Human Papillomavirus Related Neoplasia of the Ocular Adnexa
Abstract
1. Introduction
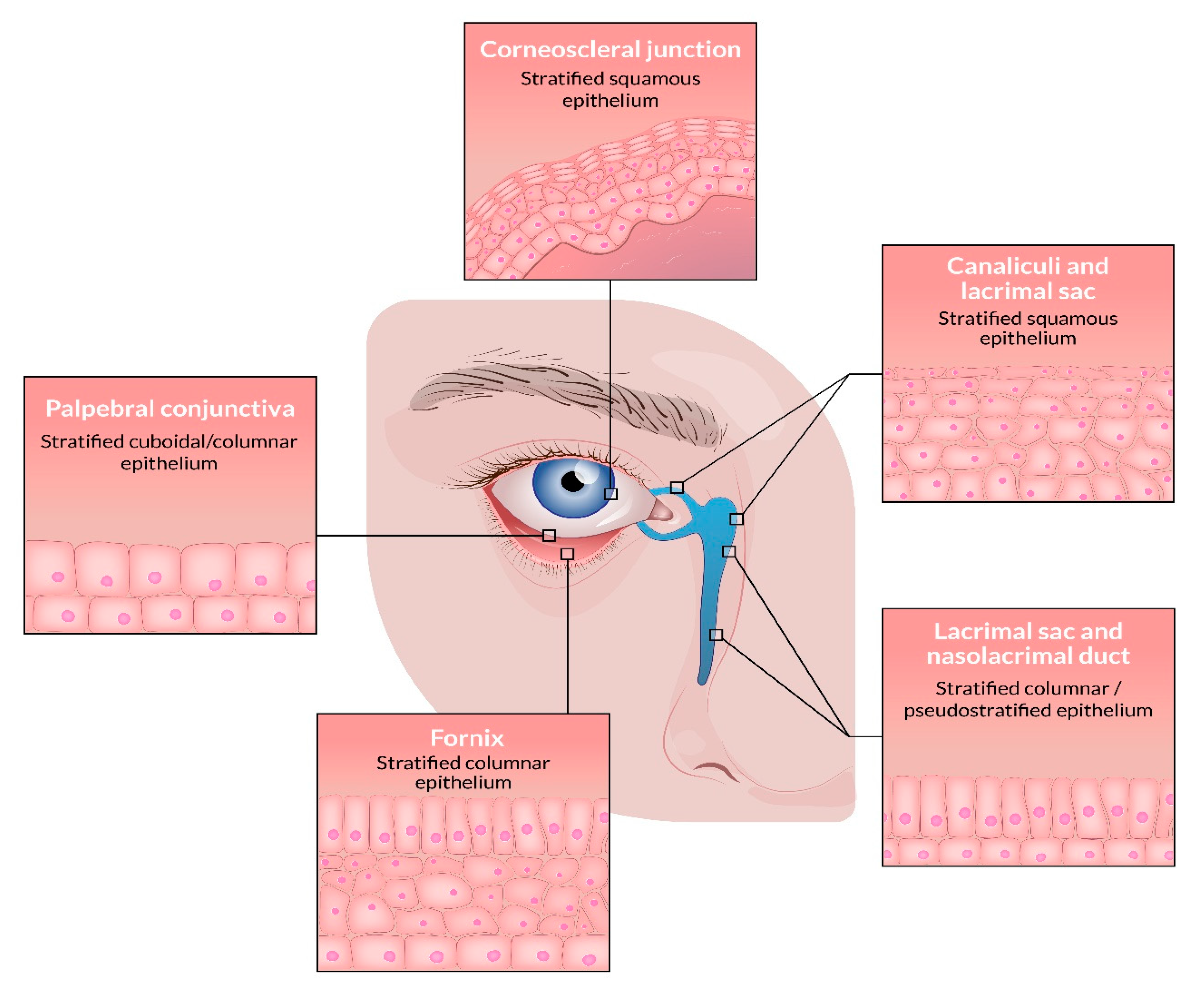
2. The Conjunctiva
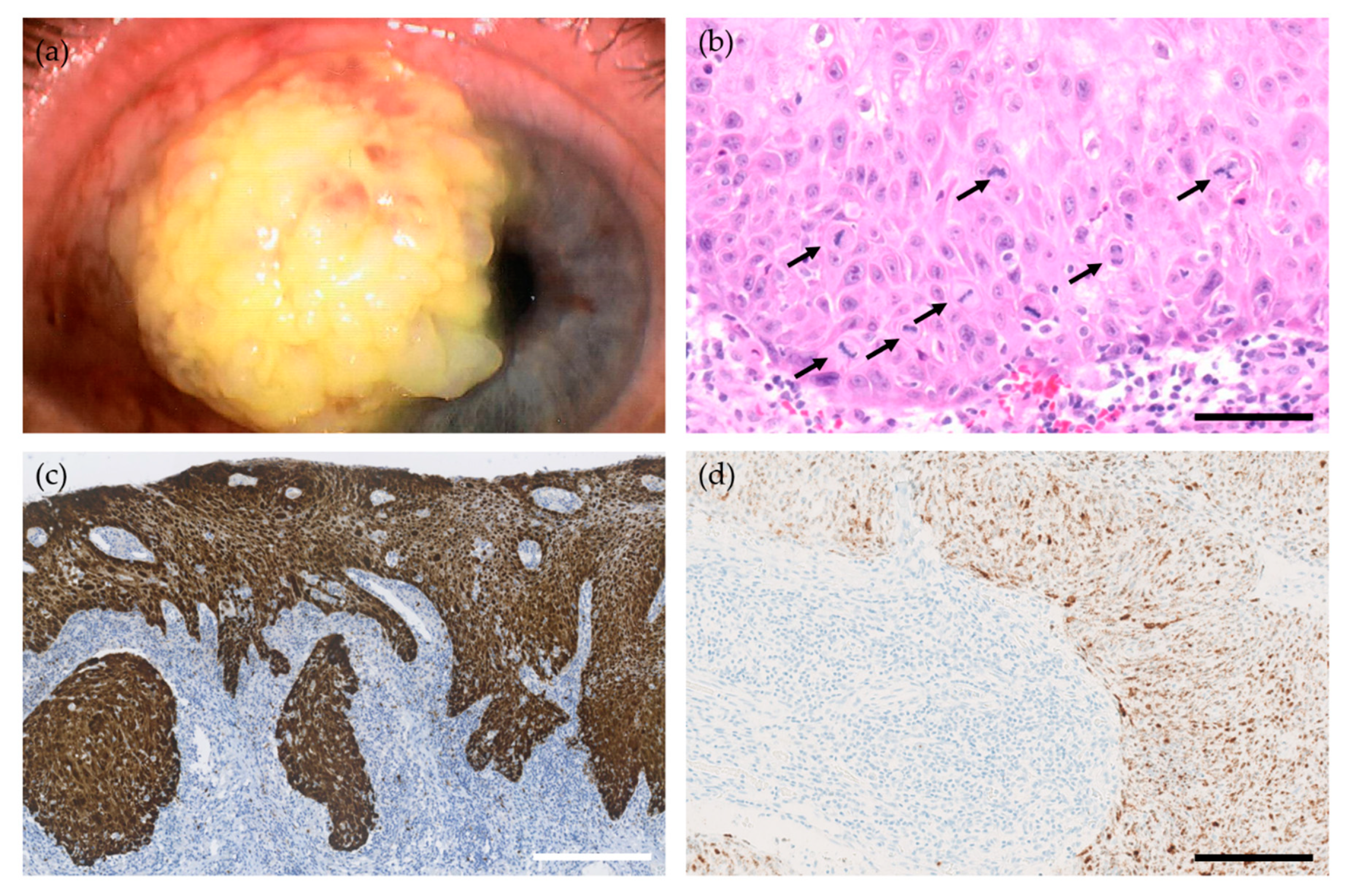
2.1. Conjunctival Papilloma
2.2. Conjunctival Intraepithelial Neoplasia and Squamous Cell Carcinoma
2.2.1. Prognosis and Treatment
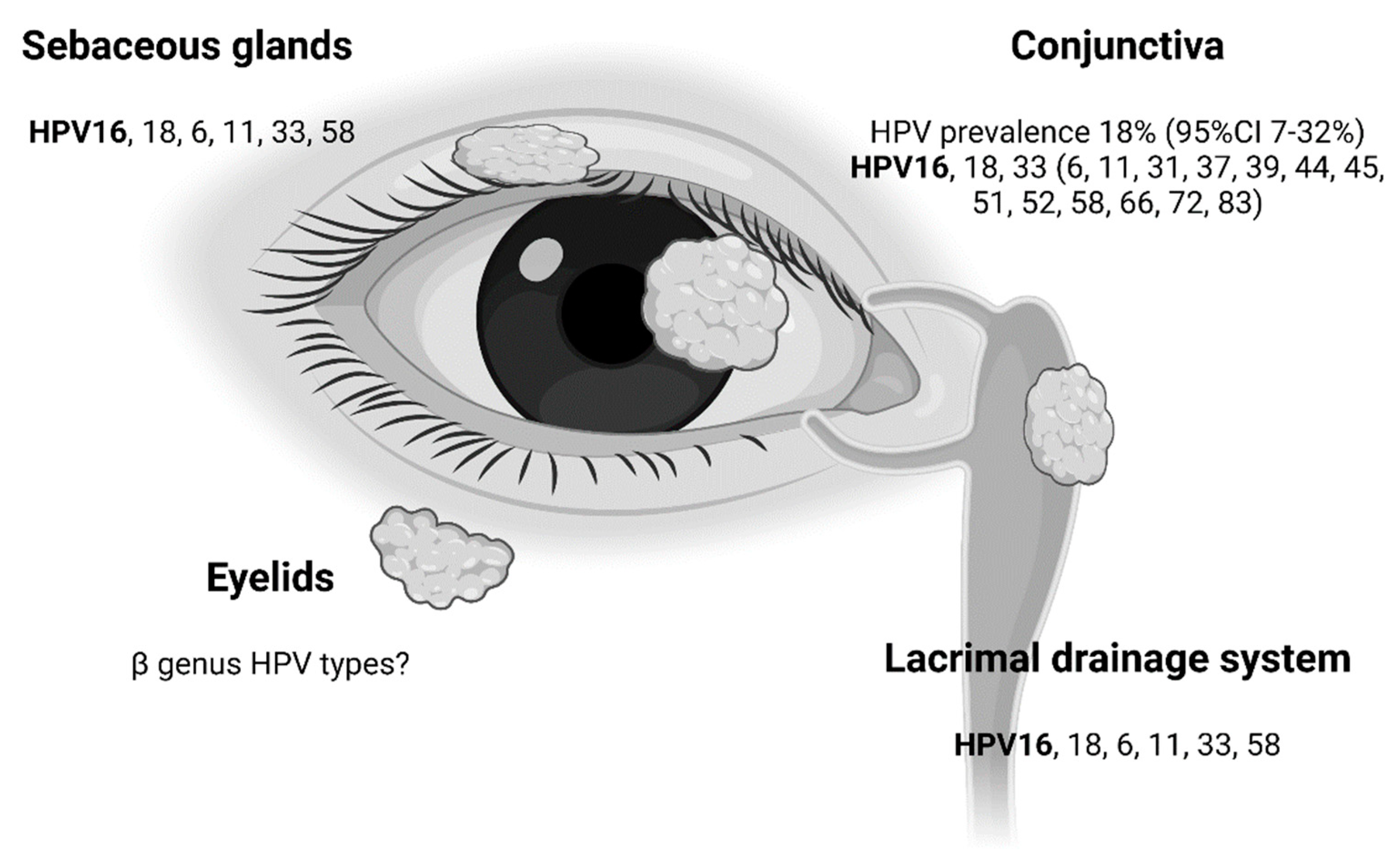
2.2.2. Human Papillomavirus in Conjunctival CIN and SCC
2.2.3. Clinical Characteristics of HPV-Related Conjunctival CIN and SCC
2.2.4. Histological and Immunohistological Markers of HPV-Related Conjunctival CIN and SCC
2.3. Transmission of HPV to the Conjunctiva
3. The Lacrimal Drainage System
4. The Eyelids
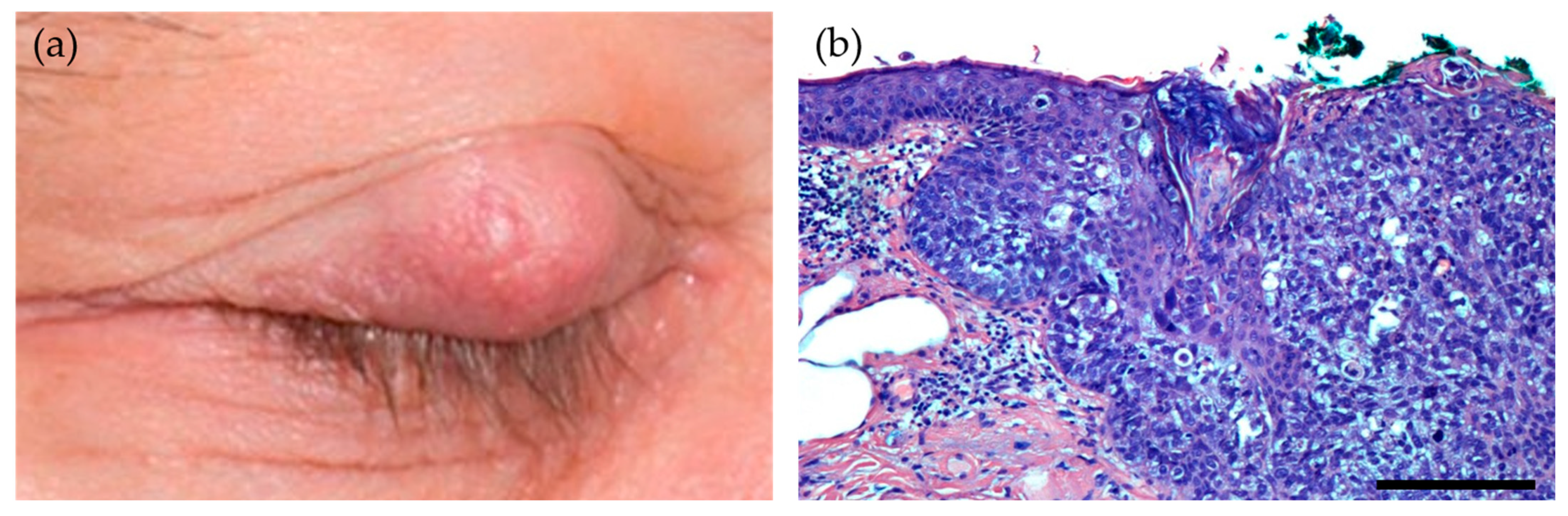
4.1. Sebaceous Gland Carcinoma
Human Papillomavirus in Ocular Adnexal Sebaceous Carcinoma
4.2. Cutaneous Squamous Cell Carcinoma
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- M’Fadyean, J.; Hobday, F. Note on the Experimental Transmission of Warts in the Dog. J. Comp. Pathol. 1898, 11, 341–344. [Google Scholar] [CrossRef]
- Stagner, A.M.; Afrogheh, A.H.; Jakobiec, F.A.; Iacob, C.E.; Grossniklaus, H.E.; Deshpande, V.; Maske, C.; Hiss, D.C.; Faquin, W.C. p16 Expression Is Not a Surrogate Marker for High-Risk Human Papillomavirus Infection in Periocular Sebaceous Carcinoma. Am. J. Ophthalmol. 2016, 170, 168–175. [Google Scholar] [CrossRef]
- Lass, J.H.; Jenson, A.B.; Papale, J.J.; Albert, D.M. Papillomavirus in human conjunctival papillomas. Am. J. Ophthalmol. 1983, 95, 364–368. [Google Scholar] [CrossRef]
- McDonnell, J.M.; McDonnell, P.J.; Mounts, P.; Wu, T.C.; Green, W.R. Demonstration of papillomavirus capsid antigen in human conjunctival neoplasia. Arch. Ophthalmol. 1986, 104, 1801–1805. [Google Scholar] [CrossRef] [PubMed]
- Sjö, N.; Heegaard, S.; Prause, J.U. Conjunctival papilloma. A histopathologically based retrospective study. Acta Ophthalmol. Scand. 2000, 78, 663–666. [Google Scholar] [CrossRef]
- Kaliki, S.; Arepalli, S.; Shields, C.L.; Klein, K.; Sun, H.; Hysenj, E.; Lally, S.E.; Shields, J.A. Conjunctival papilloma: Features and outcomes based on age at initial examination. JAMA Ophthalmol. 2013, 131, 585–593. [Google Scholar] [CrossRef] [PubMed]
- Furdova, A.; Stopkova, A.; Kapitanova, K.; Kobzova, D.; Babal, P. Conjuctival lesions—the relationship of papillomas and squamous cell carcinoma to HPV infection. Cesk. Slov. Oftalmol. 2018, 74, 92–97. [Google Scholar] [CrossRef]
- Theotoka, D.; Morkin, M.I.; Galor, A.; Karp, C.L. Update on Diagnosis and Management of Conjunctival Papilloma. Eye Vis. 2019, 6, 18. [Google Scholar] [CrossRef]
- Mlakar, J.; Kocjan, B.J.; Hosnjak, L.; Pizem, J.; Beltram, M.; Gale, N.; Drnovsek-Olup, B.; Poljak, M. Morphological characteristics of conjunctival squamous papillomas in relation to human papillomavirus infection. Br. J. Ophthalmol. 2015, 99, 431–436. [Google Scholar] [CrossRef]
- Sjö, N.C.; von Buchwald, C.; Cassonnet, P.; Norrild, B.; Prause, J.U.; Vinding, T.; Heegaard, S. Human papillomavirus in normal conjunctival tissue and in conjunctival papilloma: Types and frequencies in a large series. Br. J. Ophthalmol. 2007, 91, 1014–1015. [Google Scholar] [CrossRef] [PubMed]
