The Effect of Prophylactic HPV Vaccines on Oral and Oropharyngeal HPV Infection—A Systematic Review
Abstract
:1. Introduction
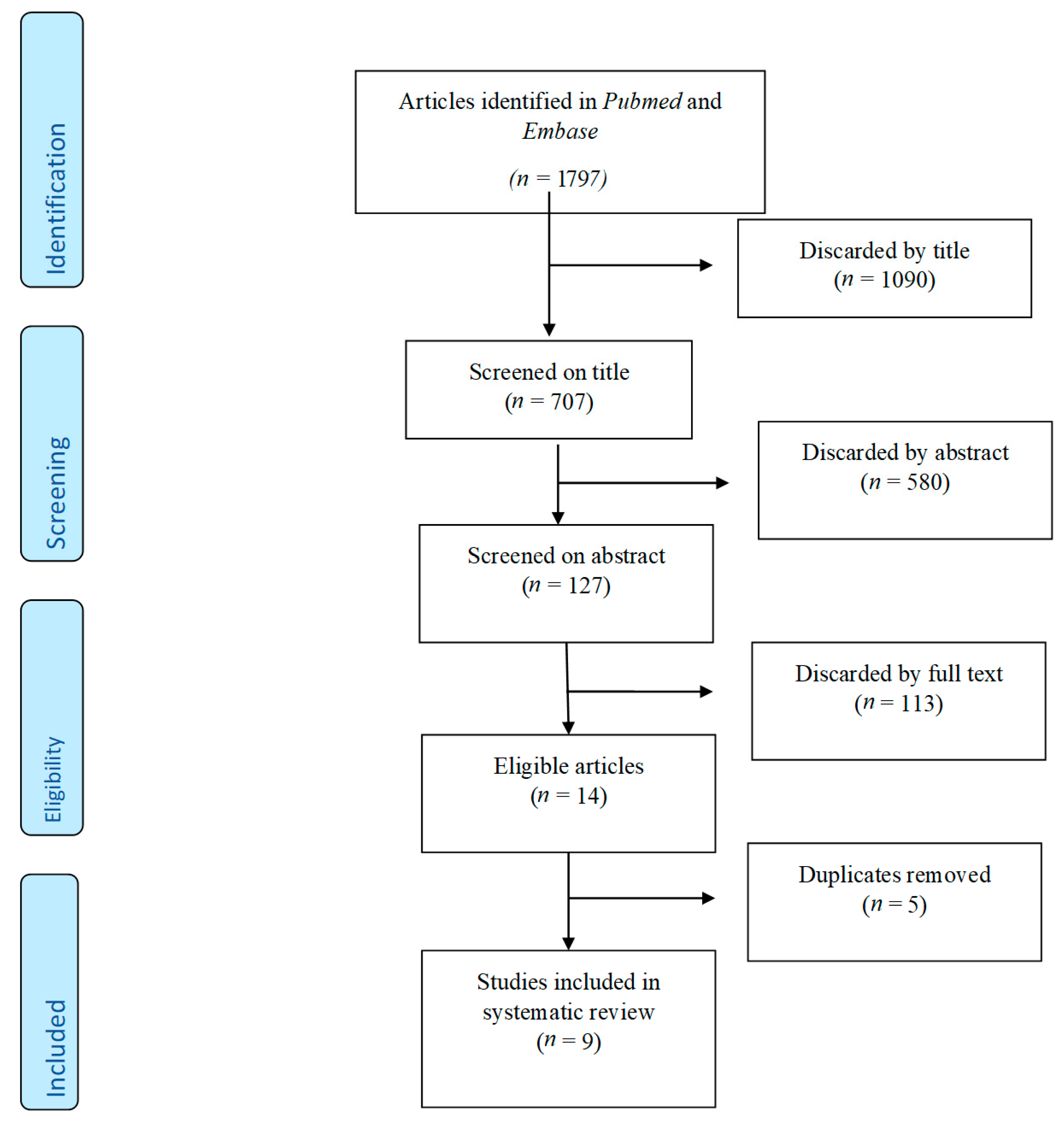
2. Materials & Methods
2.1. Systematic Literature Search
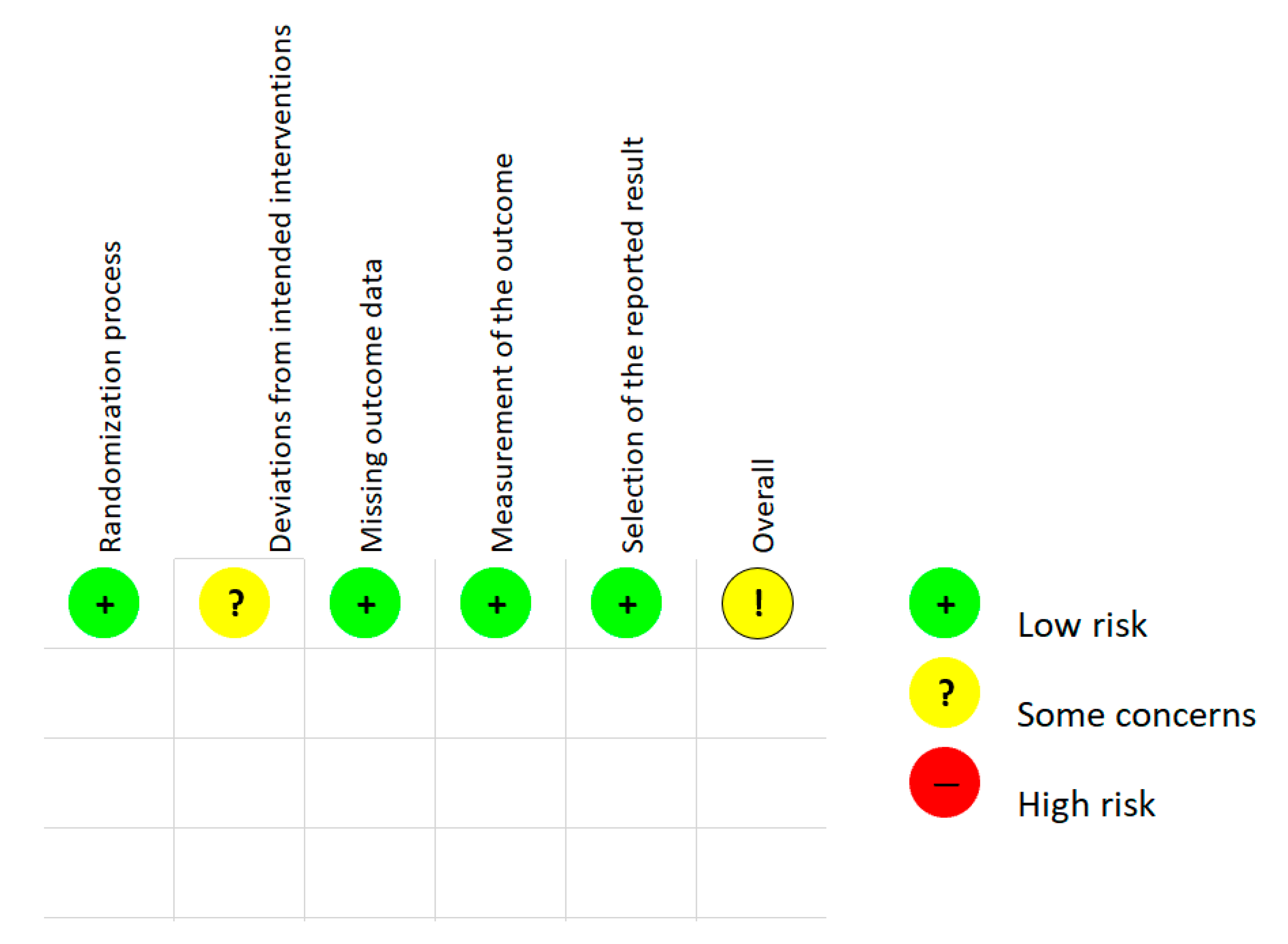
2.2. Risk of Bias Assessment in Randomized Study and Quality Assessment
3. Results
3.1. Study Characteristics
3.2. Risk of Bias Assessment and Study Quality Assessment
3.3. HPV-Specific Antibodies Pre- and Post-Vaccination
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- D’Souza, G.; Kluz, N.; Wentz, A.; Youngfellow, R.M.; Griffioen, A.; Stammer, E.; Guo, Y.; Xiao, W.; Gillison, M.L. Oral Human Papillomavirus (HPV) Infection among Unvaccinated High-Risk Young Adults. Cancers 2014, 6, 1691–1704. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chaturvedi, A.K.; Graubard, B.I.; Broutian, T.; Pickard, R.K.; Tong, Z.Y.; Xiao, W.; Kahle, L.; Gillison, M.L. Effect of prophylactic human papillomavirus (HPV) vaccination on oral HPV infections among young adults in the United States. J. Clin. Oncol. 2018, 36, 262–267. [Google Scholar] [CrossRef]
- Carlander, A.-L.F.; Larsen, C.G.; Jensen, D.H.; Garnæs, E.; Kiss, K.; Andersen, L.; Olsen, C.H.; Franzmann, M.; Høgdall, E.; Kjær, S.K.; et al. Continuing rise in oropharyngeal cancer in a high HPV prevalence area: A Danish population-based study from 2011 to 2014. Eur. J. Cancer 2017, 70, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Garnaes, E.; Kiss, K.; Andersen, L.; Therkildsen, M.H.; Franzmann, M.B.; Filtenborg-Barnkob, B.; Hoegdall, E.; Krenk, L.; Josiassen, M.; Lajer, C.B.; et al. A high and increasing HPV prevalence in tonsillar cancers in Eastern Denmark, 2000-2010: The largest registry-based study to date. Int. J. Cancer 2015, 136, 2196–2203. [Google Scholar] [CrossRef]
