Inhibitory Effect of Sargassum fusiforme and Its Components on Replication of Respiratory Syncytial Virus In Vitro and In Vivo
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture, Virus Propagation, and Plaque Assay
2.2. Plant Materials and Total Aqueous Extract Preparation
2.3. Reagents, Chemicals, and Antibodies
2.4. Antiviral Assays and Effective Concentration (EC50) Determination
2.5. Cell Viability Assay
2.6. Quantitative RT-PCR (qRT PCR)
2.7. Immunoblot Analysis
2.8. Syncytial Formation and Syncytia Counting Assay
2.9. RSV–GFP Challenge Experiment in Mouse Modal
2.10. Statistical Analysis
3. Results
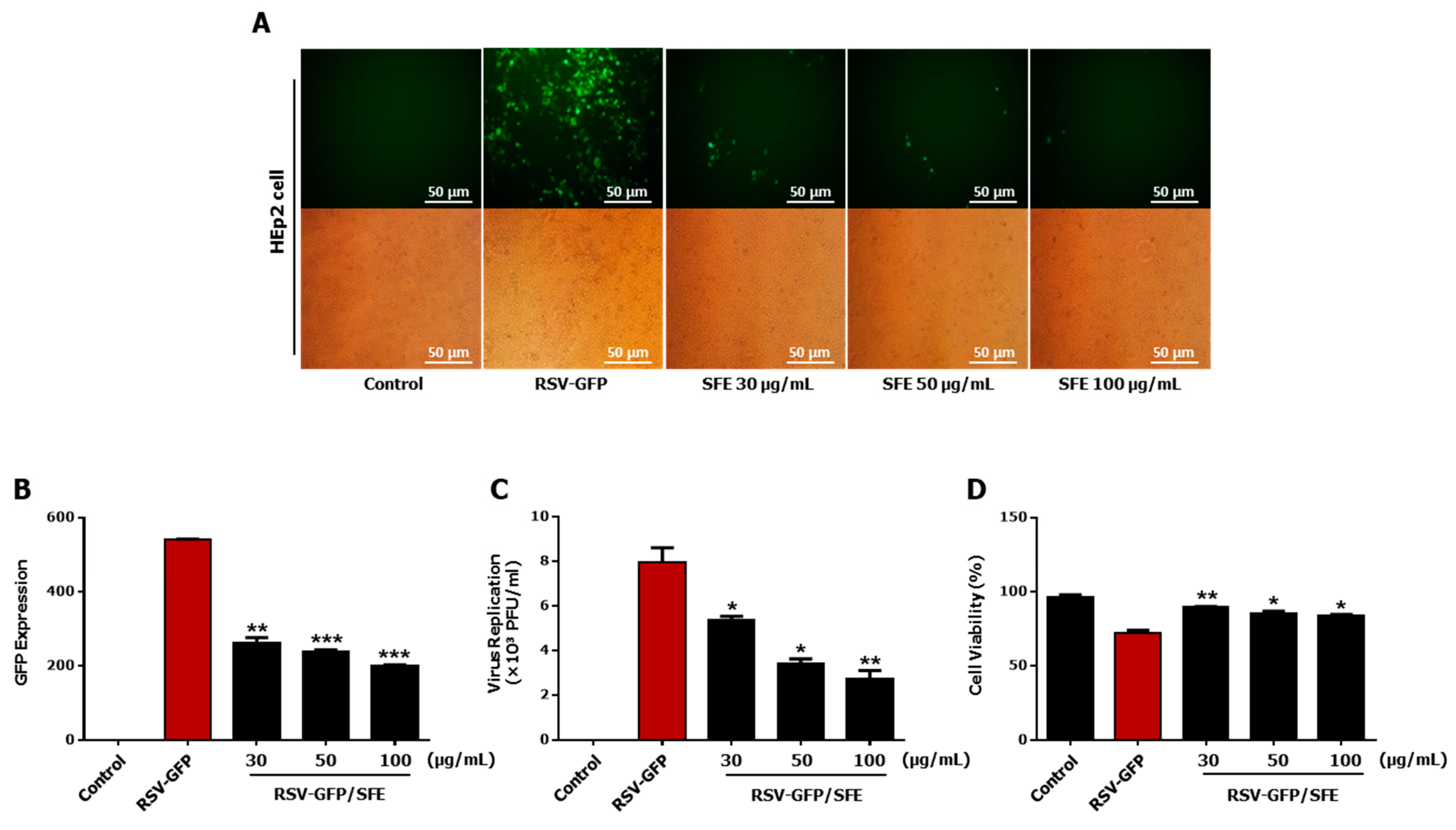
3.1. Anti-RSV Effect of SFE
3.2. Determination of the Effective Concentration (EC50) and Cytotoxic Concentration (CC50) of SFE
3.3. Therapeutic Effect of SFE against RSV Infection
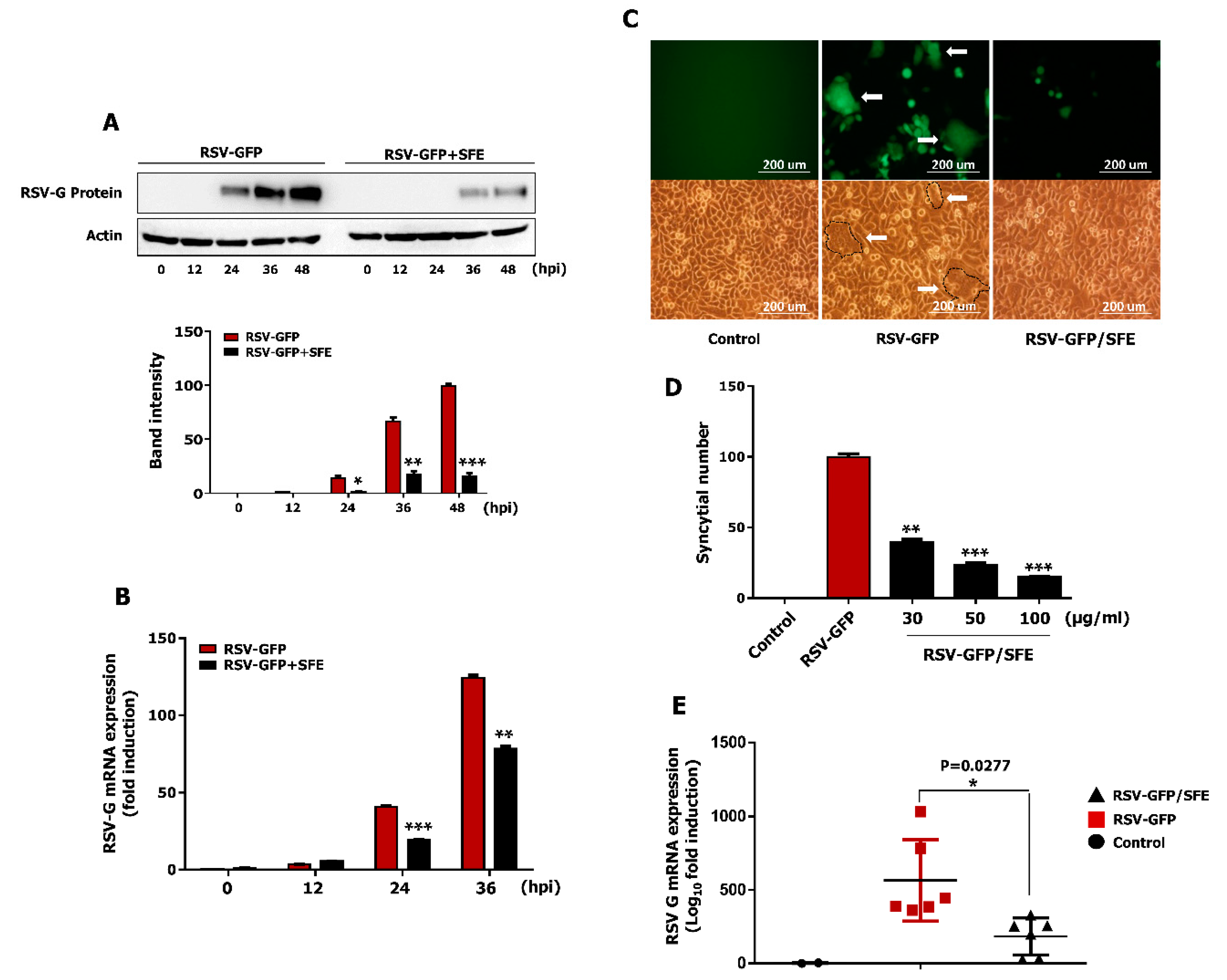
3.4. Effect of SFE on Viral Protein Synthesis and Viral RNA Expression
3.5. Effect of SFE on Syncytium Formation
3.6. Oral Administration of SFE Protects against RSV Infection in BALB/C Mice
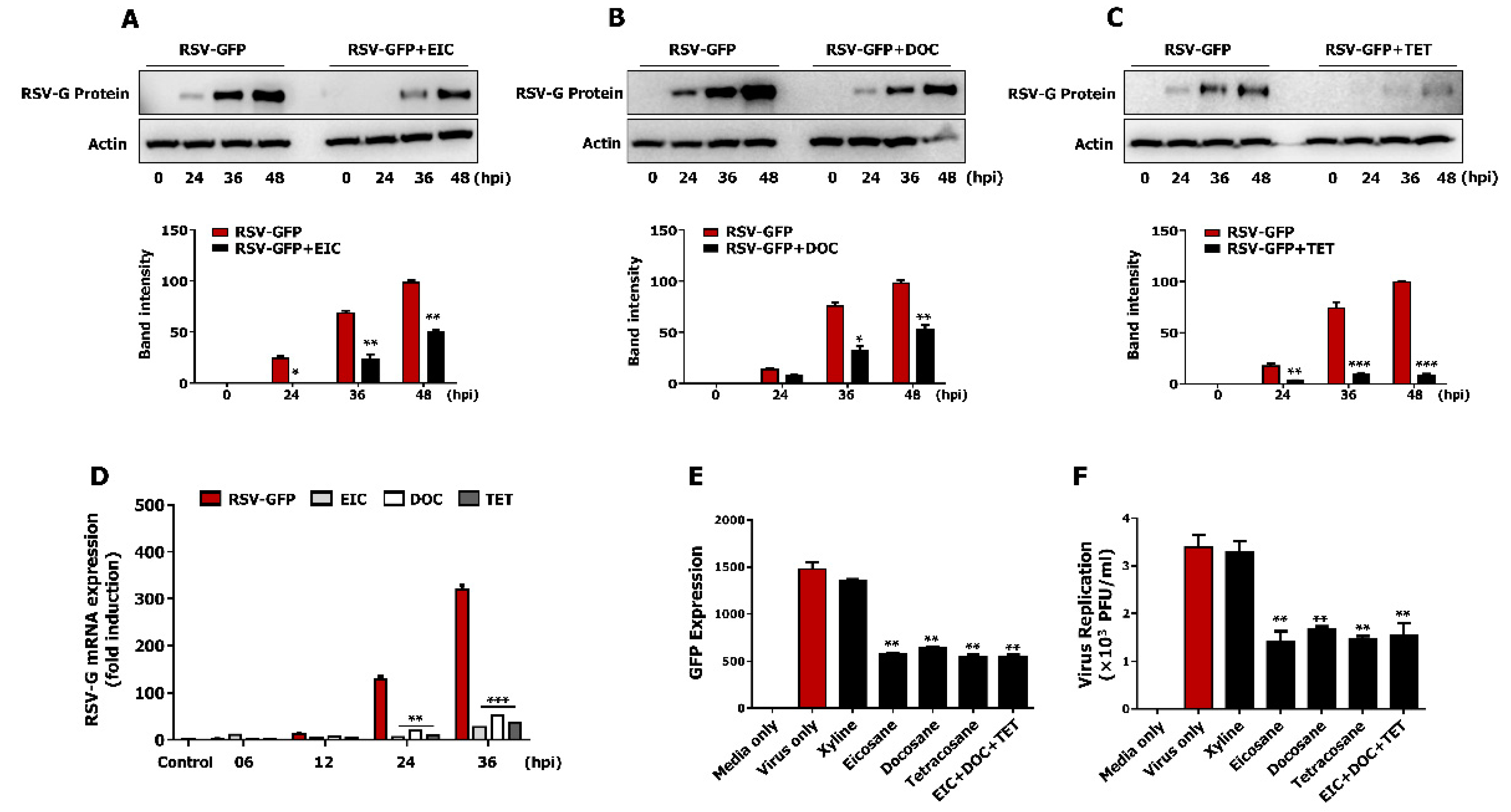
3.7. Non-Cytotoxic Concentrations of Tetracosane, Docosane, and Eicosane Inhibit RSV Replication In Vitro
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Black, C.P. Systematic review of the biology and medical management of respiratory syncytial virus infection. Respir. Care 2003, 48, 209–231. [Google Scholar]
- Polak, M.J. Respiratory syncytial virus (RSV): Overview, treatment, and prevention strategies. Newborn Infant Nurs. Rev. 2004, 4, 15–23. [Google Scholar] [CrossRef]
- Hilleman, M. Respiratory syncytial virus. Am. Rev. Respir. Dis. 1963, 88, 181–197. [Google Scholar]
- Donalisio, M.; Rusnati, M.; Cagno, V.; Civra, A.; Bugatti, A.; Giuliani, A.; Pirri, G.; Volante, M.; Papotti, M.; Landolfo, S.; et al. Inhibition of Human Respiratory Syncytial Virus Infectivity by a Dendrimeric Heparan Sulfate-Binding Peptide. Antimicrob. Agents Chemother. 2012, 56, 5278–5288. [Google Scholar] [CrossRef]