- Saegusa, M.; Takano, Y.; Hashimura, M.; Okayasu, I.; Shiga, J. HPV type 16 in conjunctival and junctional papilloma, dysplasia, and squamous cell carcinoma. J. Clin. Pathol. 1995, 48, 1106–1110. [Google Scholar] [CrossRef] [PubMed]
- Benevides Dos Santos, P.J.; Borborema Dos Santos, C.M.; Mendonça, R.R.; Vieira Do Carmo, M.A.; Astofi-Filho, S. Human papillomavirus type 13 infecting the conjunctiva. Diagn. Microbiol. Infect. Dis. 2005, 53, 71–73. [Google Scholar] [CrossRef]
- Buggage, R.R.; Smith, J.A.; Shen, D.; Chan, C.C. Conjunctival papillomas caused by human papillomavirus type 33. Arch. Ophthalmol. 2002, 120, 202–204. [Google Scholar] [CrossRef]
- Sjö, N.C.; Heegaard, S.; Prause, J.U.; Von Buchwald, C.; Lindeberg, H. Human papillomavirus in conjunctival papilloma. Br. J. Ophthalmol. 2001, 85, 785–787. [Google Scholar] [CrossRef]
- Takamura, Y.; Kubo, E.; Tsuzuki, S.; Akagi, Y. Detection of human papillomavirus in pterygium and conjunctival papilloma by hybrid capture II and PCR assays. Eye 2008, 22, 1442–1445. [Google Scholar] [CrossRef]
- Eng, H.L.; Lin, T.M.; Chen, S.Y.; Wu, S.M.; Chen, W.J. Failure to detect human papillomavirus DNA in malignant epithelial neoplasms of conjunctiva by polymerase chain reaction. Am. J. Clin. Pathol. 2002, 117, 429–436. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, Y.; Mashima, Y.; Kameyama, K.; Mukai, M.; Oguchi, Y. Detection of human papillomavirus infection in squamous tumours of the conjunctiva and lacrimal sac by immunohistochemistry, in situ hybridisation, and polymerase chain reaction. Br. J. Ophthalmol. 1997, 81, 308–313. [Google Scholar] [CrossRef] [PubMed][Green Version]
- McDonnell, P.J.; McDonnell, J.M.; Kessis, T.; Green, W.R.; Shah, K.V. Detection of human papillomavirus type 6/11 DNA in conjunctival papillomas by in situ hybridization with radioactive probes. Hum. Pathol. 1987, 18, 1115–1119. [Google Scholar] [CrossRef]
- Ramberg, I.; Sjo, N.C.; Bonde, J.H.; Heegaard, S. Inverted papilloma of the conjunctiva. BMJ Open Ophthalmol. 2019, 4, e000193. [Google Scholar] [CrossRef] [PubMed]
- Bata, B.M.; Salvi, S.M.; Mudhar, H.S. Conjunctival invasive squamous carcinoma arising from a dysplastic inverted papilloma, both positive for HPV16. Can. J. Ophthalmol. 2021. [Google Scholar] [CrossRef]
- Heuring, A.H.; Hutz, W.W.; Eckhardt, H.B.; Bohle, R.M. Invertiertes Transitionalzellpapillom der Bindehaut mit peripherer karzinomatöser Entartung. Klin. Mon. Augenheilkd. 1998, 212, 61–63. [Google Scholar] [CrossRef]
- Lassalle, S.; Maschi, C.; Caujolle, J.P.; Giordanengo, V.; Hofman, P. Inverted conjunctival papilloma: A certainly underestimated high-risk lesion for carcinomatous transformation-a case report. Can. J. Ophthalmol. 2017, 52, e30–e31. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.W.; Guo, Z.Q.; Zhang, R.X. Human papillomavirus infection and the malignant transformation of sinonasal inverted papilloma: A meta-analysis. J. Clin. Virol. 2016, 79, 36–43. [Google Scholar] [CrossRef]
- Cervantes, G.; Rodríguez, A.A.; Leal, A.G. Squamous cell carcinoma of the conjunctiva: Clinicopathological features in 287 cases. Can. J. Ophthalmol. 2002, 37, 14–20. [Google Scholar] [CrossRef]
