Feline Morbillivirus Infection in Domestic Cats: What Have We Learned So Far?
Abstract
1. Introduction
2. FeMV Is Classified in the Genus Morbillivirus within the Paramyxoviridae Family
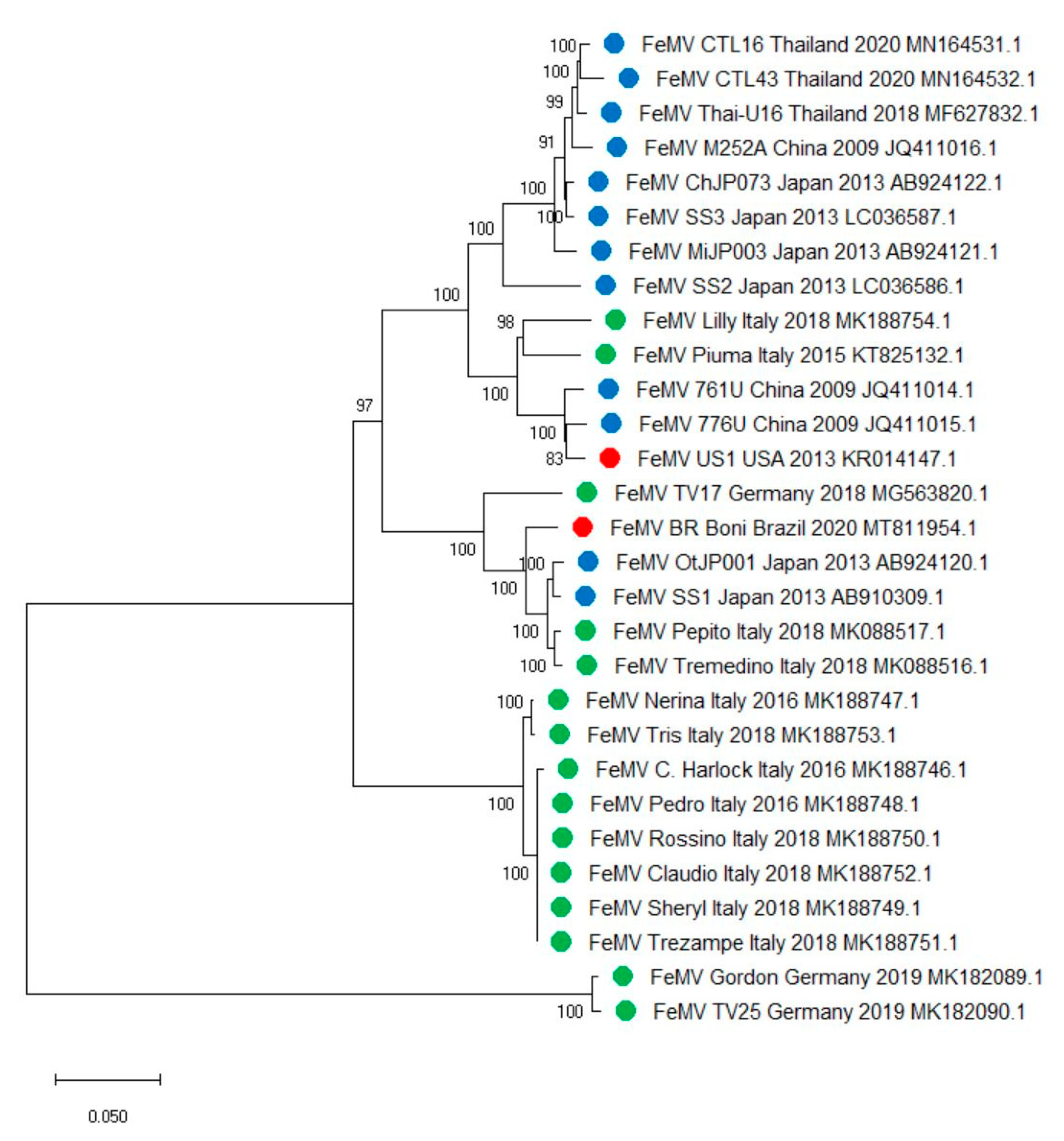
3. Genetic Heterogeneity of FeMVs
4. Epidemiology: Known and Unknown
4.1. Prevalence of FeMV
4.2. FeMV Persistent Infection
5. FeMV as the Causative Agent of Renal Disease?
6. FeMV: Not Just a Renal Pathogen?
7. FeMV Detection Methods
7.1. Virus Isolation
7.2. Reverse-Transcription Polymerase Chain Reaction
7.3. Serology
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lamb, R.A.P.G.D. Paramyxoviridae. In Fields Virology, 6th ed.; Knipe, D.M.a.H.P., Ed.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2013; pp. 957–995. [Google Scholar]
- Kumar, N.; Maherchandani, S.; Kashyap, S.K.; Singh, S.V.; Sharma, S.; Chaubey, K.K.; Ly, H. Peste des petits ruminants virus infection of small ruminants: A comprehensive review. Viruses 2014, 6, 2287–2327. [Google Scholar] [CrossRef]
- Rendon-Marin, S.; da Fontoura Budaszewski, R.; Canal, C.W.; Ruiz-Saenz, J. Tropism and molecular pathogenesis of canine distemper virus. Virol. J. 2019, 16, 30. [Google Scholar] [CrossRef] [PubMed]
- Van Bressem, M.F.; Duignan, P.J.; Banyard, A.; Barbieri, M.; Colegrove, K.M.; De Guise, S.; Di Guardo, G.; Dobson, A.; Domingo, M.; Fauquier, D.; et al. Cetacean morbillivirus: Current knowledge and future directions. Viruses 2014, 6, 5145–5181. [Google Scholar] [CrossRef] [PubMed]
- Marcacci, M.; Ancora, M.; Mangone, I.; Teodori, L.; Di Sabatino, D.; De Massis, F.; Camma, C.; Savini, G.; Lorusso, A. Whole genome sequence analysis of the arctic-lineage strain responsible for distemper in Italian wolves and dogs through a fast and robust next generation sequencing protocol. J. Virol. Methods 2014, 202, 64–68. [Google Scholar] [CrossRef]
- Woo, P.C.; Lau, S.K.; Wong, B.H.; Fan, R.Y.; Wong, A.Y.; Zhang, A.J.; Wu, Y.; Choi, G.K.; Li, K.S.; Hui, J.; et al. Feline morbillivirus, a previously undescribed paramyxovirus associated with tubulointerstitial nephritis in domestic cats. Proc. Natl. Acad. Sci. USA 2012, 109, 5435–5440. [Google Scholar] [CrossRef] [PubMed]
- Jepson, R.E. Current Understanding of the Pathogenesis of Progressive Chronic Kidney Disease in Cats. Vet. Clin. N. Am. Small Anim. Pract. 2016, 46, 1015–1048. [Google Scholar] [CrossRef]
- White, J.D.; Norris, J.M.; Baral, R.M.; Malik, R. Naturally-occurring chronic renal disease in Australian cats: A prospective study of 184 cases. Aust. Vet. J. 2006, 84, 188–194. [Google Scholar] [CrossRef] [PubMed]
