastfed Infants: New Findings and Meta-Analysis. Viruses 2020, 12, 1119. [Google Scholar] [CrossRef]
- Puranen, M.; Yliskoski, M.; Saarikoski, S.; Syrjänen, K.; Syrjänen, S. Vertical transmission of human papillomavirus from infected mothers to their newborn babies and persistence of the virus in childhood. Am. J. Obstet. Gynecol. 1996, 174, 694–699. [Google Scholar] [CrossRef]
- Puranen, M.H.; Yliskoski, M.H.; Saarikoski, S.V.; Syrjänen, K.J.; Syrjänen, S.M. Exposure of an infant to cervical human papillomavirus infection of the mother is common. Am. J. Obstet. Gynecol. 1997, 176, 1039–1045. [Google Scholar] [CrossRef]
- Peh, W.L.; Middleton, K.; Christensen, N.; Nicholls, P.; Egawa, K.; Sotlar, K.; Brandsma, J.; Percival, A.; Lewis, J.; Liu, W.J.; et al. Life cycle heterogeneity in animal models of human papillomavirus-associated disease. J. Virol. 2002, 76, 10401–10416. [Google Scholar] [CrossRef]
- Nafz, J.; Köhler, A.; Ohnesorge, M.; Nindl, I.; Stockfleth, E.; Rösl, F. Persistence of Mastomys natalensis papillomavirus in multiple organs identifies novel targets for infection. J. Gen. Virol. 2007, 88 Pt 10, 2670–2678. [Google Scholar] [CrossRef]