- Zamani, M.; Grønhøj, C.; Jensen, D.H.; Carlander, A.F.; Agander, T.; Kiss, K.; von Buchwald, C. The current epidemic of HPV-associated oropharyngeal cancer: An 18-year Danish population-based study with 2,169 patients. Eur. J. Cancer 2020, 134, 52–59. [Google Scholar] [CrossRef] [PubMed]
- Jensen, J.S.; Jensen, D.H.; Grønhøj, C.; Karnov, K.K.S.; Nørregaard, C.; Agander, T.K.; Specht, L.; von Buchwald, C. Incidence and survival of oropharyngeal cancer in Denmark: A nation-wide, population-based study from 1980 to 2014. Acta Oncol. 2018, 57, 269–275. [Google Scholar] [CrossRef] [Green Version]
- Palmer, E.; Newcombe, R.G.; Green, A.C.; Kelly, C.; Noel Gill, O.; Hall, G.; Fiander, A.N.; Pirotte, E.; Hibbitts, S.J.; Homer, J.; et al. Human papillomavirus infection is rare in nonmalignant tonsil tissue in the UK: Implications for tonsil cancer precursor lesions. Int. J. Cancer 2014, 135, 2437–2443. [Google Scholar] [CrossRef]
- Hillman, R.J.; Giuliano, A.R.; Palefsky, J.M.; Goldstone, S.; Moreira, E.D., Jr.; Vardas, E.; Aranda, C.; Jessen, H.; Ferris, D.G.; Coutlee, F.; et al. Immunogenicity of the quadrivalent human papillomavirus (type 6/11/16/18) vaccine in males 16 to 26 years old. Clin. Vac. Immunol. 2012, 19, 261–267. [Google Scholar] [CrossRef] [Green Version]
- Enerly, E.; Flingtorp, R.; Christiansen, I.K.; Campbell, S.; Hansen, M.; Myklebust, T.; Åge; Weiderpass, E.; Nygård, M. An observational study comparing HPV prevalence and type distribution between HPV-vaccinated and -unvaccinated girls after introduction of school-based HPV vaccination in Norway. PLoS ONE 2019, 14, e0223612. [Google Scholar] [CrossRef]