- Marino, C.L.; Lascelles, B.D.; Vaden, S.L.; Gruen, M.E.; Marks, S.L. Prevalence and classification of chronic kidney disease in cats randomly selected from four age groups and in cats recruited for degenerative joint disease studies. J. Feline Med. Surg. 2014, 16, 465–472. [Google Scholar] [CrossRef] [PubMed]
- Furuya, T.; Sassa, Y.; Omatsu, T.; Nagai, M.; Fukushima, R.; Shibutani, M.; Yamaguchi, T.; Uematsu, Y.; Shirota, K.; Mizutani, T. Existence of feline morbillivirus infection in Japanese cat populations. Arch. Virol. 2014, 159, 371–373. [Google Scholar] [CrossRef]
- Sakaguchi, S.; Nakagawa, S.; Yoshikawa, R.; Kuwahara, C.; Hagiwara, H.; Asai, K.I.; Kawakami, K.; Yamamoto, Y.; Ogawa, M.; Miyazawa, T. Genetic diversity of feline morbilliviruses isolated in Japan. J. Gen. Virol. 2014, 95, 1464–1468. [Google Scholar] [CrossRef] [PubMed]
- Park, E.S.; Suzuki, M.; Kimura, M.; Mizutani, H.; Saito, R.; Kubota, N.; Hasuike, Y.; Okajima, J.; Kasai, H.; Sato, Y.; et al. Epidemiological and pathological study of feline morbillivirus infection in domestic cats in Japan. BMC Vet. Res. 2016, 12, 228. [Google Scholar] [CrossRef] [PubMed]
- Sutummaporn, K.; Suzuki, K.; Machida, N.; Mizutani, T.; Park, E.S.; Morikawa, S.; Furuya, T. Association of feline morbillivirus infection with defined pathological changes in cat kidney tissues. Vet. Microbiol. 2019, 228, 12–19. [Google Scholar] [CrossRef]
- Sharp, C.R.; Nambulli, S.; Acciardo, A.S.; Rennick, L.J.; Drexler, J.F.; Rima, B.K.; Williams, T.; Duprex, W.P. Chronic Infection of Domestic Cats with Feline Morbillivirus, United States. Emerg. Infect Dis. 2016, 22, 760–762. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, H.; Tekelioglu, B.K.; Gurel, A.; Bamac, O.E.; Ozturk, G.Y.; Cizmecigil, U.Y.; Altan, E.; Aydin, O.; Yilmaz, A.; Berriatua, E.; et al. Frequency, clinicopathological features and phylogenetic analysis of feline morbillivirus in cats in Istanbul, Turkey. J. Feline Med. Surg. 2017, 19, 1206–1214. [Google Scholar] [CrossRef] [PubMed]
- Darold, G.M.; Alfieri, A.A.; Muraro, L.S.; Amude, A.M.; Zanatta, R.; Yamauchi, K.C.; Alfieri, A.F.; Lunardi, M. First report of feline morbillivirus in South America. Arch. Virol. 2017, 162, 469–475. [Google Scholar] [CrossRef] [PubMed]
- Chaiyasak, S.; Piewbang, C.; Rungsipipat, A.; Techangamsuwan, S. Molecular epidemiology and genome analysis of feline morbillivirus in household and shelter cats in Thailand. BMC Vet. Res. 2020, 16, 240. [Google Scholar] [CrossRef]
- Lorusso, A.; Di Tommaso, M.; Di Felice, E.; Zaccaria, G.; Luciani, A.; Marcacci, M.; Aste, G.; Boari, A.; Savini, G. First report of feline morbillivirus in Europe. Vet. Ital. 2015, 51, 235–237. [Google Scholar] [CrossRef]
- Marcacci, M.; De Luca, E.; Zaccaria, G.; Di Tommaso, M.; Mangone, I.; Aste, G.; Savini, G.; Boari, A.; Lorusso, A. Genome characterization of feline morbillivirus from Italy. J. Virol. Methods 2016, 234, 160–163. [Google Scholar] [CrossRef]