- Tunc, M.; Char, D.H.; Crawford, B.; Miller, T. Intraepithelial and invasive squamous cell carcinoma of the conjunctiva: Analysis of 60 cases. Br. J. Ophthalmol. 1999, 83, 98–103. [Google Scholar] [CrossRef] [PubMed]
- Merz, L.E.; Afriyie, O.; Jiagge, E.; Adjei, E.; Foltin, S.K.; Ludwig, M.L.; McHugh, J.B.; Brenner, J.C.; Merajver, S.D. Clinical characteristics, HIV status, and molecular biomarkers in squamous cell carcinoma of the conjunctiva in Ghana. Health Sci. Rep. 2019, 2, 108. [Google Scholar] [CrossRef]
- Karcioglu, Z.A.; Toth, J. Relation between p53 overexpression and clinical behavior of ocular/orbital invasion of conjunctival squamous cell carcinoma. Ophthalmic Plast. Reconstr. Surg. 2000, 16, 443–449. [Google Scholar] [CrossRef]
- Muchengeti, M.; Bohlius, J.; Dhokotera, T.G. Conjunctival cancer in people living with HIV. Curr. Opin. Infect. Dis. 2021, 34, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Gichuhi, S.; Macharia, E.; Kabiru, J.; Zindamoyen, A.M.; Rono, H.; Ollando, E.; Wachira, J.; Munene, R.; Maina, J.; Onyuma, T.; et al. Topical fluorouracil after surgery for ocular surface squamous neoplasia in Kenya: A randomised, double-blind, placebo-controlled trial. Lancet Glob. Health 2016, 4, e378–e385. [Google Scholar] [CrossRef]
- Tabin, G.; Levin, S.; Snibson, G.; Loughnan, M.; Taylor, H. Late recurrences and the necessity for long-term follow-up in corneal and conjunctival intraepithelial neoplasia. Ophthalmology 1997, 104, 485–492. [Google Scholar] [CrossRef]
- Siedlecki, A.N.; Tapp, S.; Tosteson, A.N.; Larson, R.J.; Karp, C.L.; Lietman, T.; Zegans, M.E. Surgery Versus Interferon Alpha-2b Treatment Strategies for Ocular Surface Squamous Neoplasia: A Literature-Based Decision Analysis. Cornea 2016, 35, 613–618. [Google Scholar] [CrossRef] [PubMed]
- Giannaccare, G.; Bernabei, F.; Angi, M.; Pellegrini, M.; Maestri, A.; Romano, V.; Scorcia, V.; Rothschild, P.R. Iatrogenic Ocular Surface Diseases Occurring during and/or after Different Treatments for Ocular Tumours. Cancers 2021, 13, 1933. [Google Scholar] [CrossRef] [PubMed]
- Griffin, H.; Mudhar, H.S.; Rundle, P.; Shiraz, A.; Mahmood, R.; Egawa, N.; Quint, W.; Rennie, I.G.; Doorbar, J. Human papillomavirus type 16 causes a defined subset of conjunctival in situ squamous cell carcinomas. Mod. Pathol. 2019, 33, 74–90. [Google Scholar] [CrossRef] [PubMed]
- Hirst, L.W. Randomized controlled trial of topical mitomycin C for ocular surface squamous neoplasia: Early resolution. Ophthalmology 2007, 114, 976–982. [Google Scholar] [CrossRef] [PubMed]
- Ramberg, I.; Møller-Hansen, M.; Toft, P.B.; Funding, M.; Heegaard, S. Human papillomavirus infection plays a role in conjunctival squamous cell carcinoma: A systematic review and meta-analysis of observational studies. Acta Ophthalmol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Carreira, H.; Coutinho, F.; Carrilho, C.; Lunet, N. HIV and HPV infections and ocular surface squamous neoplasia: Systematic review and meta-analysis. Br. J. Cancer 2013, 109, 1981–1988. [Google Scholar] [CrossRef]