- Chathuranga, K.; Kim, M.S.; Lee, H.-C.; Kim, T.-H.; Kim, J.-H.; Chathuranga, W.A.G.; Ekanayaka, P.; Wijerathne, H.M.S.M.; Cho, W.-K.; Kim, H.I.; et al. Anti-Respiratory Syncytial Virus Activity of Plantago asiatica and Clerodendrum trichotomum Extracts In Vitro and In Vivo. Viruses 2019, 11, 604. [Google Scholar] [CrossRef]
- Hall, C.B.; Weinberg, G.A.; Iwane, M.K.; Blumkin, A.K.; Edwards, K.M.; Staat, M.A.; Auinger, P.; Griffin, M.R.; Poehling, K.A.; Erdman, D.; et al. The Burden of Respiratory Syncytial Virus Infection in Young Children. N. Engl. J. Med. 2009, 360, 588–598. [Google Scholar] [CrossRef] [PubMed]
- Paramore, L.C.; Ciuryla, V.T.; Ciesla, G.; Liu, L. Economic Impact of Respiratory Syncytial Virus-Related Illness in the US. PharmacoEconomics 2004, 22, 275–284. [Google Scholar] [CrossRef] [PubMed]
- Harris, K.C.; Anis, A.H.; Crosby, M.C.; Cender, L.M.; Potts, J.E.; Human, D.G. Economic Evaluation of Palivizumab in Children with Congenital Heart Disease: A Canadian Perspective. Can. J. Cardiol. 2011, 27, 523.e11–523.e15. [Google Scholar] [CrossRef] [PubMed]
- Fulginiti, V.; Eller, J.; Sieber, O.; Joyner, J.; Minamitani, M.; Meiklejohn, G. Respiratory virus immunization: A field trial of two inactivated respiratory virus vaccines; an aqueous trivalent paratnfluenza virus vaccine and an alum-precipitated Respiratory Syncytial Virus vaccine. Am. J. Epidemiol. 1969, 89, 435–448. [Google Scholar] [CrossRef]
- Kapikian, A.Z.; Mitchell, R.H.; Chanock, R.M.; Shvedoff, R.A.; Stewart, C.E. An Epidemiologic Study of Altered Clinical Reactivity to Respiratory Syncytial (Rs) Virus Infection in Children Previously Vaccinated with An Inactivated Rs Virus Vaccine. Am. J. Epidemiol. 1969, 89, 405–421. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.W.; Canchola, J.G.; Brandt, C.D.; Pyles, G.; Chanock, R.M.; Jensen, K.; Parrott, R.H. Respiratory Syncytial Virus Disease in Infants Despite Prior Administration of Antigenic Inactivated Vaccine12. Am. J. Epidemiol. 1969, 89, 422–434. [Google Scholar] [CrossRef]
