Treatment Protocol for COVID-19 Based on T2R Phenotype
Abstract
1. Introduction
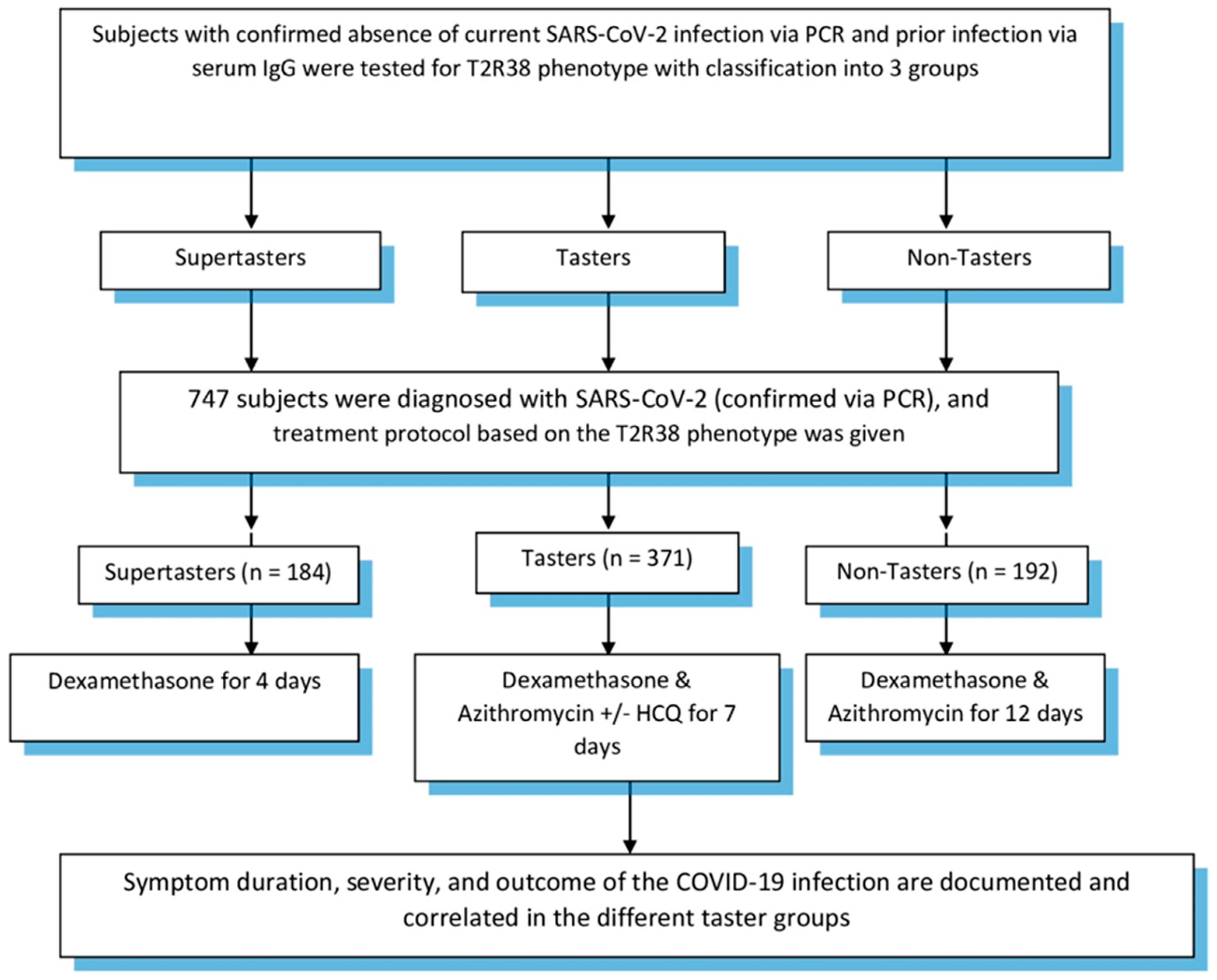
2. Methods
- Control strip
- PTC strip
- Thiourea strip
- Sodium Benzoate strip
3. Exclusions
4. Results
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Barham, H.P.; Taha, M.A.; Hall, C.A. Does phenotypic expression of bitter taste receptor T2R38 show association with COVID-19 severity? Int. Forum Allergy Rhinol. 2020, 10, 1255–1257. [Google Scholar] [CrossRef]
- Bassetti, M.; Vena, A.; Giacobbe, D.R. The novel Chinese coronavirus (2019-nCoV) infections: Challenges for fighting the storm. Eur. J. Clin. Investig. 2020, 50, e13209. [Google Scholar] [CrossRef]
- Parry, J. Wuhan: Britons to be evacuated as scientists estimate 44 000 cases of 2019-nCOV in the city. BMJ 2020, 368, m351. [Google Scholar] [CrossRef]
- Lu, H. Drug treatment options for the 2019-new coronavirus (2019-nCoV). Biosci. Trends 2020, 14, 69–71. [Google Scholar] [CrossRef]
- Zumla, A.; Hui, D.S.; Azhar, E.I.; Memish, Z.A.; Maeurer, M. Reducing mortality from 2019-nCoV: Host-directed therapies should be an option. Lancet 2020, 395, e35–e36. [Google Scholar] [CrossRef]
- Zumla, A.; Chan, J.F.W.; Azhar, E.I.; Hui, D.S.C.; Yuen, K.-Y. Coronaviruses—Drug discovery and therapeutic options. Nat. Rev. Drug Discov. 2016, 15, 327–347. [Google Scholar] [CrossRef] [PubMed]
- Rao, M.; Dodoo, E.; Zumla, A.; Maeurer, M. Immunometabolism and Pulmonary Infections: Implications for Protective Immune Responses and Host-Directed Therapies. Front. Microbiol. 2019, 10, 962. [Google Scholar] [CrossRef]
- Li, D.; Zhang, J. Diet Shapes the Evolution of the Vertebrate Bitter Taste Receptor Gene Repertoire. Mol. Biol. Evol. 2014, 31, 303–309. [Google Scholar] [CrossRef] [PubMed]
- Avau, B.; Depoortere, I. The bitter truth about bitter taste receptors: Beyond sensing bitter in the oral cavity. Acta Physiol. 2015, 216, 407–420. [Google Scholar] [CrossRef]
- Lu, P.; Zhang, C.-H.; Lifshitz, L.M.; Zhuge, R. Extraoral bitter taste receptors in health and disease. J. Gen. Physiol. 2017, 149, 181–197. [Google Scholar] [CrossRef]
- Lee, R.J.; Cohen, N.A. Taste receptors in innate immunity. Cell. Mol. Life Sci. 2015, 72, 217–236. [Google Scholar] [CrossRef] [PubMed]