- Nagarajan, P.; El-Hadad, C.; Gruschkus, S.K.; Ning, J.; Hudgens, C.W.; Sagiv, O.; Gross, N.; Tetzlaff, M.T.; Esmaeli, B. PD-L1/PD1 Expression, Composition of Tumor-Associated Immune Infiltrate, and HPV Status in Conjunctival Squamous Cell Carcinoma. Investig. Ophthalmol. Vis. Sci. 2019, 60, 2388–2398. [Google Scholar] [CrossRef]
- Ramberg, I.; Toft, P.B.; Georgsen, J.B.; Siersma, V.D.; Funding, M.; Jensen, D.H.; Von Buchwald, C.; Heegaard, S. Conjunctival intraepithelial neoplasia and carcinoma: Distinct clinical and histological features in relation to human papilloma virus status. Br. J. Ophthalmol. 2019, 105, 878–883. [Google Scholar] [CrossRef]
- Scott, I.U.; Karp, C.L.; Nuovo, G.J. Human papillomavirus 16 and 18 expression in conjunctival intraepithelial neoplasia. Ophthalmology 2002, 109, 542–547. [Google Scholar] [CrossRef]
- Egawa, N.; Egawa, K.; Griffin, H.; Doorbar, J. Human Papillomaviruses; Epithelial Tropisms, and the Development of Neoplasia. Viruses 2015, 7, 3863–3890. [Google Scholar] [CrossRef]
- Ateenyi-Agaba, C.; Franceschi, S.; Wabwire-Mangen, F.; Arslan, A.; Othieno, E.; Binta-Kahwa, J.; van Doorn, L.J.; Kleter, B.; Quint, W.; Weiderpass, E. Human papillomavirus infection and squamous cell carcinoma of the conjunctiva. Br. J. Cancer 2010, 102, 262–267. [Google Scholar] [CrossRef]
- Afrogheh, A.; Jakobiec, F.; Hammon, R.; Grossniklaus, H.; Rocco, J.; Lindeman, N.; Sadow, P.; Faquin, W. Evaluation for high-risk HPV in squamous cell carcinomas and precursor lesions arising in the conjunctiva and lacrimal sac. Am. J. Surg. Pathol. 2016, 40, 519–528. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, S.; Sen, S.; Sharma, A.; Kashyap, S.; Tandon, R.; Bajaj, M.S.; Pushker, N.; Vanathi, M.; Chauhan, S.S. p16(INK4a) overexpression as a predictor of survival in ocular surface squamous neoplasia. Br. J. Ophthalmol. 2018, 102, 840–847. [Google Scholar] [CrossRef]
- Tabrizi, S.N.; McCurrach, F.E.; Drewe, R.H.; Borg, A.J.; Garland, S.M.; Taylor, H.R. Human papillomavirus in corneal and conjunctival carcinoma. Aust. N. Z. J. Ophthalmol. 1997, 25, 211–215. [Google Scholar] [CrossRef]
- Woods, M.; Chow, S.; Heng, B.; Glenn, W.; Whitaker, N.; Waring, D.; Iwasenko, J.; Rawlinson, W.; Coroneo, M.T.; Wakefield, D.; et al. Detecting human papillomavirus in ocular surface diseases. Investig. Ophthalmol. Vis. Sci. 2013, 54, 8069–8078. [Google Scholar] [CrossRef]
- Sen, S.; Sharma, A.; Panda, A. Immunohistochemical localization of human papilloma virus in conjunctival neoplasias: A retrospective study. Indian J. Ophthalmol. 2007, 55, 361–363. [Google Scholar] [CrossRef] [PubMed]
- Auw-Haedrich, C.; Martin, G.; Spelsberg, H.; Sundmacher, R.; Freudenberg, N.; Maier, P.; Reinhard, T. Expression of p16 in conjunctival intraepithelial neoplasia does not correlate with HPV-infection. Open Ophthalmol. J. 2008, 2, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, S.; Sen, S.; Sharma, A.; Dar, L.; Kashyap, S.; Kumar, P.; Bajaj, M.S.; Tandon, R. Human papillomavirus: A predictor of better survival in ocular surface squamous neoplasia patients. Br. J. Ophthalmol. 2012, 96, 1517–1521. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.M.; Lin, H.C.; Chu, P.H.; Wu, H.H.; Shiu, T.F.; Shang, L.H.; Lai, C.H. Expression of cell cycle-regulatory proteins, MIB-1, p16, p53, and p63, in squamous cell carcinoma of conjunctiva: Not associated with human papillomavirus infection. Virchows Arch. 2006, 448, 301–305. [Google Scholar] [CrossRef]