- Kneyber, M.C.J.; Moll, H.A.; De Groot, R. Treatment and prevention of respiratory syncytial virus infection. Eur. J. Nucl. Med. Mol. Imaging 2000, 159, 399–411. [Google Scholar] [CrossRef] [PubMed]
- Solecki, R.S.; Shanidar, I.V. A Neanderthal Flower Burial in Northern Iraq. Science 1975, 190, 880–881. [Google Scholar] [CrossRef]
- Bamoniri, A.; Ebrahimabadi, A.H.; Mazoochi, A.; Behpour, M.; Kashi, F.J.; Batooli, H. Antioxidant and antimicrobial activity evaluation and essential oil analysis of Semenovia tragioides Boiss. from Iran. Food Chem. 2010, 122, 553–558. [Google Scholar] [CrossRef]
- Cumashi, A.; Ushakova, N.A.; Preobrazhenskaya, M.E.; D′Incecco, A.; Piccoli, A.; Totani, L.; Tinari, N.; Morozevich, G.E.; Berman, A.E.; Bilan, M.I.; et al. A comparative study of the anti-inflammatory, anticoagulant, antiangiogenic, and antiadhesive activities of nine different fucoidans from brown seaweeds. Glycobiology 2007, 17, 541–552. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Ooi, V.E.; Chan, P.K.; Ang, P.O., Jr. Isolation and characterization of a sulfated polysaccharide from the brown alga Sargassum patens and determination of its anti-herpes activity. Biochem. Cell Biol. 2003, 81, 25–33. [Google Scholar] [CrossRef]
- Chen, P.; Shengqin, W.; Zhang, Y.; Yang, S.; Chen, L.; Wang, S.; Zou, H.; Liao, Z.; Zhang, X.; Wu, M. Sargassum fusiforme polysaccharides activate antioxidant defense by promoting Nrf2-dependent cytoprotection and ameliorate stress insult during aging. Food Funct. 2016, 7, 4576–4588. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Xu, M.; Hu, C.; Liu, A.; Chen, J.; Gu, C.; Zhang, X.; You, C.; Tong, H.; Wu, M.; et al. Sargassum fusiforme Fucoidan SP2 Extends the Lifespan of Drosophila melanogaster by Upregulating the Nrf2-Mediated Antioxidant Signaling Pathway. Oxidative Med. Cell. Longev. 2019, 2019, 1–15. [Google Scholar] [CrossRef]
- Wang, L.; Oh, J.Y.; Kim, H.S.; Lee, W.; Cui, Y.; Lee, H.G.; Kim, Y.-T.; Jeon, Y.-J. Protective effect of polysaccharides from Celluclast-assisted extract of Hizikia fusiforme against hydrogen peroxide-induced oxidative stress in vitro in Vero cells and in vivo in zebrafish. Int. J. Biol. Macromol. 2018, 112, 483–489. [Google Scholar] [CrossRef]