- Amella, C.A.; Lofgren, L.A.; Ronn, A.M.; Nouri, M.; Shikowitz, M.J.; Steinberg, B.M. Latent infection induced with cottontail rabbit papillomavirus. A model for human papillomavirus latency. Am. J. Pathol. 1994, 144, 1167–1171. [Google Scholar] [PubMed]
- Zhang, P.; Nouri, M.; Brandsma, J.L.; Iftner, T.; Steinberg, B.M. Induction of E6/E7 expression in cottontail rabbit papillomavirus latency following UV activation. Virology 1999, 263, 388–394. [Google Scholar] [CrossRef] [PubMed]
- Selvakumar, R.; Schmitt, A.; Iftner, T.; Ahmed, R.; Wettstein, F.O. Regression of papillomas induced by cottontail rabbit papillomavirus is associated with infiltration of CD8+ cells and persistence of viral DNA after regression. J. Virol. 1997, 71, 5540–5548. [Google Scholar] [CrossRef] [PubMed]
- Pao, C.C.; Lin, S.S.; Lin, C.Y.; Maa, J.S.; Lai, C.H.; Hsieh, T.T. Identification of human papillomavirus DNA sequences in peripheral blood mononuclear cells. Am. J. Clin. Pathol. 1991, 95, 540–546. [Google Scholar] [CrossRef]