- De Luca, E.; Crisi, P.E.; Di Domenico, M.; Malatesta, D.; Vincifori, G.; Di Tommaso, M.; Di Guardo, G.; Di Francesco, G.; Petrini, A.; Savini, G.; et al. A real-time RT-PCR assay for molecular identification and quantitation of feline morbillivirus RNA from biological specimens. J. Virol. Methods 2018, 258, 24–28. [Google Scholar] [CrossRef]
- Stranieri, A.; Lauzi, S.; Dallari, A.; Gelain, M.E.; Bonsembiante, F.; Ferro, S.; Paltrinieri, S. Feline morbillivirus in Northern Italy: Prevalence in urine and kidneys with and without renal disease. Vet. Microbiol. 2019, 233, 133–139. [Google Scholar] [CrossRef]
- De Luca, E.; Crisi, P.E.; Marcacci, M.; Malatesta, D.; Di Sabatino, D.; Cito, F.; D’Alterio, N.; Puglia, I.; Berjaoui, S.; Colaianni, M.L.; et al. Epidemiology, pathological aspects and genome heterogeneity of feline morbillivirus in Italy. Vet. Microbiol. 2020, 240, 108484. [Google Scholar] [CrossRef]
- Muratore, E.; Cerutti, F.; Colombino, E.; Biasibetti, E.; Caruso, C.; Brovida, C.; Cavana, P.; Poncino, L.; Caputo, M.P.; Peletto, S.; et al. Feline morbillivirus in northwestern Italy: First detection of genotype 1-B. J. Feline Med. Surg. 2020. [Google Scholar] [CrossRef]
- McCallum, K.E.; Stubbs, S.; Hope, N.; Mickleburgh, I.; Dight, D.; Tiley, L.; Williams, T.L. Detection and seroprevalence of morbillivirus and other paramyxoviruses in geriatric cats with and without evidence of azotemic chronic kidney disease. J. Vet. Intern. Med. 2018, 32, 1100–1108. [Google Scholar] [CrossRef] [PubMed]
- Sieg, M.; Busch, J.; Eschke, M.; Bottcher, D.; Heenemann, K.; Vahlenkamp, A.; Reinert, A.; Seeger, J.; Heilmann, R.; Scheffler, K.; et al. A New Genotype of Feline Morbillivirus Infects Primary Cells of the Lung, Kidney, Brain and Peripheral Blood. Viruses 2019, 11, 146. [Google Scholar] [CrossRef] [PubMed]
- Sieg, M.; Heenemann, K.; Ruckner, A.; Burgener, I.; Oechtering, G.; Vahlenkamp, T.W. Discovery of new feline paramyxoviruses in domestic cats with chronic kidney disease. Virus Genes 2015, 51, 294–297. [Google Scholar] [CrossRef] [PubMed]
- Mohd Isa, N.H.; Selvarajah, G.T.; Khor, K.H.; Tan, S.W.; Manoraj, H.; Omar, N.H.; Omar, A.R.; Mustaffa-Kamal, F. Molecular detection and characterisation of feline morbillivirus in domestic cats in Malaysia. Vet. Microbiol. 2019, 236, 108382. [Google Scholar] [CrossRef] [PubMed]
- Ou, J.; Ye, S.; Xu, H.; Zhao, J.; Ren, Z.; Lu, G.; Li, S. First report of feline morbillivirus in mainland China. Arch. Virol. 2020, 165, 1837–1841. [Google Scholar] [CrossRef] [PubMed]