- Cohen, N.A. The genetics of the bitter taste receptor T2R38 in upper airway innate immunity and implications for chronic rhinosinusitis. Laryngoscope 2017, 127, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.-C.; Chen, Z.-H.; Xue, J.-B.; Zhao, D.-X.; Lu, C.; Li, Y.-H.; Li, S.-M.; Du, Y.-W.; Liu, Q.; Wang, P.; et al. Infection by the parasitic helminth Trichinella spiralis activates a Tas2r-mediated signaling pathway in intestinal tuft cells. Proc. Natl. Acad. Sci. USA 2019, 116, 5564–5569. [Google Scholar] [CrossRef] [PubMed]
- Behrens, M.; Meyerhof, W. Bitter taste receptor research comes of age: From characterization to modulation of TAS2Rs. Semin. Cell Dev. Biol. 2013, 24, 215–221. [Google Scholar] [CrossRef]
- Kim, U.K.; Drayna, D. Genetics of individual differences in bitter taste perception: Lessons from the PTC gene. Clin. Genet. 2004, 67, 275–280. [Google Scholar] [CrossRef]
- Lee, R.J.; Kofonow, J.M.; Rosen, P.L.; Siebert, A.P.; Chen, B.; Doghramji, L.; Xiong, G.; Adappa, N.D.; Palmer, J.N.; Kennedy, D.W.; et al. Bitter and sweet taste receptors regulate human upper respiratory innate immunity. J. Clin. Investig. 2014, 124, 1393–1405. [Google Scholar] [CrossRef]
- Åkerström, S.; Gunalan, V.; Keng, C.T.; Tan, Y.-J.; Mirazimi, A. Dual effect of nitric oxide on SARS-CoV replication: Viral RNA production and palmitoylation of the S protein are affected. Virology 2009, 395, 1–9. [Google Scholar] [CrossRef]
- Bufe, B.; Breslin, P.A.S.; Kuhn, C.; Reed, D.R.; Tharp, C.D.; Slack, J.P.; Kim, U.-K.; Drayna, D.; Meyerhof, W. The molecular basis of individual differences in phenylthiocarbamide and propylthiouracil bitterness perception. Curr. Biol. 2005, 15, 322–327. [Google Scholar] [CrossRef]
- Meyerhof, W.; Batram, C.; Kuhn, C.; Brockhoff, A.; Chudoba, E.; Bufe, B.; Appendino, G.; Behrens, M. The Molecular Receptive Ranges of Human TAS2R Bitter Taste Receptors. Chem. Senses 2010, 35, 157–170. [Google Scholar] [CrossRef]
- Adappa, N.D.; Truesdale, C.M.; Ba, A.D.W.; Rn, L.D.; Mansfield, C.; Kennedy, D.W.; Palmer, J.N.; Cowart, B.J.; Cohen, N.A. Correlation of T2R38 taste phenotype and in vitro biofilm formation from nonpolypoid chronic rhinosinusitis patients. Int. Forum Allergy Rhinol. 2016, 6, 783–791. [Google Scholar] [CrossRef]
- Rom, D.; Christensen, J.; Alvarado, R.; Sacks, R.; Harvey, R. The impact of bitter taste receptor genetics on culturable bacteria in chronic rhinosinusitis. Rhinol. J. 2017, 55, 90–94. [Google Scholar] [CrossRef]