- Cladel, N.M.; Jiang, P.; Li, J.J.; Peng, X.; Cooper, T.K.; Majerciak, V.; Balogh, K.K.; Thomas, J.; Meyer, T.J.; Brendle, S.A.; et al. Papillomavirus can be transmitted through the blood and produce infections in blood recipients: Evidence from two animal models. Emerg. Microbes Infect. 2019, 8, 1108–1121. [Google Scholar] [CrossRef] [PubMed]
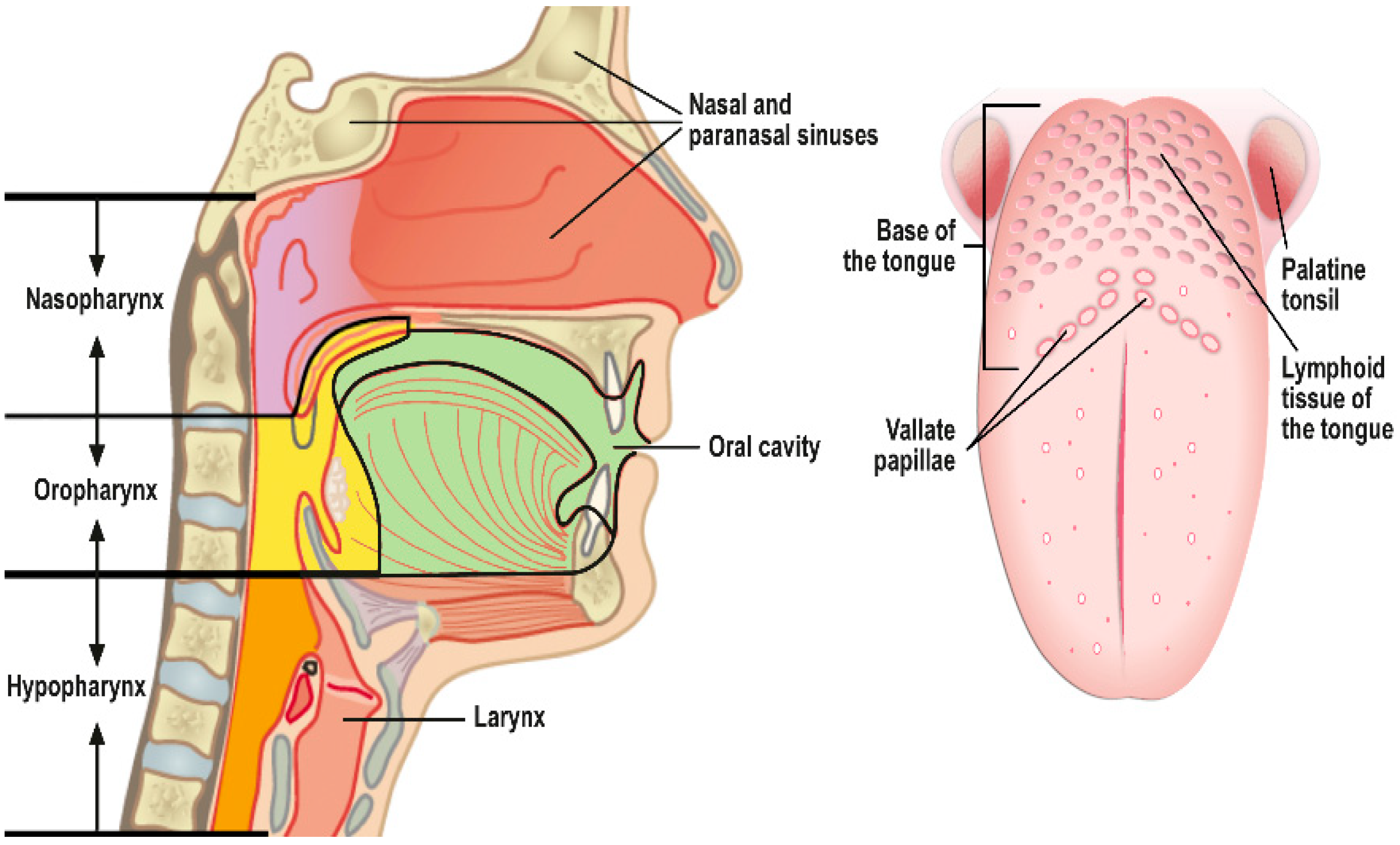
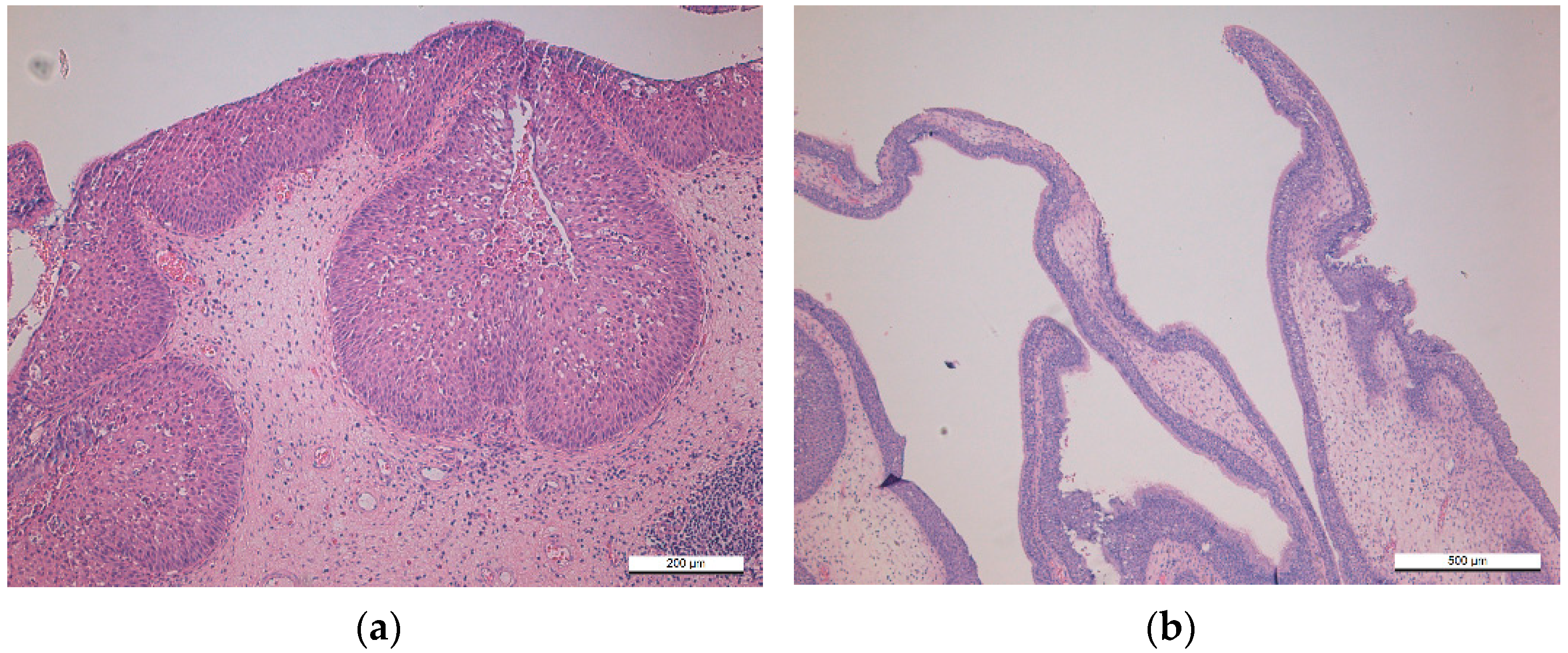
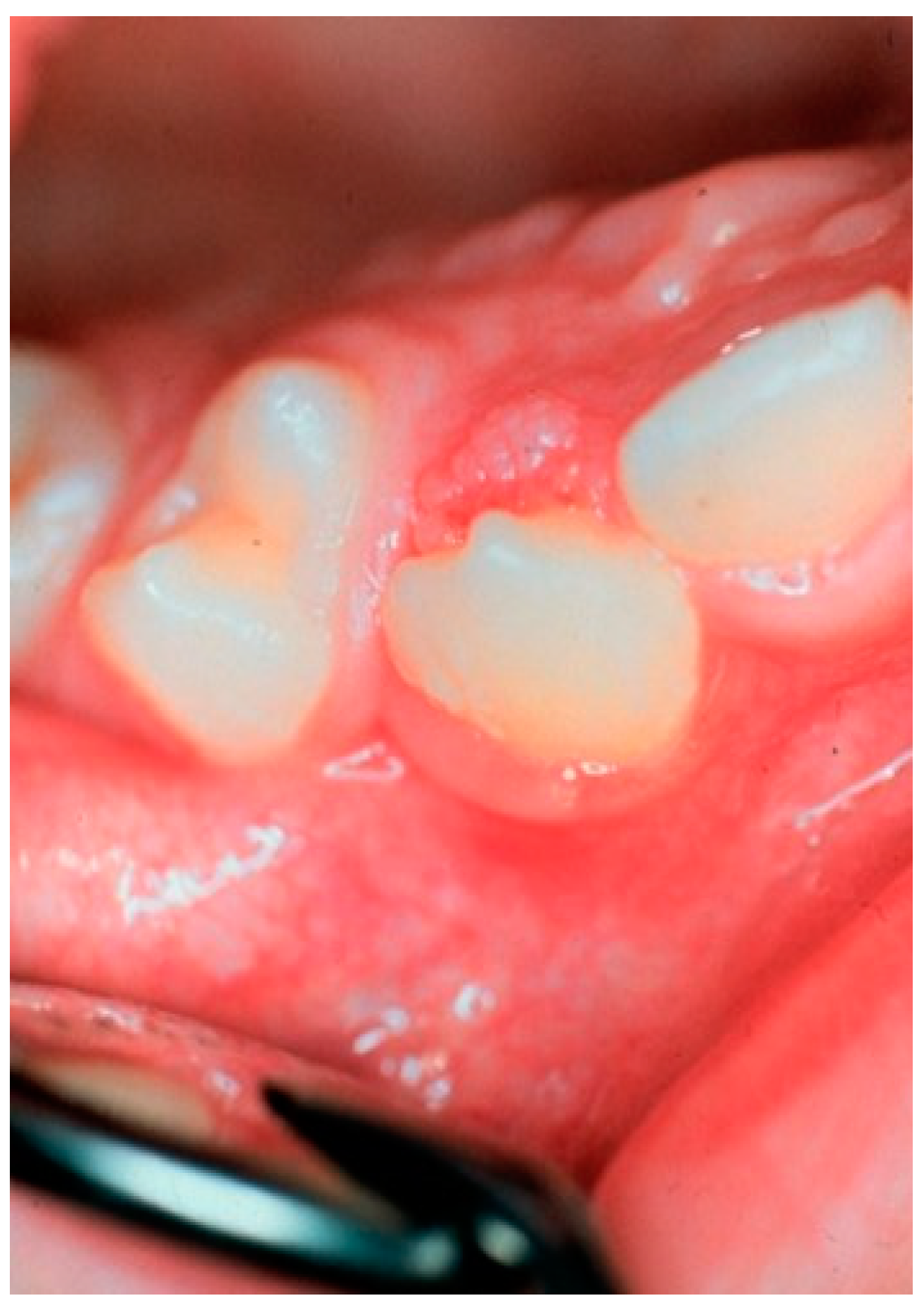
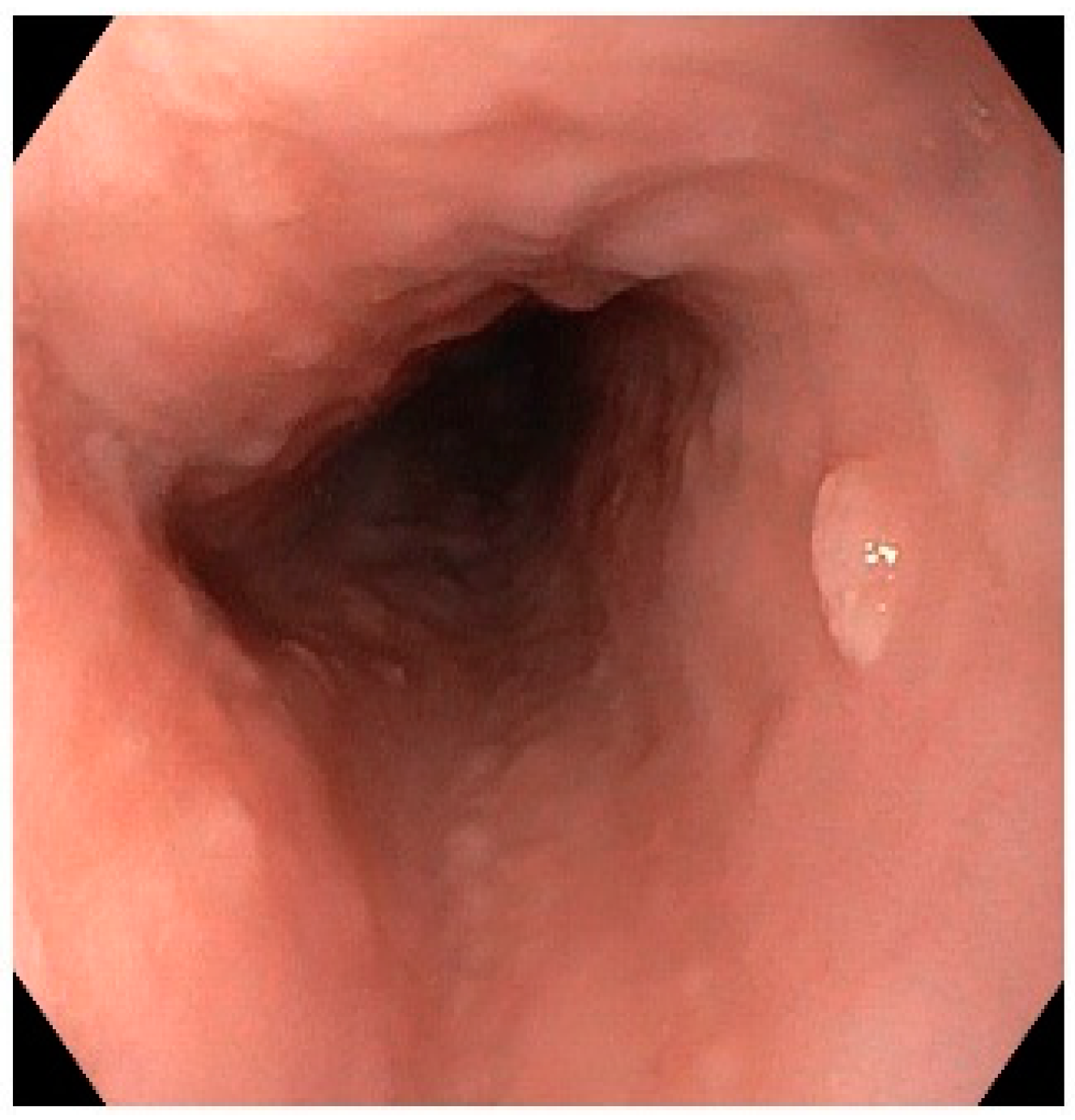
| Characteristic | Sinonasal Papillomas: Inverted/Exophytic/Oncocytic | Oral Papillomas | Laryngeal Papillomas | Esophageal Papillomas |
|---|---|---|---|---|
| Frequency Proportion among sinonasal suptypes | Sinonasal overall 0.74–2.3/100,000 children extremely low incidence SNIP 65–70% SNEP 20–25% SNOP 3–6% | children common, but prevalence unknown adults 0.5/100,000 | children 0.6–4.3/100,000 adults 1.8–2.3/100,000 | children 0.08% adults 0.01–0.45% |