- Crisi, P.E.; Dondi, F.; De Luca, E.; Di Tommaso, M.; Vasylyeva, K.; Ferlizza, E.; Savini, G.; Luciani, A.; Malatesta, D.; Lorusso, A.; et al. Early Renal Involvement in Cats with Natural Feline Morbillivirus Infection. Animals 2020, 10, 828. [Google Scholar] [CrossRef] [PubMed]
- Conceidao, C.; Bailey, D. Animal Morbilliviruses. Ref. Modul. Life Sci. 2019. [Google Scholar] [CrossRef]
- Kumar, S.; Nayak, B.; Collins, P.L.; Samal, S.K. Complete genome sequence of avian paramyxovirus type 3 reveals an unusually long trailer region. Virus Res. 2008, 137, 189–197. [Google Scholar] [CrossRef]
- Samuel, A.S.; Paldurai, A.; Kumar, S.; Collins, P.L.; Samal, S.K. Complete genome sequence of avian paramyxovirus (APMV) serotype 5 completes the analysis of nine APMV serotypes and reveals the longest APMV genome. PLoS ONE 2010, 5, e9269. [Google Scholar] [CrossRef][Green Version]
- Sato, H.; Yoneda, M.; Honda, T.; Kai, C. Morbillivirus receptors and tropism: Multiple pathways for infection. Front. Microbiol. 2012, 75. [Google Scholar] [CrossRef]
- Rima, B.K.; Duprex, W.P. The measles virus replication cycle. Curr. Top Microbiol. Immunol. 2009, 329, 77–102. [Google Scholar] [CrossRef]
- Liljeroos, L.; Huiskonen, J.T.; Ora, A.; Susi, P.; Butcher, S.J. Electron cryotomography of measles virus reveals how matrix protein coats the ribonucleocapsid within intact virions. Proc. Natl. Acad. Sci. USA 2011, 108, 18085–18090. [Google Scholar] [CrossRef] [PubMed]
- Suryanarayana, K.; Baczko, K.; ter Meulen, V.; Wagner, R.R. Transcription inhibition and other properties of matrix proteins expressed by M genes cloned from measles viruses and diseased human brain tissue. J. Virol. 1994, 68, 1532–1543. [Google Scholar] [CrossRef]
- Banyard, A.C.; Grant, R.J.; Romero, C.H.; Barrett, T. Sequence of the nucleocapsid gene and genome and antigenome promoters for an isolate of porpoise morbillivirus. Virus Res. 2008, 132, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Wild, T.F.; Malvoisin, E.; Buckland, R. Measles virus: Both the haemagglutinin and fusion glycoproteins are required for fusion. J Gen. Virol. 1991, 72 Pt 2, 439–442. [Google Scholar] [CrossRef]
- Delpeut, S.; Noyce, R.S.; Richardson, C.D. The tumor-associated marker, PVRL4 (nectin-4), is the epithelial receptor for morbilliviruses. Viruses 2014, 6, 2268–2286. [Google Scholar] [CrossRef]
- Melia, M.M.; Earle, J.P.; Abdullah, H.; Reaney, K.; Tangy, F.; Cosby, S.L. Use of SLAM and PVRL4 and identification of pro-HB-EGF as cell entry receptors for wild type phocine distemper virus. PLoS ONE 2014, 9, e106281. [Google Scholar] [CrossRef] [PubMed]
- Park, E.S.; Suzuki, M.; Kimura, M.; Maruyama, K.; Mizutani, H.; Saito, R.; Kubota, N.; Furuya, T.; Mizutani, T.; Imaoka, K.; et al. Identification of a natural recombination in the F and H genes of feline morbillivirus. Virology 2014, 468–470, 524–531. [Google Scholar] [CrossRef][Green Version]