- Kuo, K.T.; Chang, H.C.; Hsiao, C.H.; Lin, M.C. Increased Ki-67 proliferative index and absence of P16INK4 in CIN-HPV related pathogenic pathways different from cervical squamous intraepithelial lesion. Br. J. Ophthalmol. 2006, 90, 894–899. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, T.; Choi, W.; Kim, G.E.; Yang, J.M.; Yoon, K.C. Human papilloma virus identification in ocular surface squamous neoplasia by p16 immunohistochemistry and DNA chip test: A strobe-compliant article. Medicine 2019, 98, e13944. [Google Scholar] [CrossRef] [PubMed]
- Moyer, A.B.; Roberts, J.; Olsen, R.J.; Chevez-Barrios, P. Human papillomavirus-driven squamous lesions: High-risk genotype found in conjunctival papillomas, dysplasia, and carcinoma. Am. J. Dermatopathol. 2018, 40, 486–490. [Google Scholar] [CrossRef]
- Naghashfar, Z.; McDonnell, P.J.; McDonnell, J.M.; Green, W.R.; Shah, K.V. Genital tract papillomavirus type 6 in recurrent conjunctival papilloma. Arch. Ophthalmol. 1986, 104, 1814–1815. [Google Scholar] [CrossRef] [PubMed]
- Egbert, J.E.; Kersten, R.C. Female genital tract papillomavirus in conjunctival papillomas of infancy. Am. J. Ophthalmol. 1997, 123, 551–552. [Google Scholar] [CrossRef]
- Minchiotti, S.; Masucci, L.; Serapiao Dos Santos, M.; Perrella, E.; Graffeo, R.; Lambiase, A.; Bonini, S. Conjunctival papilloma and human papillomavirus: Identification of HPV types by PCR. Eur. J. Ophthalmol. 2006, 16, 473–477. [Google Scholar] [CrossRef] [PubMed]
- Trottier, H.; Mayrand, M.H.; Coutlée, F.; Monnier, P.; Laporte, L.; Niyibizi, J.; Carceller, A.M.; Fraser, W.D.; Brassard, P.; Lacroix, J.; et al. Human papillomavirus (HPV) perinatal transmission and risk of HPV persistence among children: Design, methods and preliminary results of the HERITAGE study. Papillomavirus Res. 2016, 2, 145–152. [Google Scholar] [CrossRef]
- Sonnex, C.; Strauss, S.; Gray, J.J. Detection of human papillomavirus DNA on the fingers of patients with genital warts. Sex. Transm. Dis. 1999, 75, 317–319. [Google Scholar] [CrossRef]
- Iovieno, A.; Piana, S.; Chiesi, L.; Fodero, C.; Fontana, L. Human papillomavirus (HPV)-associated trilateral squamous neoplasia in immunocompetent individual. Int. Ophthalmol. 2018, 38, 1347–1350. [Google Scholar] [CrossRef]
- Gichuhi, S.; Sagoo, M.S.; Weiss, H.A.; Burton, M.J. Epidemiology of ocular surface squamous neoplasia in Africa. Trop. Med. Int. Health 2013, 18, 1424–1443. [Google Scholar] [CrossRef]
- McGrath, L.A.; Salvi, S.M.; Sandramouli, S.; Bhatt, R.; Cuschieri, K.; Mudhar, H.S. Squamous cell carcinoma in the anophthalmic socket: A series of four cases with HPV-16 profiling. Br. J. Ophthalmol. 2018, 103, 680–685. [Google Scholar] [CrossRef]
- Ramberg, I.; Toft, P.B.; Heegaard, S. Carcinomas of the lacrimal drainage system. Surv. Ophthalmol. 2020, 65, 691–707. [Google Scholar] [CrossRef] [PubMed]
- Kroll, J.; Busse, H. Tumoren der ableitenden tranenwege. Klin. Mon. Augenheilkd. 2008, 225, 91–95. [Google Scholar] [CrossRef] [PubMed]