- Castillo, A.; Osorio, J.C.; Fernández, A.; Méndez, F.; Alarcón, L.; Arturo, G.; Herrero, R.; Bravo, L.E. Effect of vaccination against oral HPV-16 infection in high school students in the city of Cali, Colombia. Papillomavirus Res. 2019, 7, 112–117. [Google Scholar] [CrossRef]
- Pinto, L.A.; Kemp, T.J.; Torres, B.N.; Isaacs-Soriano, K.; Ingles, D.; Abrahamsen, M.; Pan, Y.; Lazcano-Ponce, E.; Salmeron, J.; Giuliano, A.R. Quadrivalent Human Papillomavirus (HPV) Vaccine Induces HPV-Specific Antibodies in the Oral Cavity: Results From the Mid-Adult Male Vaccine Trial. J. Infect. Dis. 2016, 214, 1276–1283. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLOS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [Green Version]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.-Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cantrell, A.; Croot, E.; Johnson, M.; Wong, R.; Chambers, D.; Baxter, S.K.; Booth, A. Access to primary and community health-care services for people 16 years and over with intellectual disabilities: A mapping and targeted systematic review. Health Serv. Deliv. Res. 2020, 8, 1–142. [Google Scholar] [CrossRef] [Green Version]
- Mehanna, H.; Bryant, T.S.; Babrah, J.; Louie, K.; Bryant, J.L.; Spruce, R.J.; Batis, N.; Olaleye, O.; Jones, J.; Struijk, L.; et al. Human Papillomavirus (HPV) Vaccine Effectiveness and Potential Herd Immunity for Reducing Oncogenic Oropharyngeal HPV-16 Prevalence in the United Kingdom: A Cross-sectional Study. Clin. Infect. Dis. 2018, 69, 