- Farquhar, D.R.; Kovatch, K.J.; Palmer, J.N.; Shofer, F.S.; Adappa, N.D.; Cohen, N.A. Phenylthiocarbamide taste sensitivity is associated with sinonasal symptoms in healthy adults. Int. Forum Allergy Rhinol. 2014, 5, 111–118. [Google Scholar] [CrossRef]
- Workman, A.D.; Brooks, S.G.; Kohanski, M.A.; Blasetti, M.T.; Cowart, B.J.; Mansfield, C.; Kennedy, D.W.; Palmer, J.N.; Adappa, N.D.; Reed, D.R.; et al. Bitter and sweet taste tests are reflective of disease status in chronic rhinosinusitis. J. Allergy Clin. Immunol. Pract. 2018, 6, 1078–1080. [Google Scholar] [CrossRef]
- Douglas, J.E.; Cohen, N.A. Taste Receptors Mediate Sinonasal Immunity and Respiratory Disease. Int. J. Mol. Sci. 2017, 18, 437. [Google Scholar] [CrossRef]
- Kohanski, M.A.; Brown, L.; Orr, M.; Tan, L.H.; Adappa, N.D.; Palmer, J.N.; Rubenstein, R.C.; Cohen, N.A. Bitter taste receptor agonists regulate epithelial two-pore potassium channels via cAMP signaling. Respir. Res. 2021, 22, 31. [Google Scholar] [CrossRef] [PubMed]
- Clark, A.A.; Liggett, S.B.; Munger, S.D. Extraoral bitter taste receptors as mediators of off-target drug effects. FASEB J. 2012, 26, 4827–4831. [Google Scholar] [CrossRef]
- Levit, A.; Nowak, S.; Peters, M.; Wiener, A.; Meyerhof, W.; Behrens, M.; Niv, M.Y. The bitter pill: Clinical drugs that activate the human bitter taste receptor TAS2R14. FASEB J. 2013, 28, 1181–1197. [Google Scholar] [CrossRef] [PubMed]
- Dotson, C.D.; Zhang, L.; Xu, H.; Shin, Y.-K.; Vigues, S.; Ott, S.H.; Elson, A.E.T.; Choi, H.J.; Shaw, H.; Egan, J.M.; et al. Bitter Taste Receptors Influence Glucose Homeostasis. PLoS ONE 2008, 3, e3974. [Google Scholar] [CrossRef] [PubMed]
- Jaggupilli, A.; Singh, N.; De Jesus, V.C.; Gounni, M.S.; Dhanaraj, P.; Chelikani, P. Chemosensory bitter taste receptors (T2Rs) are activated by multiple antibiotics. FASEB J. 2018, 33, 501–517. [Google Scholar] [CrossRef]
- Manson, M.L.; Säfholm, J.; Al-Ameri, M.; Bergman, P.; Orre, A.-C.; Swärd, K.; James, A.; Dahlén, S.-E.; Adner, M. Bitter taste receptor agonists mediate relaxation of human and rodent vascular smooth muscle. Eur. J. Pharmacol. 2014, 740, 302–311. [Google Scholar] [CrossRef]