| Common location | SNIP nasal cavity and maxillary sinus SNEP lower anterior nasal septum SNOP lateral nasal wall | tongue, gingiva, under lip (oropharynx: soft palate, uvula, tonsil) | vocal cords and ventricles, false cords | proximal esophagus |
| Male to female ratio | children 3:1 SNIP 2.5–3:1 SNEP 2–10:1 SNOP 1:15 | 1:1 | children 1:1 adults 3:2 | children 1:2.3 adults 5:2 to 1:5 |
| Age at presentation | children 6–15 years adults: SNIP 50–60 years SNEP 30–50 years SNOP > 50 years | children adults 30–50 years | JO-RRP < 5–8 years AO-RRP 20–40 years | children 2–17 years adults 40–50 years |
| Prevalence of HPV | SNIP 25–39% SNEP 65.3% SNOP 22.5% | overall 27–48% HPV+ samples: 48% alpha-HPV 23% gamma-HPV | 76%-93.7% | 10.5–57% |
| Mucosal Alpha HPVs low-risk high risk | HPV6, 11, 42 HPV16, 58, 83 | HPV6, HPV11 HPV16, 18, 35, 51, 74 | HPV6, 11 HPV16,18, 31, 33, 35, HPV45, 55, 70, 76, 84 | HPV6 HPV16,18, 31, 81 |
| Cutaneous HPV beta-HPV gamma-HPV | NA | HPV5, HPV12, HPV22, HPV23, HPV120 HPV121, HPV123, HPV130, HPV131, HPV161 | HPV8 NA | NA |
| Recurrence | SNIP 25–30% SNEP 22–50% SNOP 39% | unusual approx. 4.1% | aggressive course in 20% of JO-RRP, distal spread 5–48% | NA |
| Risk of malignant transformation | incidence 0.38/106 SNIP 7–11% SNEP SNOP | no evidence | JO-RRP < 1% AO-RRP 3–6% | rare |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Syrjänen, S.; Syrjänen, K. HPV-Associated Benign Squamous Cell Papillomas in the Upper Aero-Digestive Tract and Their Malignant Potential. Viruses 2021, 13, 1624. https://doi.org/10.3390/v13081624
Syrjänen S, Syrjänen K. HPV-Associated Benign Squamous Cell Papillomas in the Upper Aero-Digestive Tract and Their Malignant Potential. Viruses. 2021; 13(8):1624. https://doi.org/10.3390/v13081624
Chicago/Turabian StyleSyrjänen, Stina, and Kari Syrjänen. 2021. "HPV-Associated Benign Squamous Cell Papillomas in the Upper Aero-Digestive Tract and Their Malignant Potential" Viruses 13, no. 8: 1624. https://doi.org/10.3390/v13081624
APA StyleSyrjänen, S., & Syrjänen, K. (2021). HPV-Associated Benign Squamous Cell Papillomas in the Upper Aero-Digestive Tract and Their Malignant Potential. Viruses, 13(8), 1624. https://doi.org/10.3390/v13081624