1296–1302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hirth, J.M.; Chang, M.; Resto, V.A.; Guo, F.; Berenson, A.B. Prevalence of oral human papillomavirus by vaccination status among young adults (18–30 years old). Vaccine 2017, 35, 3446–3451. [Google Scholar] [CrossRef] [PubMed]
- Lehtinen, M.; Apter, D.; Eriksson, T.; Harjula, K.; Hokkanen, M.; Lehtinen, T.; Natunen, K.; Damaso, S.; Soila, M.; Bi, D.; et al. Effectiveness of the AS04-adjuvanted HPV-16/18 vaccine in reducing oropharyngeal HPV infections in young females—Results from a community-randomized trial. Int. J. Cancer 2020, 147, 170–174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schlecht, N.F.; Masika, M.; Diaz, A.; Nucci-Sack, A.; Salandy, A.; Pickering, S.; Strickler, H.D.; Shankar, V. and Burk, R.D. Risk of oral human papillomavirus infection among sexually active female adolescents receiving the quadrivalent vaccine. JAMA Netw. Open 2019, 2, e1914031. [Google Scholar] [CrossRef]
- Eggersmann, T.K.; Sharaf, K.; Baumeister, P.; Thaler, C.; Dannecker, C.J.; Jeschke, U.; Mahner, S.; Weyerstahl, K.; Bergauer, F.; Gallwas, J.K.S. Prevalence of oral HPV infection in cervical HPV positive women and their sexual partners. Arch. Gynecol. Obstet. 2019, 299, 1659–1665. [Google Scholar] [CrossRef]
- Handisurya, A.; Schellenbacher, C.; Haitel, A.; Senger, T.; Kirnbauer, R. Human papillomavirus vaccination induces neutralising antibodies in oral mucosal fluids. Br. J. Cancer 2016, 114. [Google Scholar] [CrossRef] [Green Version]
- Pasteur, S.; Vaccines, A.J. Det danske Børne Vaccinations Program. 2020, pp. 2–4. Available online: https://www.ssi.dk/vaccinationer/boernevaccination (accessed on 16 March 2021).