- Sieg, M.; Vahlenkamp, A.; Baums, C.G.; Vahlenkamp, T.W. First Complete Genome Sequence of a Feline Morbillivirus Isolate from Germany. Genome Announc. 2018, 19. [Google Scholar] [CrossRef]
- Nei, M.; Kumar, S. Molecular Evolution and Phylogenetics; Oxford University Press: New York, NY, USA, 2000. [Google Scholar]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across Computing Platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef] [PubMed]
- Busch, J.; Sacristan, I.; Cevidanes, A.; Millan, J.; Vahlenkamp, T.W.; Napolitano, C.; Sieg, M. High seroprevalence of feline morbilliviruses in free-roaming domestic cats in Chile. Arch. Virol 2020, 166, 281–285. [Google Scholar] [CrossRef]
- Gaskin, J.M. Canine distemper virus in domesticated cats and pigs. Adv. Enzymol. Relat. Areas Mol. Biol. 1974, 40, 803–806. [Google Scholar] [PubMed]
- Ikeda, Y.; Nakamura, K.; Miyazawa, T.; Chen, M.C.; Kuo, T.F.; Lin, J.A.; Mikami, T.; Kai, C.; Takahashi, E. Seroprevalence of canine distemper virus in cats. Clin. Diagn. Lab. Immunol. 2001, 8, 641–644. [Google Scholar] [CrossRef]
- Bartges, J.W. Chronic kidney disease in dogs and cats. Vet. Clin. N. Am. Small Anim. Pract. 2012, 42, 669–692. [Google Scholar] [CrossRef] [PubMed]
- Sparkes, A.H.; Caney, S.; Chalhoub, S.; Elliott, J.; Finch, N.; Gajanayake, I.; Langston, C.; Lefebvre, H.P.; White, J.; Quimby, J. ISFM Consensus Guidelines on the Diagnosis and Management of Feline Chronic Kidney Disease. J. Feline Med. Surg. 2016, 18, 219–239. [Google Scholar] [CrossRef]
- Ferlizza, E.; Campos, A.; Neagu, A.; Cuoghi, A.; Bellei, E.; Monari, E.; Dondi, F.; Almeida, A.M.; Isani, G. The effect of chronic kidney disease on the urine proteome in the domestic cat (Felis catus). Vet. J. 2015, 204, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Martorelli, C.R.; Kogika, M.M.; Chacar, F.C.; Caragelasco, D.S.; de Campos Fonseca Pinto, A.C.B.; Lorigados, C.A.B.; Andrade, L.C. Urinary Fractional Excretion of Phosphorus in Dogs with Spontaneous Chronic Kidney Disease. Vet. Sci. 2017, 4, 67. [Google Scholar] [CrossRef]
- Miyazaki, M.; Soeta, S.; Yamagishi, N.; Taira, H.; Suzuki, A.; Yamashita, T. Tubulointerstitial nephritis causes decreased renal expression and urinary excretion of cauxin, a major urinary protein of the domestic cat. Res. Vet. Sci. 2007, 82, 76–79. [Google Scholar] [CrossRef] [PubMed]
- Greene, C.E. Infectious Disease of the Dog and Cat, 3rd ed.; Saunders, Ed.; Elsevier: St. Louis, MO, USA, 2011; pp. 92–149. [Google Scholar]
- Poli, A.; Tozon, N.; Guidi, G.; Pistello, M. Renal alterations in feline immunodeficiency virus (FIV)-infected cats: A natural model of lentivirus-induced renal disease changes. Viruses 2012, 4, 1372–1389. [Google Scholar] [CrossRef] [PubMed]
