Detection of Atypical Porcine Pestivirus in Piglets from Danish Sow Herds
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Detection of APPV
3.2. Survey
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hause, B.M.; Collin, E.A.; Peddireddi, L.; Yuan, F.; Chen, Z.; Hesse, R.A.; Gauger, P.C.; Clement, T.; Fang, Y.; Anderson, G. Discovery of a Novel Putative Atypical Porcine Pestivirus in Pigs in the USA. J. Gen. Virol. 2015, 96, 2994–2998. [Google Scholar] [CrossRef]
- Kaufmann, C.; Stalder, H.; Sidler, X.; Renzullo, S.; Gurtner, C.; Grahofer, A.; Schweizer, M. Long-Term Circulation of Atypical Porcine Pestivirus (APPV) within Switzerland. Viruses 2019, 11, 653. [Google Scholar] [CrossRef]
- Schwarz, L.; Riedel, C.; Högler, S.; Sinn, L.J.; Voglmayr, T.; Wöchtl, B.; Dinhopl, N.; Rebel-Bauder, B.; Weissenböck, H.; Ladinig, A.; et al. Congenital Infection with Atypical Porcine Pestivirus (APPV) Is Associated with Disease and Viral Persistence. Vet. Res. 2017, 48, 1. [Google Scholar] [CrossRef] [PubMed]
- Gatto, I.R.H.; Harmon, K.; Bradner, L.; Silva, P.; Linhares, D.C.L.; Arruda, P.H.; de Oliveira, L.G.; Arruda, B.L. Detection of Atypical Porcine Pestivirus in Brazil in the Central Nervous System of Suckling Piglets with Congenital Tremor. Transbound. Emerg. Dis. 2018, 65, 375–380. [Google Scholar] [CrossRef] [PubMed]
- Dessureault, F.G.; Choinière, M.; Provost, C.; Gagnon, C.A. Cross-Canada Disease Report First report of atypical porcine pestivirus in piglets with congenital tremor in Canada. Can. Vet. J. 2018, 59, 429–432. [Google Scholar] [PubMed]
- Yuan, J.; Han, Z.; Li, J.; Huang, Y.; Yang, J.; Ding, H.; Zhang, J.; Zhu, M.; Zhang, Y.; Liao, J.; et al. Atypical Porcine Pestivirus as a Novel Type of Pestivirus in Pigs in China. Front. Microbiol. 2017, 8, 862. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, T.B.; Johnston, C.M.; Strandbygaard, B.; Belsham, G.J.; Bøtner, A. First Detection of Atypical Porcine Pestivirus in Piglets with Congenital Tremor in Denmark. In Proceedings of the 11th International Congress for Veterinary Virology, Vienna, Austria, 27–30 August 2018. [Google Scholar]
- Beer, M.; Wernike, K.; Dräger, C.; Höper, D.; Pohlmann, A.; Bergermann, C.; Schröder, C.; Klinkhammer, S.; Blome, S.; Hoffmann, B. High Prevalence of Highly Variable Atypical Porcine Pestiviruses Found in Germany. Transbound. Emerg. Dis. 2017, 64, e22–e26. [Google Scholar] [CrossRef]