- Cossellu, G.; Fedele, L.; Badaoui, B.; Angiero, F.; Farronato, G.; Monti, E.; Liverani, C.A.; Gorni, C.; Botti, S. Prevalence and concordance of oral and genital HPV in women positive for cervical HPV infection and in their sexual stable partners: An Italian screening study. PLoS ONE 2018, 13, e0205574. [Google Scholar] [CrossRef] [PubMed]
- Chikandiwa, A.; Pisa, P.T.; Chersich, M.F.; E Muller, E.; Mayaud, P.; Delany-Moretlwe, S. Oropharyngeal HPV infection: Prevalence and sampling methods among HIV-infected men in South Africa. Int. J. STD AIDS 2018, 29, 776–780. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eggersmann, T.K.; Baumeister, P.; Kumbrink, J.; Mayr, D.; Schmoeckel, E.; Thaler, C.J.; Dannecker, C.; Jeschke, U.; Nagler, T.; Mahner, S.; et al. Oropharyngeal HPV Detection Techniques in HPV-associated Head and Neck Cancer Patients. Anticancer. Res. 2020, 40, 2117–2123. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-H.; Koo, B.-S.; Kang, S.; Park, K.; Kim, H.; Lee, K.R.; Lee, M.J.; Kim, J.M.; Choi, E.C.; Cho, N.H. HPV integration begins in the tonsillar crypt and leads to the alteration of p16, EGFR and c-myc during tumor formation. Int. J. Cancer 2007, 120, 1418–1425. [Google Scholar] [CrossRef]
| Study | Study Design | Participants | Key Findings | Odds/Risk Ratio/Prevalence Ratio | p-Value | Method |
|---|---|---|---|---|---|---|
| Espen Enerly [9] 2019 | Observational study- cross sectional study | 312 | In total four oral samples were positive for any type of HPV, and all of these participants had received at least one HPV vaccine before oral sexual debut. Results for infections other than oral HPV infection are not included in the article. | N/A * Results of vaccine effect on oral HPV infection are disregarded due to small sample size. | % | Facebook advertisement to recruit participants. Self-sampled oral and vaginal specimens using Evalyn brush and FLOQ-swab. Sexual behavior ascertained through questionnaire. |
| Anil K. Chaturvedi [2] 2017 | Cross-sectional study | 2627 | The prevalence of oral HPV (16/18/6/11) infections was significantly reduced in vaccinated vs. unvaccinated individuals. This corresponds to an estimated 82.2% (5.7%–98.5%) reduction on the prevalence after adjustments were made. | 0.11% of vaccinated vs. 1.61% of unvaccinated individuals had an oral HPV infection. | Adjusted p-value = 0.008 | Patient data was collected from NHANES (2011–2014). Vaccination status was self-reported. |
| Matti Lehtinen [17] 2019 | Community randomized trial | 38,631 | Vaccine effectiveness on HPV (16/18, 31/45 and 31/33/45) was, respectively, 82.4% CI: (47.3–94.1); 75.3% CI: (12.7–93.0); and 69.9% CI: (29.6–87.1). The AS04 vaccine showed effectiveness on HPV infections in adolescent females up to 6 years post-vaccination. | Relative reduction in HPV16/18: 82.4% | % | Three arms of 11 communities were enrolled and compared. Participants were blinded to vaccine allocation. HPV DNA prevalence was determined by SPF-10 LiPA and Multiplex type-specific PCR. |
| Andres Castillo [10] 2019 | Cross-sectional study | 1784 | HPV vaccination was associated with the reduction of HPV-16 exposure percentages in the oral and oropharyngeal cavity. 72% reduction in HPV-16 detection in students immunized with two doses. | ODDS RATIO HPV16 in vaccinated vs. unvaccinated: 0.28 (95% CI: 0.07–0.88). | p = 0.01; calculated using regression model. | HPV-16 DNA was detected in samples from the oral cavity and throat of 1784 high school students of both genders, aged 14–17 years old. The number of vaccinated girls was 944 vs. 95 unvaccinated girls and 745 unvaccinated boys. |