- Pulkkinen, V.; Manson, M.L.; Säfholm, J.; Adner, M.; Dahlén, S.-E. The bitter taste receptor (TAS2R) agonists denatonium and chloroquine display distinct patterns of relaxation of the guinea pig trachea. Am. J. Physiol. Cell. Mol. Physiol. 2012, 303, L956–L966. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Yi, R.; Nayak, A.P.; Wang, N.; Tang, F.; Knight, M.J.; Pan, S.; Oliver, B.; Deshpande, D.A. Bitter Taste Receptor Agonists Mitigate Features of Allergic Asthma in Mice. Sci. Rep. 2017, 7, srep46166. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Panebra, A.; Pera, T.; Tiegs, B.C.; Hershfeld, A.; Kenyon, L.C.; Deshpande, D.A. Antimitogenic effect of bitter taste receptor agonists on airway smooth muscle cells. Am. J. Physiol. Cell. Mol. Physiol. 2016, 310, L365–L376. [Google Scholar] [CrossRef] [PubMed]
- McAlinden, K.D.; Deshpande, D.A.; Ghavami, S.; Xenaki, D.; Sohal, S.S.; Oliver, B.G.; Haghi, M.; Sharma, P. Autophagy Activation in Asthma Airways Remodeling. Am. J. Respir. Cell Mol. Biol. 2019, 60, 541–553. [Google Scholar] [CrossRef] [PubMed]
- Brockhoff, A.; Behrens, M.; Massarotti, A.; Appendino, G.B.; Meyerhof, W. Broad Tuning of the Human Bitter Taste Receptor hTAS2R46 to Various Sesquiterpene Lactones, Clerodane and Labdane Diterpenoids, Strychnine, and Denatonium. J. Agric. Food Chem. 2007, 55, 6236–6243. [Google Scholar] [CrossRef]
- Hansen, J.L.; Reed, D.R.; Wright, M.J.; Martin, N.G.; Breslin, P.A.S. Heritability and Genetic Covariation of Sensitivity to PROP, SOA, Quinine HCl, and Caffeine. Chem. Senses 2006, 31, 403–413. [Google Scholar] [CrossRef]
- Lee, R.J.; Xiong, G.; Kofonow, J.M.; Chen, B.; Lysenko, A.; Jiang, P.; Abraham, V.; Doghramji, L.; Adappa, N.D.; Palmer, J.N.; et al. T2R38 taste receptor polymorphisms underlie susceptibility to upper respiratory infection. J. Clin. Investig. 2012, 122, 4145–4159. [Google Scholar] [CrossRef] [PubMed]
- Parker, D.; Prince, A. Innate Immunity in the Respiratory Epithelium. Am. J. Respir. Cell Mol. Biol. 2011, 45, 189–201. [Google Scholar] [CrossRef]
- Zhang, Y.; Hoon, M.A.; Chandrashekar, J.; Mueller, K.L.; Cook, B.; Wu, D.; Zuker, C.S.; Ryba, N.J.P. Coding of sweet, bitter, and umami tastes: Different receptor cells sharing similar signaling pathways. Cell 2003, 112, 293e301. [Google Scholar] [CrossRef]
- Iwata, S.; Yoshida, R.; Ninomiya, Y. Taste transductions in taste receptor cells: Basic tastes and moreover. Curr. Pharm. Des. 2014, 20, 2684–2692. [Google Scholar] [CrossRef]
- Sollai, G.; Melis, M.; Pani, D.; Cosseddu, P.; Usai, I.; Crnjar, R.; Bonfiglio, A.; Barbarossa, I.T. First objective evaluation of taste sensitivity to 6-n-propylthiouracil (PROP), a paradigm gustatory stimulus in humans. Sci. Rep. 2017, 7, 40353. [Google Scholar] [CrossRef]