- Ryan, S.J.; Font, R.L. Primary epithelial neoplasms of the lacrimal sac. Am. J. Ophthalmol. 1973, 76, 73–88. [Google Scholar] [CrossRef]
- Stefanyszyn, M.A.; Hidayat, A.A.; Pe’er, J.J.; Flanagan, J.C. Lacrimal sac tumors. Ophthalmic Plast. Reconstr. Surg. 1994, 10, 169–184. [Google Scholar] [CrossRef]
- Anderson, K.K.; Lessner, A.M.; Hood, I.; Mendenhall, W.; Stringer, S.; Warren, R. Invasive transitional cell carcinoma of the lacrimal sac arising in an inverted papilloma. Arch. Ophthalmol. 1994, 112, 306–307. [Google Scholar] [CrossRef] [PubMed]
- Sjö, N.C.; von Buchwald, C.; Cassonnet, P.; Flamant, P.; Heegaard, S.; Norrild, B.; Prause, J.U.; Orth, G. Human papillomavirus: Cause of epithelial lacrimal sac neoplasia? Acta Ophthalmol. 2007, 85, 551–556. [Google Scholar] [CrossRef]
- Jones, H.; Gane, S.; Rimmer, J.; Cuschieri, K.; Lund, V.J. HPV may not play a role in all lacrimal transitional cell papilloma. Rhinology 2020. [Google Scholar] [CrossRef] [PubMed]
- Madreperla, S.A.; Green, W.R.; Daniel, R.; Shah, K.V. Human papillomavirus in primary epithelial tumors of the lacrimal sac. Ophthalmology 1993, 100, 569–573. [Google Scholar] [CrossRef]
- Vickers, J.L.; Matherne, R.J.; Allison, A.W.; Wilkerson, M.G.; Tyring, S.K.; Bartlett, B.L.; Rady, P.L.; Kelly, B.C. Transitional cell neoplasm of the nasolacrimal duct associated with human papillomavirus type 11. J. Cutan. Pathol. 2010, 37, 793–796. [Google Scholar] [CrossRef]
- Buchwald, C.; Skoedt, V.; Tos, M. An expansive papilloma of the nasolachrymal drainage system harbouring human papilloma virus. Rhinology 1996, 34, 184–185. [Google Scholar]
- Alam, M.S.; Mukherjee, B.; Krishnakumar, S. Clinical profile and management outcomes of lacrimal drainage system malignancies. Orbit 2021, 1–8. [Google Scholar] [CrossRef]
- Hayashi, N.; Furihata, M.; Ohtsuki, Y.; Ueno, H. Search for accumulation of p53 protein and detection of human papillomavirus genomes in sebaceous gland carcinoma of the eyelid. Virchows Arch. 1994, 424, 503–509. [Google Scholar] [CrossRef]
- North, J.P.; Golovato, J.; Vaske, C.J.; Sanborn, J.Z.; Nguyen, A.; Wu, W.; Goode, B.; Stevers, M.; McMullen, K.; Perez White, B.E.; et al. Cell of origin and mutation pattern define three clinically distinct classes of sebaceous carcinoma. Nat. Commun. 2018, 9, 1894. [Google Scholar] [CrossRef] [PubMed]
- Kwon, M.J.; Shin, H.S.; Nam, E.S.; Cho, S.J.; Lee, M.J.; Lee, S.; Park, H.R. Comparison of HER2 gene amplification and KRAS alteration in eyelid sebaceous carcinomas with that in other eyelid tumors. Pathol. Res. Pract. 2015, 211, 349–355. [Google Scholar] [CrossRef]
- Gonzalez-Fernandez, F.; Kaltreider, S.A.; Patnaik, B.D.; Retief, J.D.; Bao, Y.; Newman, S.; Stoler, M.H.; Levine, P.A. Sebaceous carcinoma. Tumor progression through mutational inactivation of p53. Ophthalmology 1998, 105, 497–506. [Google Scholar] [CrossRef]
- Chauhan, S.; Sen, S.; Singh, N.; Sharma, A.; Pushker, N.; Kashyap, S.; Chawla, B. Human papillomavirus in ocular malignant tumours: A study from a tertiary eye care centre in North India. Can. J. Ophthalmol. 2019, 54, 688–693. [Google Scholar] [CrossRef]