- Reinacher, M. Diseases associated with spontaneous feline leukemia virus (FeLV) infection in cats. Vet. Immunol. Immunopathol. 1989, 21, 85–95. [Google Scholar] [CrossRef]
- Fujii, Y.; Tochitani, T.; Kouchi, M.; Matsumoto, I.; Yamada, T.; Funabashi, H. Glomerulonephritis in a ferret with feline coronavirus infection. J. Vet. Diagn. Investig. 2015, 27, 637–640. [Google Scholar] [CrossRef] [PubMed]
- Francis, D.P.; Essex, M.; Jakowski, R.M.; Cotter, S.M.; Lerer, T.J.; Hardy, W.D., Jr. Increased risk for lymphoma and glomerulonephritis in a closed population of cats exposed to feline leukemia virus. Am. J. Epidemiol. 1980, 111, 337–346. [Google Scholar] [CrossRef]
- Tomashefski, J.F.F.C.; Fraire, A.E. Dail and Hammar’s Pulmonary Pathology; Springer: New York, NY, USA, 2008; pp. 432–434. [Google Scholar]
- Gu, J.; Korteweg, C. Pathology and pathogenesis of severe acute respiratory syndrome. Am. J. Pathol. 2007, 170, 1136–1147. [Google Scholar] [CrossRef]
- MacLachlan, N.J.; Dubovi, E.J. Fenner’s Veterinary Virology, 5th ed.; Academic: London, UK; Boston, MA, USA, 2011. [Google Scholar]
- da Fontoura Budaszewski, R.; von Messling, V. Morbillivirus Experimental Animal Models: Measles Virus Pathogenesis Insights from Canine Distemper Virus. Viruses 2016, 8, 274. [Google Scholar] [CrossRef] [PubMed]
- Tatsuo, H.; Ono, N.; Yanagi, Y. Morbilliviruses use signaling lymphocyte activation molecules (CD150) as cellular receptors. J. Virol. 2001, 75, 5842–5850. [Google Scholar] [CrossRef]
- Muhlebach, M.D.; Mateo, M.; Sinn, P.L.; Prufer, S.; Uhlig, K.M.; Leonard, V.H.; Navaratnarajah, C.K.; Frenzke, M.; Wong, X.X.; Sawatsky, B.; et al. Adherens junction protein nectin-4 is the epithelial receptor for measles virus. Nature 2011, 480, 530–533. [Google Scholar] [CrossRef]
- Noyce, R.S.; Bondre, D.G.; Ha, M.N.; Lin, L.T.; Sisson, G.; Tsao, M.S.; Richardson, C.D. Tumor cell marker PVRL4 (nectin 4) is an epithelial cell receptor for measles virus. PLoS Pathog. 2011, 7, e1002240. [Google Scholar] [CrossRef]
- Koide, R.; Sakaguchi, S.; Miyazawa, T. Basic biological characterization of feline morbillivirus. J. Vet. Med. Sci. 2015, 77, 565–569. [Google Scholar] [CrossRef]
- MacLachlan, N.J.; Edward, J.D. Fenner’s Veterinary Virology, 4th ed.; Academic: London, UK; Boston, MA, USA, 2011. [Google Scholar]
- Donato, G.; De Luca, E.; Crisi, P.E.; Pizzurro, F.; Masucci, M.; Marcacci, M.; Cito, F.; Di Sabatino, D.; Boari, A.; D’Alterio, N.; et al. Isolation and genome sequences of two Feline Morbillivirus genotype 1 strains from Italy. Vet. Ital. 2019, 55, 179–182. [Google Scholar] [CrossRef] [PubMed]