- Jeong, S.C.; Jeong, Y.T.; Lee, S.M.; Kim, J.H. Immune-modulating activities of polysaccharides extracted from brown algae Hizikia fusiforme. Biosci. Biotechnol. Biochem. 2015, 79, 1362–1365. [Google Scholar] [CrossRef]
- Chen, X.; Nie, W.; Fan, S.; Zhang, J.; Wang, Y.; Lu, J.; Jin, L. A polysaccharide from Sargassum fusiforme protects against immunosuppression in cyclophosphamide-treated mice. Carbohydr. Polym. 2012, 90, 1114–1119. [Google Scholar] [CrossRef]
- Chen, P.; Yang, S.; Hu, C.; Zhao, Z.; Liu, J.; Cheng, Y.; Wang, S.; Chen, Q.; Yu, P.; Zhang, X.; et al. Sargassum fusiforme Polysaccharide Rejuvenat es the Small Intestine in Mice Through Altering its Physiol ogy and Gut Microbiota Composition. Curr. Mol. Med. 2018, 17, 1. [Google Scholar] [CrossRef] [PubMed]
- Fan, S.; Zhang, J.; Nie, W.; Zhou, W.; Jin, L.; Chen, X.; Lu, J. Antitumor effects of polysaccharide from Sargassum fusiforme against human hepatocellular carcinoma HepG2 cells. Food Chem. Toxicol. 2017, 102, 53–62. [Google Scholar] [CrossRef]
- Zhang, J.-W.; He, L.-J.; Cao, S.-J.; Yang, Q.; Yang, S.-W.; Zhou, Y.-J. Effect of glycemic variability on short term prognosis in acute myocardial infarction subjects undergoing primary percutaneous coronary interventions. Diabetol. Metab. Syndr. 2014, 6, 76. [Google Scholar] [CrossRef]
- Dobashi, K.; Nishino, T.; Fujihara, M.; Nagumo, T. Isolation and preliminary characterization of fucose-containing sulfated polysaccharides with blood-anticoagulant activity from the brown seaweed Hizikia fusiforme. Carbohydr. Res. 1989, 194, 315–320. [Google Scholar] [CrossRef]
- Sugiura, Y.; Kinoshita, Y.; Abe, M.; Murase, N.; Tanaka, R.; Matsushita, T.; Usui, M.; Hanaoka, K.-I.; Miyata, M. Suppressive effects of the diethyl ether fraction from a brown alga Sargassum fusiforme on allergic and inflammatory reactions. Fish. Sci. 2016, 82, 369–377. [Google Scholar] [CrossRef]