| Hisham Mehanna [15] 2019 | Cross-sectional study | 940 | Overall, oropharyngeal HPV-16 prevalence was significantly lower in vaccinated vs. unvaccinated females. In contrast, prevalence of any oropharyngeal HPV type was similar in vaccinated and unvaccinated females. Oropharyngeal HPV-16 prevalence in unvaccinated males was similar to vaccinated females. | HPV-16 Prevalence in vaccinated vs. unvaccinated women: 0.5% vs. 5.6%. Prevalence in unvaccinated males was similar to vaccinated females (0% vs 0.5%, p > 0.99). | p = 0.04 p > 0.99 | Subjects aged 0–65 years undergoing tonsillectomy for nonmalignant indications were recruited in six hospitals in the United Kingdom. Vaccination status obtained from health authorities. All samples were centrally tested for HPV DNA by polymerase chain reaction. |
| A Handisurya [20] 2019 | Case-control study | 34 | HPV vaccination induced type-specific antibody response in oral fluid and sera. In vitro the antibodies in oral fluid were capable of neutralizing HPV pseudovirions indicating protection from infection. | N/A | p < 0.001 | Oral fluid and sera were collected from females before and after administration of the quadrivalent vaccine. IgG and neutralizing antibodies of HPV 6 and 16/18 were analyzed pre- and post-vaccination and compared to unvaccinated females. |
| Jacqueline M. Hirth [16] 2017 | Cross-sectional study | 3040 | Lower prevalence of oral HPV types in vaccinated vs. unvaccinated individuals. Prevalence was the same amongst participants on non-vaccine type oral HPV infection. | HPV16 vaccinated vs. unvaccinated prevalence: 0.09 vs. 0.84. HPV18 0.07 vs. 0.29 | 16: 0.01; 18: 0.15; Any high risk: 0.04. | Cross-sectional data obtained from NHANES. Participants provided oral samples and questionnaires were used to ascertain vaccination status amongst participants. |
| Ligia A. Pinto [11] 2016 | Case-control study | 150 | 100% of men seroconverted, and the majority of individuals developed detectable anti-HPV-16 and anti-HPV-18 antibodies (up to 96% and 72%, respectively) at the oral cavity following vaccination. | % | % | Sera and saliva collected in mouthwash and Merocel sponges at day 1 and month 7 were obtained from 150 men who received Gardasil at day 1 and months 2 and 6. Specimens were tested for anti-HPV-16 and anti-HPV-18 IgG levels by an L1 virus-like particle-based enzyme-linked immunosorbent assay. |
| Nicolas F. Schlecht [18] 2019 | Longitudinal cohort study | 1259 | Large risk of oral HPV-infection in unvaccinated women. Vaccination associated with significant decrease in prevalence of oral HPV-infection. (83% decrease in risk of oral HPV infection in vaccinated individuals.) | Odds ratio: 0.17 CI: (0.04–0.998). | % | Repeated collection of oral rinse specimens from sexually active female adolescents in healthcare clinics. Included vaccinated and unvaccinated individuals. HPV-DNA was analyzed using PCR. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nielsen, K.J.; Jakobsen, K.K.; Jensen, J.S.; Grønhøj, C.; Von Buchwald, C. The Effect of Prophylactic HPV Vaccines on Oral and Oropharyngeal HPV Infection—A Systematic Review. Viruses 2021, 13, 1339. https://doi.org/10.3390/v13071339
Nielsen KJ, Jakobsen KK, Jensen JS, Grønhøj C, Von Buchwald C. The Effect of Prophylactic HPV Vaccines on Oral and Oropharyngeal HPV Infection—A Systematic Review. Viruses. 2021; 13(7):1339. https://doi.org/10.3390/v13071339
Chicago/Turabian StyleNielsen, Kristoffer Juul, Kathrine Kronberg Jakobsen, Jakob Schmidt Jensen, Christian Grønhøj, and Christian Von Buchwald. 2021. "The Effect of Prophylactic HPV Vaccines on Oral and Oropharyngeal HPV Infection—A Systematic Review" Viruses 13, no. 7: 1339. https://doi.org/10.3390/v13071339
APA StyleNielsen, K. J., Jakobsen, K. K., Jensen, J. S., Grønhøj, C., & Von Buchwald, C. (2021). The Effect of Prophylactic HPV Vaccines on Oral and Oropharyngeal HPV Infection—A Systematic Review. Viruses, 13(7), 1339. https://doi.org/10.3390/v13071339