- Salathe, M. Regulation of Mammalian Ciliary Beating. Annu. Rev. Physiol. 2007, 69, 401–422. [Google Scholar] [CrossRef]
- Hariri, B.M.; McMahon, D.B.; Chen, B.; Freund, J.R.; Mansfield, C.J.; Doghramji, L.J.; Adappa, N.D.; Palmer, J.N.; Kennedy, D.W.; Reed, D.R.; et al. Flavones modulate respiratory epithelial innate immunity: Anti-inflammatory effects and activation of the T2R14 receptor. J. Biol. Chem. 2017, 292, 8484–8497. [Google Scholar] [CrossRef] [PubMed]
- Yan, C.H.; Hahn, S.; McMahon, D.; Bonislawski, D.; Kennedy, D.W.; Adappa, N.D.; Palmer, J.N.; Jiang, P.; Lee, R.J.; Cohen, N.A. Nitric Oxide Production is Stimulated by Bitter Taste Receptors Ubiquitously Expressed in the Sinonasal Cavity. Am. J. Rhinol. Allergy 2017, 31, 85–92. [Google Scholar] [CrossRef]
- Hume, D.A.; Underhill, D.M.; Sweet, M.J.; Ozinsky, A.O.; Liew, F.Y.; Aderem, A. Macrophages exposed continuously to lipopolysaccharide and other agonists that act via toll-like receptors exhibit a sustained and additive activation state. BMC Immunol. 2001, 2, 11. [Google Scholar] [CrossRef] [PubMed]
- Culic, O.; Erakovic, V.; Parnham, M.J. Anti-inflammatory effects of macrolide antibiotics. Eur. J. Pharmacol. 2001, 429, 209–229. [Google Scholar] [CrossRef]
- Good, J.T.; Rollins, D.R.; Martin, R.J. Macrolides in the treatment of asthma. Curr. Opin. Pulm. Med. 2012, 18, 76–84. [Google Scholar] [CrossRef] [PubMed]
- Rollins, D.R.; Beuther, D.A.; Martin, R.J. Update on Infection and Antibiotics in Asthma. Curr. Allergy Asthma Rep. 2010, 10, 67–73. [Google Scholar] [CrossRef]
- Gao, X.; Ray, R.; Xiao, Y.; Ishida, K.; Ray, P. Macrolide antibiotics improve chemotactic and phagocytic capacity as well as reduce inflammation in sulfur mustard-exposed monocytes. Pulm. Pharmacol. Ther. 2010, 23, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Sleigh, M.A.; Blake, J.R.; Liron, N. The propulsion of mucus by cilia. Am. Rev. Respir. Dis. 1988, 137, 726e741. [Google Scholar] [CrossRef]
- Kato, A.; Schleimer, R.P. Beyond inflammation: Airway epithelial cells are at the interface of innate and adaptive immunity. Curr. Opin. Immunol. 2007, 19, 711–720. [Google Scholar] [CrossRef]
- Patel, N.N.; Kohanski, M.A.; Maina, I.W.; Bs, V.T.; Bs, A.D.W.; Tong, C.C.; Kuan, E.C.; Bosso, J.V.; Adappa, N.D.; Palmer, J.N.; et al. Solitary chemosensory cells producing interleukin-25 and group-2 innate lymphoid cells are enriched in chronic rhinosinusitis with nasal polyps. Int. Forum Allergy Rhinol. 2018, 8, 900–906. [Google Scholar] [CrossRef]