- Arikawa, K.; Wachi, A.; Imura, Y.; Sutummaporn, K.; Kai, C.; Park, E.S.; Morikawa, S.; Uematsu, Y.; Yamaguchi, T.; Furuya, T. Development of an ELISA for serological detection of feline morbillivirus infection. Arch. Virol. 2017, 162, 2421–2425. [Google Scholar] [CrossRef]
- Sakaguchi, S.; Koide, R.; Miyazawa, T. In vitro host range of feline morbillivirus. J. Vet. Med. Sci. 2015, 77, 1485–1487. [Google Scholar] [CrossRef] [PubMed]
- Seki, F.; Ono, N.; Yamaguchi, R.; Yanagi, Y. Efficient isolation of wild strains of canine distemper virus in Vero cells expressing canine SLAM (CD150) and their adaptability to marmoset B95a cells. J. Virol. 2003, 77, 9943–9950. [Google Scholar] [CrossRef]
- Koide, R.; Sakaguchi, S.; Ogawa, M.; Miyazawa, T. Rapid detection of feline morbillivirus by a reverse transcription loop-mediated isothermal amplification. J. Vet. Med. Sci. 2016, 78, 105–108. [Google Scholar] [CrossRef]
- Tong, S.; Chern, S.W.; Li, Y.; Pallansch, M.A.; Anderson, L.J. Sensitive and broadly reactive reverse transcription-PCR assays to detect novel paramyxoviruses. J. Clin. Microbiol. 2008, 46, 2652–2658. [Google Scholar] [CrossRef]
- Cox, R.; Plemper, R.K. The paramyxovirus polymerase complex as a target for next-generation anti-paramyxovirus therapeutics. Front. Microbiol. 2015, 12, 459. [Google Scholar] [CrossRef] [PubMed]
- Das, M.; Kumar, S. Recombinant phosphoprotein based single serum dilution ELISA for rapid serological detection of Newcastle disease virus. J. Virol. Methods 2015, 225, 64–69. [Google Scholar] [CrossRef] [PubMed]
| Reference | Population | Total | Sample | FeMV RNA-Positive Tissues | Prevalence per Type of Sample | Prevalence c | Country |
|---|---|---|---|---|---|---|---|
| Woo et al., 2012 | Stray cats | 457 | Urine | 11.6% | 12.3% | Hong Kong | |
| Blood | 0.2% | ||||||
| Feces | 0.8% | ||||||
| 16 | Oral swab | 6.2% | 6.2% | Mainland China | |||
| Rectal swab | 6.2% | ||||||
| Furuya et al., 2014 | Household cats | 82 | Urine | 6.1% | 9.8% | Japan | |
| 10 | Blood | 10% | |||||
| 10 | Tissues | Kidney | 40% | ||||
| Sakaguchi et al., 2014 | Household cats | 13 | Urine | 23% | 23% | Japan | |
| Furuya et al., 2016 | Household cats | 166 | Urine | 15.1% | 15.1% | Japan | |
| Sharp et al., 2016 | Household cats | 327 | Urine | 3% | 3% | USA | |
| Park et al., 2016 | Stray/household cats | 100 | Urine | 17% | 22% | Japan | |
| Tissues | Kidney | 18% | |||||
| Darold et al., 2017 | Colony cats a | 17 | Urine | 52.9% | 23% | Brazil | |
| Household cats | 35 | 8.6% | |||||
| Yilmaz et al., 2017 | Household cats | 96 | Urine | 3.1% | 5.4% | Turkey | |
| 15 | Tissues | Kidney | 26% | ||||
| Lymph nodes | 13% | ||||||
| Lung | 6% | ||||||
| Spleen | 6% | ||||||
| Intestine | 6% | ||||||
| Liver | 6% | ||||||
| McCallum et al., 2017 | Household cats | 40 | Urine | 12.5% | 12.5% | United Kingdom | |