- Postel, A.; Meyer, D.; Cagatay, G.N.; Feliziani, F.; De Mia, G.M.; Fischer, N.; Grundhoff, A.; Milićević, V.; Deng, M.-C.; Chang, C.-Y.; et al. High Abundance and Genetic Variability of Atypical Porcine Pestivirus in Pigs from Europe and Asia. Emerg. Infect. Dis. Dis. 2017, 23, 2104–2107. [Google Scholar] [CrossRef]
- Dénes, L.; Biksi, I.; Albert, M.; Szeredi, L.; Knapp, D.G.; Szilasi, A.; Bálint, Á.; Balka, G. Detection and Phylogenetic Characterization of Atypical Porcine Pestivirus Strains in Hungary. Transbound. Emerg. Dis. 2018, 65, 2039–2042. [Google Scholar] [CrossRef]
- Sozzi, E.; Salogni, C.; Lelli, D.; Barbieri, I.; Moreno, A.; Alborali, G.L.; Lavazza, A. Molecular Survey and Phylogenetic Analysis of Atypical Porcine Pestivirus (APPV) Identified in Swine and Wild Boar from Northern Italy. Viruses 2019, 11, 1142. [Google Scholar] [CrossRef]
- Kim, S.-C.; Jeong, C.-G.; Yoon, S.-M.; Lee, K.-H.; Yang, M.-S.; Kim, B.; Lee, S.-Y.; Kang, S.-J.; Kim, W.-I. Detection of atypical porcine pestivirus (APPV) from a case of congenital tremor in Korea. Korean J. Vet. Serv. 2017, 40, 209–213. [Google Scholar] [CrossRef]
- De Groof, A.; Deijs, M.; Guelen, L.; van Grinsven, L.; van Os-Galdos, L.; Vogels, W.; Derks, C.; Cruijsen, T.; Geurts, V.; Vrijenhoek, M.; et al. Atypical Porcine Pestivirus: A Possible Cause of Congenital Tremor Type A-II in Newborn Piglets. Viruses 2016, 8, 271. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-González, S.; Canturri, A.; Pérez-Simó, M.; Bohórquez, J.A.; Rosell, R.; Cabezón, O.; Segalés, J.; Domingo, M.; Ganges, L. First Report of the Novel Atypical Porcine Pestivirus in Spain and a Retrospective Study. Transbound. Emerg. Dis. 2017, 64, 1645–1649. [Google Scholar] [CrossRef]
- Blomström, A.-L.; Fossum, C.; Wallgren, P.; Berg, M. Viral Metagenomic Analysis Displays the Co-Infection Situation in Healthy and PMWS Affected Pigs. PLoS ONE 2016, 11, e0166863. [Google Scholar] [CrossRef]
- Stenberg, H.; Jacobson, M.; Malmberg, M. Detection of Atypical Porcine Pestivirus in Swedish Piglets with Congenital Tremor Type A-II. BMC Vet. Res. 2020, 16, 260. [Google Scholar] [CrossRef] [PubMed]
- Arruda, B.L.; Arruda, P.H.; Magstadt, D.R.; Schwartz, K.J.; Dohlman, T.; Schleining, J.A.; Patterson, A.R.; Visek, C.A.; Victoria, J.G. Identification of a Divergent Lineage Porcine Pestivirus in Nursing Piglets with Congenital Tremors and Reproduction of Disease Following Experimental Inoculation. PLoS ONE 2016, 11, e0150104. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Wang, L.; Qiao, S.; Deng, R.; Zhang, G. Genetic Characterization and Recombination Analysis of Atypical Porcine Pestivirus. Infect. Genet. Evol. 2020, 81, 104259. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wen, W.; Hao, G.; Hu, Y.; Chen, H.; Qian, P.; Li, X. Phylogenetic and Genomic Characterization of a Novel Atypical Porcine Pestivirus in China. Transbound. Emerg. Dis. 2018, 65, e202–e204. [Google Scholar] [CrossRef]