- Kohanski, M.A.; Workman, A.D.; Patel, N.N.; Hung, L.-Y.; Shtraks, J.P.; Chen, B.; Blasetti, M.; Doghramji, L.; Kennedy, D.W.; Adappa, N.D.; et al. Solitary chemosensory cells are a primary epithelial source of IL-25 in patients with chronic rhinosinusitis with nasal polyps. J. Allergy Clin. Immunol. 2018, 142, 460–469.e7. [Google Scholar] [CrossRef] [PubMed]
- Deshpande, D.A.; Wang, W.C.H.; McIlmoyle, E.L.; Robinett, K.S.; Schillinger, R.M.; An, S.S.; Sham, J.S.K.; Liggett, S.B. Bitter taste receptors on airway smooth muscle bronchodilate by localized calcium signaling and reverse obstruction. Nat. Med. 2010, 16, 1299–1304. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.S.; Ben-Shahar, Y.; Moninger, T.O.; Kline, J.N.; Welsh, M.J. Motile Cilia of Human Airway Epithelia Are Chemosensory. Science 2009, 325, 1131–1134. [Google Scholar] [CrossRef] [PubMed]
- Grassin-Delyle, S.; Abrial, C.; Brollo, M.; Fayad-Kobeissi, S.; Naline, E.; Devillier, P. Characterization of the expression and the role of bitter taste receptors in human lung parenchyma and macrophages. Am. J. Respir. Crit. Care Med. 2014, 189, A5749. [Google Scholar]
- Upadhyaya, J.D.; Singh, N.; Sikarwar, A.S.; Chakraborty, R.; Pydi, S.P.; Bhullar, R.P.; Dakshinamurti, S.; Chelikani, P. Dextromethorphan mediated bitter taste receptor activation in the pulmonary circuit causes vasoconstriction. PLoS ONE 2014, 9, e110373. [Google Scholar] [CrossRef] [PubMed]
- Kaufman, A.C.; Colquitt, L.; Ruckenstein, M.J.; Bigelow, D.C.; Eliades, S.J.; Xiong, G.; Lin, C.; Reed, D.R.; Cohen, N.A. Bitter Taste Receptors and Chronic Otitis Media. Otolaryngol. Neck Surg. 2021, 12. [Google Scholar] [CrossRef]
- Florindo, H.F.; Kleiner, R.; Vaskovich-Koubi, D.; Acúrcio, R.C.; Carreira, B.; Yeini, E.; Tiram, G.; Liubomirski, Y.; Satchi-Fainaro, R. Immune-mediated approaches against COVID-19. Nat. Nanotechnol. 2020, 15, 630–645. [Google Scholar] [CrossRef]
- Savarino, A.; Boelaert, J.R.; Cassone, A.; Majori, G.; Cauda, R. Effects of chloroquine on viral infections: An old drug against today’s diseases. Lancet Infect. Dis. 2003, 3, 722–727. [Google Scholar] [CrossRef]
- Vincent, M.J.; Bergeron, E.; Benjannet, S.; Erickson, B.R.; Rollin, P.E.; Ksiazek, T.G.; Seidah, N.G.; Nichol, S.T. Chloroquine is a potent inhibitor of SARS coronavirus infection and spread. Virol. J. 2005, 2, 69. [Google Scholar] [CrossRef]