| Stranieri et al., 2019 | Stray cats | 6 | Urine | 16.6% | 3.2% | Italy | |
| Household cats | 59 | 0% | |||||
| Stray/household cats | 27 | Tissues | Kidney | 7.4% | |||
| Mohd et al., 2019 | Stray/household cats | 124 | Urine | 50.8% | 39.4% | Malaysia | |
| 25 | Tissues | Kidney | 80% | ||||
| Sieg et al., 2019 | na | 723 | Urine | 0.8% | 0.83% | Germany | |
| De Luca et al., 2020 | Colony cats b | 69 | Urine | 31.8% | 16.8% | Italy | |
| Household cats | 127 | 8.6% | |||||
| Colony cats b | 7 | Tissues | Kidney | 57.1% | 22.8% | ||
| Bladder | 14.2% | ||||||
| Spleen | 28.5% | ||||||
| Lymph nodes | 14.2% | ||||||
| Household cats | 28 | Tissues | Kidney | 10.7% | |||
| Bladder | 10.7% | ||||||
| Spleen | 3.5% | ||||||
| Brain | 3.5% | ||||||
| Muratore et al., 2020 | Household cats | 127 | Urine | 3.9% | 7.3% | ||
| Colony cats b | 40 | Tissues | Kidney | 7.5% | |||
| Household cats | 23 | Urine | 26% | 8% | |||
| Colony cats b | 10 | Tissues | Kidney | 10% | |||
| Ou et al., 2020 | n.a. | 64 | Urine | 9.3% | 9.37% | Mainland China | |
| Chaiyasak et al., 2020 | Colony cats b | 31 | Urine | 19.3% | 11.9% | Thailand | |
| Household cats | 100 | Urine | 13% | ||||
| Colony cats b | 61 | Blood | 19.6% | ||||
| Household cats | 200 | Blood | 0% | ||||
| Reference | Population | Total | Seroprevalence | Country |
|---|---|---|---|---|
| Woo et al., 2012 | Stray cats | 457 | 27.8% | China |
| Sakaguchi et al., 2014 | Household cats | 13 | 23% | Japan |
| Park et al., 2016 | Stray and household cats | 100 | 21% | Japan |
| Arikawa et al., 2017 | n.a. | 100 | 22% | Japan |
| McCallum et al., 2017 | Household cats | 72 | 31% | United Kingdom |
| De Luca et al., 2020 | Colony cats a | 69 | 21.73% | Italy |
| Household cats | 127 | 17.32% | ||
| Busch et al., 2020 | Colony cats a | 112 | 63% | Chile |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Luca, E.; Sautto, G.A.; Crisi, P.E.; Lorusso, A. Feline Morbillivirus Infection in Domestic Cats: What Have We Learned So Far? Viruses 2021, 13, 683. https://doi.org/10.3390/v13040683
De Luca E, Sautto GA, Crisi PE, Lorusso A. Feline Morbillivirus Infection in Domestic Cats: What Have We Learned So Far? Viruses. 2021; 13(4):683. https://doi.org/10.3390/v13040683
Chicago/Turabian StyleDe Luca, Eliana, Giuseppe Andrea Sautto, Paolo Emidio Crisi, and Alessio Lorusso. 2021. "Feline Morbillivirus Infection in Domestic Cats: What Have We Learned So Far?" Viruses 13, no. 4: 683. https://doi.org/10.3390/v13040683
APA StyleDe Luca, E., Sautto, G. A., Crisi, P. E., & Lorusso, A. (2021). Feline Morbillivirus Infection in Domestic Cats: What Have We Learned So Far? Viruses, 13(4), 683. https://doi.org/10.3390/v13040683