- Tetzlaff, M.T.; Curry, J.L.; Ning, J.; Sagiv, O.; Kandl, T.; Peng, B.; Bell, D.; Routbort, M.J.; Hudgens, C.W.; Ivan, D.; et al. Distinct biological types of ocular adnexal sebaceous carcinoma: HPV-driven and virus-negative tumors arise through non-overlapping molecular-genetic alterations. Clin. Cancer Res. 2018, 25, 1280–1290. [Google Scholar] [CrossRef] [PubMed]
- Moore, R.F.; Zhang, X.R.; Allison, D.B.; Rooper, L.M.; Campbell, A.A.; Eberhart, C.G. High-risk human papillomavirus and ZEB1 in ocular adnexal sebaceous carcinoma. J. Cutan. Pathol. 2021, 48, 1027–1033. [Google Scholar] [CrossRef] [PubMed]
- Liau, J.Y.; Liao, S.L.; Hsiao, C.H.; Lin, M.C.; Chang, H.C.; Kuo, K.T. Hypermethylation of the CDKN2A gene promoter is a frequent epigenetic change in periocular sebaceous carcinoma and is associated with younger patient age. Hum. Pathol. 2014, 45, 533–539. [Google Scholar] [CrossRef] [PubMed]
- Reifler, D.M.; Hornblass, A. Squamous cell carcinoma of the eyelid. Surv. Ophthalmol. 1986, 30, 349–365. [Google Scholar] [CrossRef]
- Cook, B.E., Jr.; Bartley, G.B. Epidemiologic characteristics and clinical course of patients with malignant eyelid tumors in an incidence cohort in Olmsted County, Minnesota. Ophthalmology 1999, 106, 746–750. [Google Scholar] [CrossRef]
- Arroyo Mühr, L.S.; Hultin, E.; Dillner, J. Transcription of Human Papillomaviruses in Non-Melanoma Skin Cancers of the Immunosuppressed. Int J. Cancer 2021, 149, 1341–1347. [Google Scholar] [CrossRef]
- Ramezani, M.; Baharzadeh, F.; Almasi, A.; Sadeghi, M. A Systematic Review and Meta-Analysis: Evaluation of the β-Human Papillomavirus in Immunosuppressed Individuals with Cutaneous Squamous Cell Carcinoma. BioMedicine 2020, 10, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Rollison, D.E.; Viarisio, D.; Amorrortu, R.P.; Gheit, T.; Tommasino, M. An Emerging Issue in Oncogenic Virology: The Role of Beta Human Papillomavirus Types in the Development of Cutaneous Squamous Cell Carcinoma. J. Virol. 2019, 93, e01003-18. [Google Scholar] [CrossRef] [PubMed]
- Doorbar, J.; Egawa, N.; Griffin, H.; Kranjec, C.; Murakami, I. Human papillomavirus molecular biology and disease association. Rev. Med. Virol. 2015, 25 (Suppl. 1), 2–23. [Google Scholar] [CrossRef] [PubMed]
| Author, Year | Median Age Years (Range), Gender | HPV+ (DNA PCR) | HPV+ (RNA ISH) | HPV Genotypes | HPV Detection Modality |
|---|---|---|---|---|---|
| Mlakar et al., 2015 [9] | 49 (28–77), 17M/8F | 19/25 (76%) | NA | 6, 11 | PCR, in-situ hybridization |
| Takamura et al., 2008 [15] | 40 (28–76), 4M/2F | 6/6 (100%) | NA | NA | PCR, hybrid capture II |
| Sjö et al., 2007 [10] | 27 (18–65) | 86/106 (81%) | NA | 6, 11, 45 | PCR |
| Eng et al., 2002 [16] | (9–80), 21M/3F | 14/24 (58%) | NA | 6, 11 | PCR |
| Nakamura et al., 1997 [17] | 51 (20–73), 6M/2F | 4/8 (50%) | NA | 6 | PCR, in-situ hybridization |
| Saegusa et al., 1995 [11] | 38 (14–73), 5M/11F | 12/16 (75%) | NA | 16 | PCR, in-situ hybridization |
| McDonnell et al., 1987 [18] | 25 (1–71) | 15/23 (65%) | NA | 6 | In-situ hybridization |
| Author, Year | Median Age Years (Range)/Gender | HPV+ (DNA) | HPV+ (RNA) | HPV Genotypes | HPV Detection Modality |