- Fuchimoto, J.; Kojima, T.; Okabayashi, T.; Masaki, T.; Ogasawara, N.; Obata, K.; Nomura, K.; Hirakawa, S.; Kobayashi, N.; Shigyo, T.; et al. Humulone suppresses replication of respiratory syncytial virus and release of IL-8 and RANTES in normal human nasal epithelial cells. Med. Mol. Morphol. 2013, 46, 203–209. [Google Scholar] [CrossRef]
- Le Nouën, C.; Brock, L.G.; Luongo, C.; Mccarty, T.; Yang, L.; Mehedi, M.; Wimmer, E.; Mueller, S.; Collins, P.L.; Buchholz, U.J.; et al. Attenuation of Human Respiratory Syncytial Virus by Genome-Scale Codon-Pair Deoptimization; National Academy of Sciences: Washington, DC, USA, 2014; Volume 111, pp. 13169–13174. [Google Scholar]
- Moon, H.-J.; Lee, J.-S.; Choi, Y.-K.; Park, J.-Y.; Talactac, M.R.; Chowdhury, M.Y.; Poo, H.; Sung, M.-H.; Lee, J.-H.; Jung, J.U.; et al. Induction of type I interferon by high-molecular poly-γ-glutamate protects B6.A2G-Mx1 mice against influenza A virus. Antivir. Res. 2012, 94, 98–102. [Google Scholar] [CrossRef]
- Nguyen, D.; De Witte, L.; Ludlow, M.; Yüksel, S.; Wiesmüller, K.; Geijtenbeek, T.; Osterhaus, A.; De Swart, R. The Synthetic Bacterial Lipopeptide Pam3CSK4 Modulates Respiratory Syncytial Virus Infection Independent of TLR Activation. PLoS Pathog. 2010, 6, e1001049. [Google Scholar] [CrossRef] [PubMed]
- Strober, W. Trypan Blue Exclusion Test of Cell Viability. Curr. Protoc. Immunol. 2015, 111, A3.B.1–A3.B.3. [Google Scholar] [CrossRef]
- Kuhn, D.M.; Balkis, M.; Chandra, J.; Mukherjee, P.K.; Ghannoum, M.A. Uses and Limitations of the XTT Assay in Studies of Candida Growth and Metabolism. J. Clin. Microbiol. 2003, 41, 506–508. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2− ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Lee, B.-H.; Chathuranga, K.; Uddin, B.; Weeratunga, P.; Kim, M.S.; Cho, W.-K.; Kim, H.I.; Ma, J.Y.; Lee, J.-S. Coptidis Rhizoma extract inhibits replication of respiratory syncytial virus in vitro and in vivo by inducing antiviral state. J. Microbiol. 2017, 55, 488–498. [Google Scholar] [CrossRef]