- Mennella, J.A.; Pepino, M.Y.; Duke, F.F.; Reed, D.R. Age modifies the genotype-phenotype relationship for the bitter receptor TAS2R38. BMC Genet. 2010, 11, 60–69. [Google Scholar] [CrossRef]
- Mennella, J.A.; Reed, D.R.; Roberts, K.M.; Mathew, P.S.; Mansfield, C.J. Age-Related Differences in Bitter Taste and Efficacy of Bitter Blockers. PLoS ONE 2014, 9, e103107. [Google Scholar] [CrossRef]
- Whissell-Buechy, D. Effects of age and sex on taste sensitivity to phenylthiocarbamide (PTC) in the Berkeley Guidance sample. Chem. Senses 1990, 15, 39–57. [Google Scholar] [CrossRef]
- Whissell-Buechy, D.; Wills, C. Male and female correlations for taster (P.T.C.) phenotypes and rate of adolescent development. Ann. Hum. Biol. 1989, 16, 131–146. [Google Scholar] [CrossRef] [PubMed]
- Caly, L.; Druce, J.D.; Catton, M.G.; Jans, D.A.; Wagstaff, K.M. The FDAapproved Drug Ivermectin inhibits the replication of SARS-CoV-2 in vitro. Antivir. Res. 2020, 178, 104787. [Google Scholar] [CrossRef] [PubMed]
| COVID-19 Patients (n = 747) | ||||
|---|---|---|---|---|
| Variable | All | Supertasters | Tasters | Nontasters |
| Number | 747 | 184 | 371 | 192 |
| Age | 46.3 (13.7) | 41.1 (11.0) | 46.5 (13.7) | 50.2 (15.8) |
| Sex (F) | 423 (56.6%) | 122 (66.3%) | 189 (50.9%) | 112 (58.3%) |
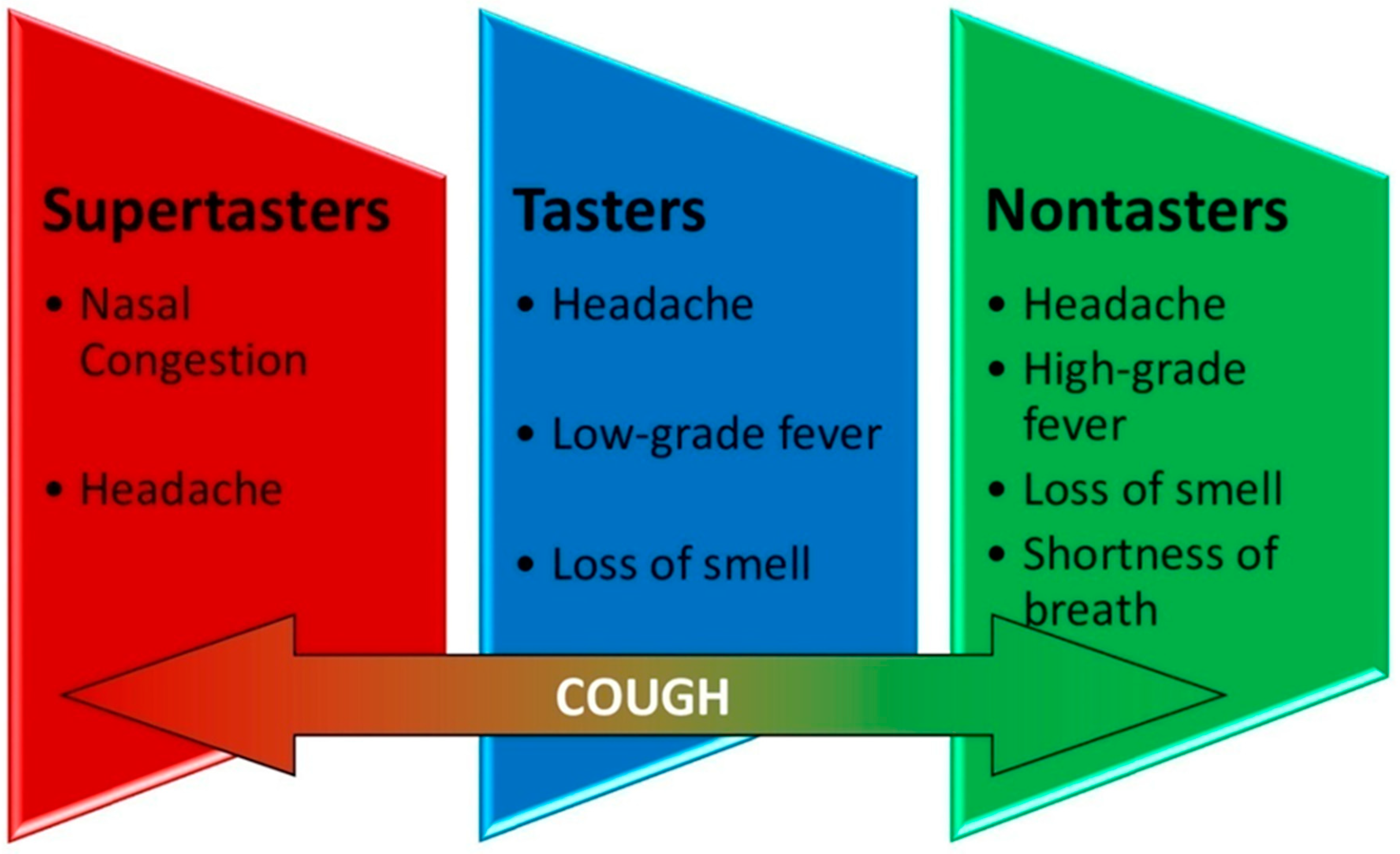
| Duration of symptoms | 5 | 8.1 | 16.2 | |
| Most common symptoms | Nasal congestion Headache | Low-grade fever Headache Loss of smell | High-grade fever Headache Loss of smell Shortness of breath | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Taha, M.A.; Hall, C.A.; Shortess, C.J.; Rathbone, R.F.; Barham, H.P. Treatment Protocol for COVID-19 Based on T2R Phenotype. Viruses 2021, 13, 503. https://doi.org/10.3390/v13030503
Taha MA, Hall CA, Shortess CJ, Rathbone RF, Barham HP. Treatment Protocol for COVID-19 Based on T2R Phenotype. Viruses. 2021; 13(3):503. https://doi.org/10.3390/v13030503
Chicago/Turabian StyleTaha, Mohamed A., Christian A. Hall, Colin J. Shortess, Richard F. Rathbone, and Henry P. Barham. 2021. "Treatment Protocol for COVID-19 Based on T2R Phenotype" Viruses 13, no. 3: 503. https://doi.org/10.3390/v13030503
APA StyleTaha, M. A., Hall, C. A., Shortess, C. J., Rathbone, R. F., & Barham, H. P. (2021). Treatment Protocol for COVID-19 Based on T2R Phenotype. Viruses, 13(3), 503. https://doi.org/10.3390/v13030503