- Smith, D.B.; Meyers, G.; Bukh, J.; Gould, E.A.; Monath, T.; Scott Muerhoff, A.; Pletnev, A.; Rico-Hesse, R.; Stapleton, J.T.; Simmonds, P.; et al. Proposed Revision to the Taxonomy of the Genus Pestivirus, Family Flaviviridae. J. Gen. Virol. 2017, 98, 2106–2112. [Google Scholar] [CrossRef] [PubMed]
- Lamp, B.; Schwarz, L.; Högler, S.; Riedel, C.; Sinn, L.; Rebel-Bauder, B.; Weissenböck, H.; Ladinig, A.; Rümenapf, T. Novel Pestivirus Species in Pigs, Austria, 2015. Emerg. Infect. Dis. 2017, 23, 1176–1179. [Google Scholar] [CrossRef]
- Tautz, N.; Tews, B.A.; Meyers, G. Chapter Two—The Molecular Biology of Pestiviruses. In Advances in Virus Research; Elsevier: Amsterdam, The Netherlands, 2015; Volume 93, pp. 47–160. ISBN 978-0-12-802179-8. [Google Scholar]
- Postel, A.; Hansmann, F.; Baechlein, C.; Fischer, N.; Alawi, M.; Grundhoff, A.; Derking, S.; Tenhündfeld, J.; Pfankuche, V.M.; Herder, V.; et al. Presence of Atypical Porcine Pestivirus (APPV) Genomes in Newborn Piglets Correlates with Congenital Tremor. Sci. Rep. 2016, 6, 27735. [Google Scholar] [CrossRef] [PubMed]
- Patterson, D.S.P.; Sweasey, D.; Brush, P.J.; Harding, J.D.J. Neurochemistry of the Spinal Cord in Congenital Tremor of Piglets (Type Aii), a Spinal Dysmyeltmogenesis of Infectious Origin. J. Neurochem. 1973, 21, 397–406. [Google Scholar] [CrossRef] [PubMed]
- Sutton, K.M.; Lahmers, K.K.; Harris, S.P.; Wijesena, H.R.; Mote, B.E.; Kachman, S.D.; Borza, T.; Ciobanu, D.C. Detection of Atypical Porcine Pestivirus Genome in Newborn Piglets Affected by Congenital Tremor and High Preweaning Mortality1. J. Anim. Sci. 2019, 97, 4093–4100. [Google Scholar] [CrossRef] [PubMed]
- Cagatay, G.N.; Meyer, D.; Wendt, M.; Becher, P.; Postel, A. Characterization of the Humoral Immune Response Induced after Infection with Atypical Porcine Pestivirus (APPV). Viruses 2019, 11, 880. [Google Scholar] [CrossRef] [PubMed]
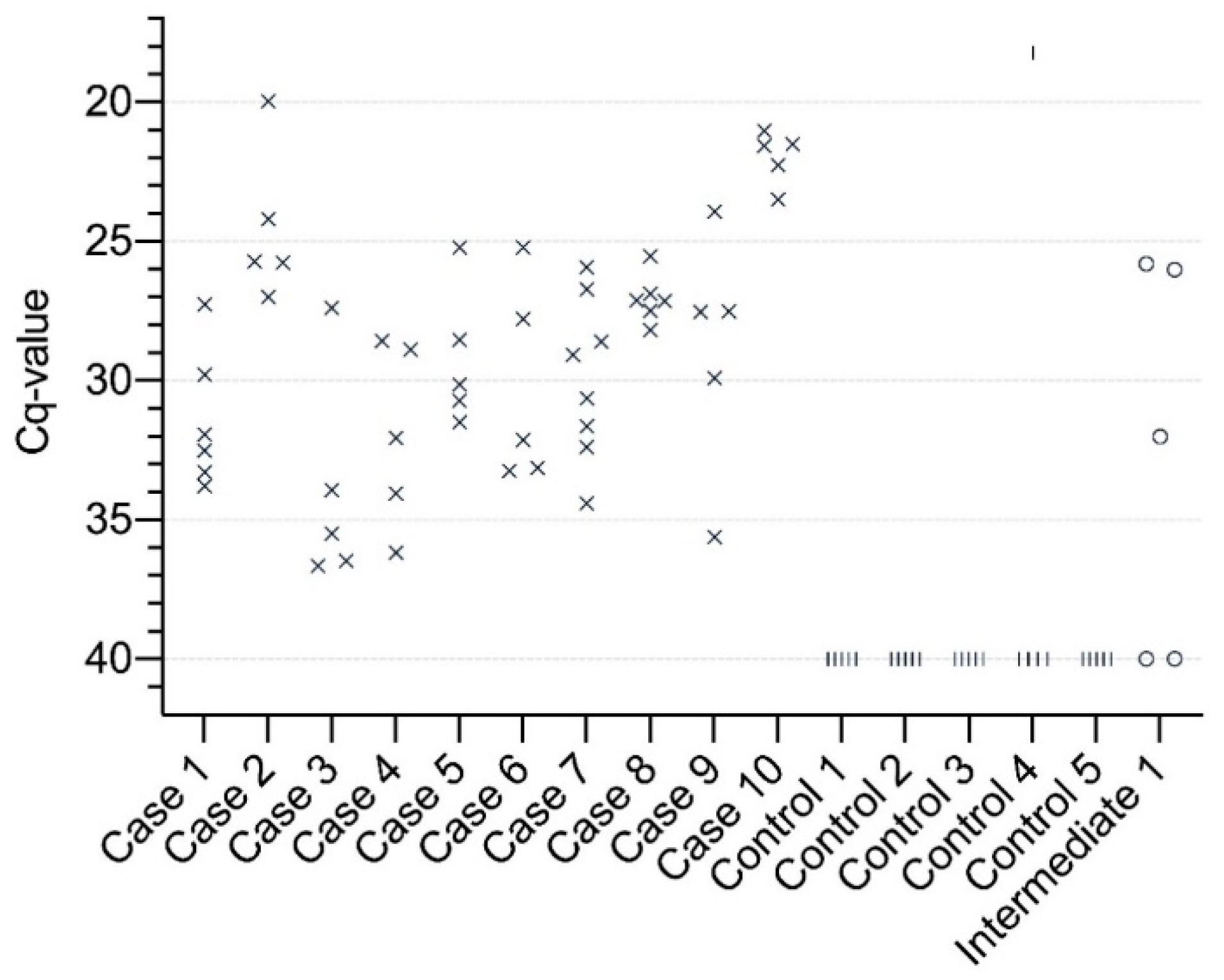
| Sample Origin | Serum Positive/Tested (%) | Pool Positive/Tested (%) | Date of Sample Collection | Region |
|---|---|---|---|---|
| Case 1 | 6/6 (100) | 1/1 (100) | 7 August 2019 | Southern Denmark |
| Case 2 | 5/5 (100) | 1/1 (100) | 21 August 2019 | Southern Denmark |