- El Shafay, S.M.; Ali, S.S.; El-Sheekh, M.M. Antimicrobial activity of some seaweeds species from Red sea, against multidrug resistant bacteria. Egypt. J. Aquat. Res. 2016, 42, 65–74. [Google Scholar] [CrossRef]
- Jassim, S.; Naji, M. Novel antiviral agents: A medicinal plant perspective. J. Appl. Microbiol. 2003, 95, 412–427. [Google Scholar] [CrossRef]
- Fajarningsih, N.D. An Emerging Marine Biotechnology: Marine Drug Discovery. Squalen Bull. Mar. Fish. Postharvest Biotechnol. 2012, 7, 89–96. [Google Scholar] [CrossRef]
- Pan, S.-Y.; Zhou, S.-F.; Gao, S.-H.; Yu, Z.-L.; Zhang, S.-F.; Tang, M.-K.; Sun, J.-N.; Ma, D.-L.; Han, Y.-F.; Fong, W.-F. New perspectives on how to discover drugs from herbal medicines: CAM’s outstanding contribution to modern therapeutics. Evid. Based Complementary Altern. Med. 2013, 2013, 627375. [Google Scholar] [CrossRef] [PubMed]
- Nugent-Head, J. The First Materia Medica: The Shen Nong Ben Cao Jing. J. Chin. Med. 2014, 104, 24–28. [Google Scholar]
- Feng, N.-P.; Di Bin, L.W.-Y. Pharmacopoeia of the People′s Republic of China Pharmacopoeia of the People’s Republic of China. Chem. Pharm. Bull. 2005, 53, 978–983. [Google Scholar] [CrossRef][Green Version]
- Zhang, R.; Zhang, X.; Tang, Y.; Mao, J. Composition, isolation, purification and biological activities of Sargassum fusiforme polysaccharides: A review. Carbohydr. Polym. 2020, 228, 115381. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.-J.; Shi, M.-J.; Cui, S.; Hao, S.-X.; Hider, R.C.; Zhou, T. Improved antioxidant and anti-tyrosinase activity of polysaccharide from Sargassum fusiforme by degradation. Int. J. Biol. Macromol. 2016, 92, 715–722. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Nie, W.; Yu, G.; Li, Y.; Hu, Y.; Lu, J.; Jin, L. Antitumor and immunomodulatory activity of polysaccharides from Sargassum fusiforme. Food Chem. Toxicol. 2012, 50, 695–700. [Google Scholar] [CrossRef]
- Ye, Y.; Ji, D.; You, L.; Zhou, L.; Zhao, Z.; Brennan, C. Structural properties and protective effect of Sargassum fusiforme polysaccharides against ultraviolet B radiation in hairless Kun Ming mice. J. Funct. Foods 2018, 43, 8–16. [Google Scholar] [CrossRef]