|---|---|---|---|---|---|
| Griffin et al., 2019 [33] | 61 (21–103)/25M, 16F | 17/41 (41%) | 11/13 (85%) * | 16 | PCR, RNA ISH, p16INK4a |
| Nagarajan et al., 2019 [37] | 62 (36–81)/16M, 15F | NA | 8/31 (26%) | High-risk genotypes | RNA ISH |
| Ramberg et al., 2019 [38] | 65 (30–97)/81M, 31F | 24/112 (21%) | 18/19 (95%) ** | 6, 11, 16, 33, 39 | PCR, RNA ISH, p16INK4a |
| Scott et al., 2002 [39] | NA | 10/10 (100%) | 10/10 (100%) | 16, 18 | RT-PCR, DNA ISH |
| Author, Year | Median Age Years (Range)/Gender | HPV+ (DNA PCR) | HPV+ (RNA ISH) | HPV Genotypes | HPV Detection Modality |
|---|---|---|---|---|---|
| Jones et al., 2020 [67] | - | 3/10 (30%) | NA | 6, 11, 16 | PCR |
| Madreperla et al., 1993 [68] | 38 (36–54)/3M | 2/2 (100%) | NA | 11 | PCR, DNA ISH |
| Sjö et al., 2007 [66] | 37 (30–56) | 4/4 (100%) | 2/2 (100%) | 6, 11 | PCR, DNA ISH, RNA ISH |
| Vickers et al., 2010 [69] | 53/F | 1/1 (100%) | NA | 11 | PCR |
| Nakamura et al., 1997 [17] | 38 (26–50)/1F, 1M | 1/2 (50%) | NA | 16 | DNA ISH, PCR |
| Buchwald et al., 1996 [70] | - | 1/1 (100%) | NA | 6/11 | DNA ISH |
| Author, Year | Median Age Years (Range)/Gender | HPV+ (DNA PCR) | HPV+ (RNA ISH) | HPV Genotypes | HPV Detection Modality |
|---|---|---|---|---|---|
| Afrogheh et al., 2016 [42] | 60 (34–75)/4M, 5F | 8/9 (89%) | NA | 16, 33, 58 | DNA ISH, PCR, p16INK4a |
| Madreperla et al., 1993 [68] | - | 1/2 (50%) * | NA | 18 | DNA ISH, PCR |
| Sjö et al., 2007 [66] | 61 (33–86)/4M, 2F | 4/6 (67%) | 0/4 (0%) | 6, 11, 16 ** | PCR, DNA ISH, RNA ISH |
| Jones et al., 2020 [67] | - | 2/4 (50%) | NA | 16 | PCR |
| Author, Year | Median Age Years (Range)/Gender | HPV+ (DNA PCR) | HPV+ (RNA ISH) | HPV Genotypes | HPV Detection Modality |
|---|---|---|---|---|---|
| Hayashi et al., 1994 [72] | 63 (52–83)/6M:7F | 13/21 (62%) | NA | 16, 18, 31, 33, 6, 11 | DNA ISH |
| Gonzalez-Fernandez et al., 1998 [75] | 72 (32–90)/7F | 0/7 (0%) | NA | NA | DNA ISH, PCR |
| Kwon et al., 2015 [74] | 72 (45–86)/4M:10F | 0/14 (0%) | NA | NA | HPV chip test |
| Liau et al., 2014 [79] | NA/8M:16F | 1/24 (4%) | NA | 16 | PCR |
| Stagner et al., 2016 [2] | NA | 1/24 (4%) | 0/18 (0%) | 16 | PCR, RNA ISH |
| Tetzlaff et al., 2019 [77] | 68 (44–93)/13M:16F | 4/29 (14%) | 4/29 (14%) | 16, 18 | RNA ISH, RNA sequencing |
| Chauhan et al., 2019 [76] | mean 56.8±13.9 (25–88)/16M:14F | 0/30 (0%) | NA | NA | PCR |
| Moore et al., 2021 [78] | mean 73 (27–98)/8M:10F | NA | 2/11 (18%) | High-risk HPV | RNA ISH |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramberg, I.; Heegaard, S. Human Papillomavirus Related Neoplasia of the Ocular Adnexa. Viruses 2021, 13, 1522. https://doi.org/10.3390/v13081522
Ramberg I, Heegaard S. Human Papillomavirus Related Neoplasia of the Ocular Adnexa. Viruses. 2021; 13(8):1522. https://doi.org/10.3390/v13081522
Chicago/Turabian StyleRamberg, Ingvild, and Steffen Heegaard. 2021. "Human Papillomavirus Related Neoplasia of the Ocular Adnexa" Viruses 13, no. 8: 1522. https://doi.org/10.3390/v13081522
APA StyleRamberg, I., & Heegaard, S. (2021). Human Papillomavirus Related Neoplasia of the Ocular Adnexa. Viruses, 13(8), 1522. https://doi.org/10.3390/v13081522