| Case 3 | 5/5 (100) | 1/1 (100) | 26 August 2019 | Central Jutland |
| Case 4 | 5/5 (100) | 1/1 (100) | 5 September 2019 | Northern Jutland |
| Case 5 | 5/5 (100) | 1/1 (100) | 14 October 2019 | Northern Jutland |
| Case 6 | 5/5 (100) | 1/1 (100) | 14 November 2019 | Southern Denmark |
| Case 7 | 8/8 (100) | 1/1 (100) | 5 December 2019 | Zealand |
| Case 8 | 6/6 (100) | 1/1 (100) | 16 December 2019 | Central Jutland |
| Case 9 | 5/5 (100) | 1/1 (100) | 23 December 2019 | Northern Jutland |
| Case 10 | 5/5 (100) | 1/1 (100) | 10 January 2020 | Southern Denmark |
| Control 1 | 0/5 (0) | 0/1 (0) | 27 January 2020 | Central Jutland |
| Control 2 | 0/5 (0) | 0/1 (0) | 28 January 2020 | Central Jutland |
| Control 3 | 0/5 (0) | 0/1 (0) | 28 January 2020 | Central Jutland |
| Control 4 | 1/5 (20) | 1/1 (100) | 18 March 2020 | Northern Jutland |
| Control 5 | 0/5 (0) | 0/1 (0) | 23 March 2020 | Zealand |
| Intermediate | 3/5 (60) | 1/1 (100) | 27 February 2020 | Northern Jutland |
| Duration of CT Presentation in the Herd | Number of Herds | ||
| -Few sow batches | 2 | ||
| -Every batch for a period of one to three months | 6 | ||
| -Ongoing issue for six months | 2 | ||
| Parity of Sows with CT-Affected Litters | Number of Herds | ||
| -Gilts | 9 | ||
| -1st–3rd parity | 1 | ||
| -All parities | 0 | ||
| Strategy for New Breeding Stock | Case Herds | Control Herds | Intermediate Herd |
| -Own breeding stock | 2 | 2 | - |
| -Own breeding stock, but supplied by other herds | 3 | - | - |
| -Purchased breeding animals from other herds | 5 | 3 | 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pedersen, K.; Kristensen, C.S.; Strandbygaard, B.; Bøtner, A.; Rasmussen, T.B. Detection of Atypical Porcine Pestivirus in Piglets from Danish Sow Herds. Viruses 2021, 13, 717. https://doi.org/10.3390/v13050717
Pedersen K, Kristensen CS, Strandbygaard B, Bøtner A, Rasmussen TB. Detection of Atypical Porcine Pestivirus in Piglets from Danish Sow Herds. Viruses. 2021; 13(5):717. https://doi.org/10.3390/v13050717
Chicago/Turabian StylePedersen, Kasper, Charlotte Sonne Kristensen, Bertel Strandbygaard, Anette Bøtner, and Thomas Bruun Rasmussen. 2021. "Detection of Atypical Porcine Pestivirus in Piglets from Danish Sow Herds" Viruses 13, no. 5: 717. https://doi.org/10.3390/v13050717
APA StylePedersen, K., Kristensen, C. S., Strandbygaard, B., Bøtner, A., & Rasmussen, T. B. (2021). Detection of Atypical Porcine Pestivirus in Piglets from Danish Sow Herds. Viruses, 13(5), 717. https://doi.org/10.3390/v13050717