- Cong, Q.; Chen, H.; Liao, W.; Xiao, F.; Wang, P.; Qin, Y.; Dong, Q.; Ding, K. Structural characterization and effect on anti-angiogenic activity of a fucoidan from Sargassum fusiforme. Carbohydr. Polym. 2016, 136, 899–907. [Google Scholar] [CrossRef]
- Chen, L.; Chen, P.; Liu, J.; Hu, C.; Yang, S.; He, D.; Yu, P.; Wu, M.; Zhang, X. Sargassum fusiforme polysaccharide SFP-F2 activates the NF-κB signaling pathway via CD14/IKK and P38 axes in RAW264. 7 cells. Mar. Drugs 2018, 16, 264. [Google Scholar] [CrossRef] [PubMed]
- Shekar, S.P.; Rojas, E.E.; D’Angelo, C.C.; Gillenwater, S.R.; Galvis, N.P.M. Legally Lethal Kratom: A Herbal Supplement with Overdose Potential. J. Psychoact. Drugs 2018, 51, 28–30. [Google Scholar] [CrossRef] [PubMed]
- Heminway, B.; Yu, Y.; Tanaka, Y.; Perrine, K.; Gustafson, E.; Bernstein, J.; Galinski, M. Analysis of Respiratory Syncytial Virus F, G, and SH Proteins in Cell Fusion. Virology 1994, 200, 801–805. [Google Scholar] [CrossRef]
- Boukhvalova, M.S.; Yim, K.C.; Prince, G.A.; Blanco, J.C. Methods for Monitoring Dynamics of Pulmonary RSV Replication by Viral Culture and by Real-Time Reverse Transcription–PCR In Vivo: Detection of Abortive Viral Replication. Curr. Protoc. Cell Biol. 2010, 46, 26.6.1–26.6.19. [Google Scholar] [CrossRef]
- DeVincenzo, J.P.; El Saleeby, C.M.; Bush, A.J. Respiratory Syncytial Virus Load Predicts Disease Severity in Previously Healthy Infants. J. Infect. Dis. 2005, 191, 1861–1868. [Google Scholar] [CrossRef]
- Karron, R.A.; Wright, P.F.; Crowe, J.E., Jr.; Mann, M.L.C.; Thompson, J.; Makhene, M.; Casey, R.; Murphy, B.R. Evaluation of two live, cold-passaged, temperature-sensitive respiratory syncytial virus vaccines in chimpanzees and in human adults, infants, and children. J. Infect. Dis. 1997, 176, 1428–1436. [Google Scholar] [CrossRef]
- Liu, L.; Heinrich, M.; Myers, S.; Dworjanyn, S.A. Towards a better understanding of medicinal uses of the brown seaweed Sargassum in Traditional Chinese Medicine: A phytochemical and pharmacological review. J. Ethnopharmacol. 2012, 142, 591–619. [Google Scholar] [CrossRef] [PubMed]
- Fitzgerald, C.; Gallagher, E.; Tasdemir, D.; Hayes, M. Heart Health Peptides from Macroalgae and Their Potential Use in Functional Foods. J. Agric. Food Chem. 2011, 59, 6829–6836. [Google Scholar] [CrossRef] [PubMed]
- Uma, B.; Parvathavarthini, R. Antibacterial effect of hexane extract of sea urchin, Temnopleurus alexandri (Bell, 1884). Int. J. PharmTech Res. 2010, 2, 1677–1680. [Google Scholar]
- Uddin, S.J.; Grice, D.; Tiralongo, E. Evaluation of cytotoxic activity of patriscabratine, tetracosane and various flavonoids isolated from the Bangladeshi medicinal plant Acrostichum aureum. Pharm. Biol. 2012, 50, 1276–1280. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chathuranga, K.; Weerawardhana, A.; Dodantenna, N.; Ranathunga, L.; Cho, W.-K.; Ma, J.Y.; Lee, J.-S. Inhibitory Effect of Sargassum fusiforme and Its Components on Replication of Respiratory Syncytial Virus In Vitro and In Vivo. Viruses 2021, 13, 548. https://doi.org/10.3390/v13040548
Chathuranga K, Weerawardhana A, Dodantenna N, Ranathunga L, Cho W-K, Ma JY, Lee J-S. Inhibitory Effect of Sargassum fusiforme and Its Components on Replication of Respiratory Syncytial Virus In Vitro and In Vivo. Viruses. 2021; 13(4):548. https://doi.org/10.3390/v13040548
Chicago/Turabian StyleChathuranga, Kiramage, Asela Weerawardhana, Niranjan Dodantenna, Lakmal Ranathunga, Won-Kyung Cho, Jin Yeul Ma, and Jong-Soo Lee. 2021. "Inhibitory Effect of Sargassum fusiforme and Its Components on Replication of Respiratory Syncytial Virus In Vitro and In Vivo" Viruses 13, no. 4: 548. https://doi.org/10.3390/v13040548
APA StyleChathuranga, K., Weerawardhana, A., Dodantenna, N., Ranathunga, L., Cho, W.-K., Ma, J. Y., & Lee, J.-S. (2021). Inhibitory Effect of Sargassum fusiforme and Its Components on Replication of Respiratory Syncytial Virus In Vitro and In Vivo. Viruses, 13(4), 548. https://doi.org/10.3390/v13040548








