Antibody Responses in Cats Following Primary and Annual Vaccination against Feline Immunodeficiency Virus (FIV) with an Inactivated Whole-Virus Vaccine (Fel-O-Vax® FIV)
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Populations
2.2. Vaccination
2.3. Determination of FIV Infection Status
2.4. Sampling Procedure
2.5. Evaluation of Antibodies to p24 Using a Microsphere Immunoassay (MIA)
2.6. Evaluation of Antibodies to gp40 Using a Laboratory ELISA
2.7. ‘Positive’ and ‘False-Positive’ Terminology for Antibody Test Results
2.8. Ethics Approval
2.9. Statistical Analysis
3. Results
3.1. Sample Populations
3.2. FIV Point-of-Care Testing (Primary Vaccination)
3.3. FIV Point-of-Care Testing (Annual Vaccination)
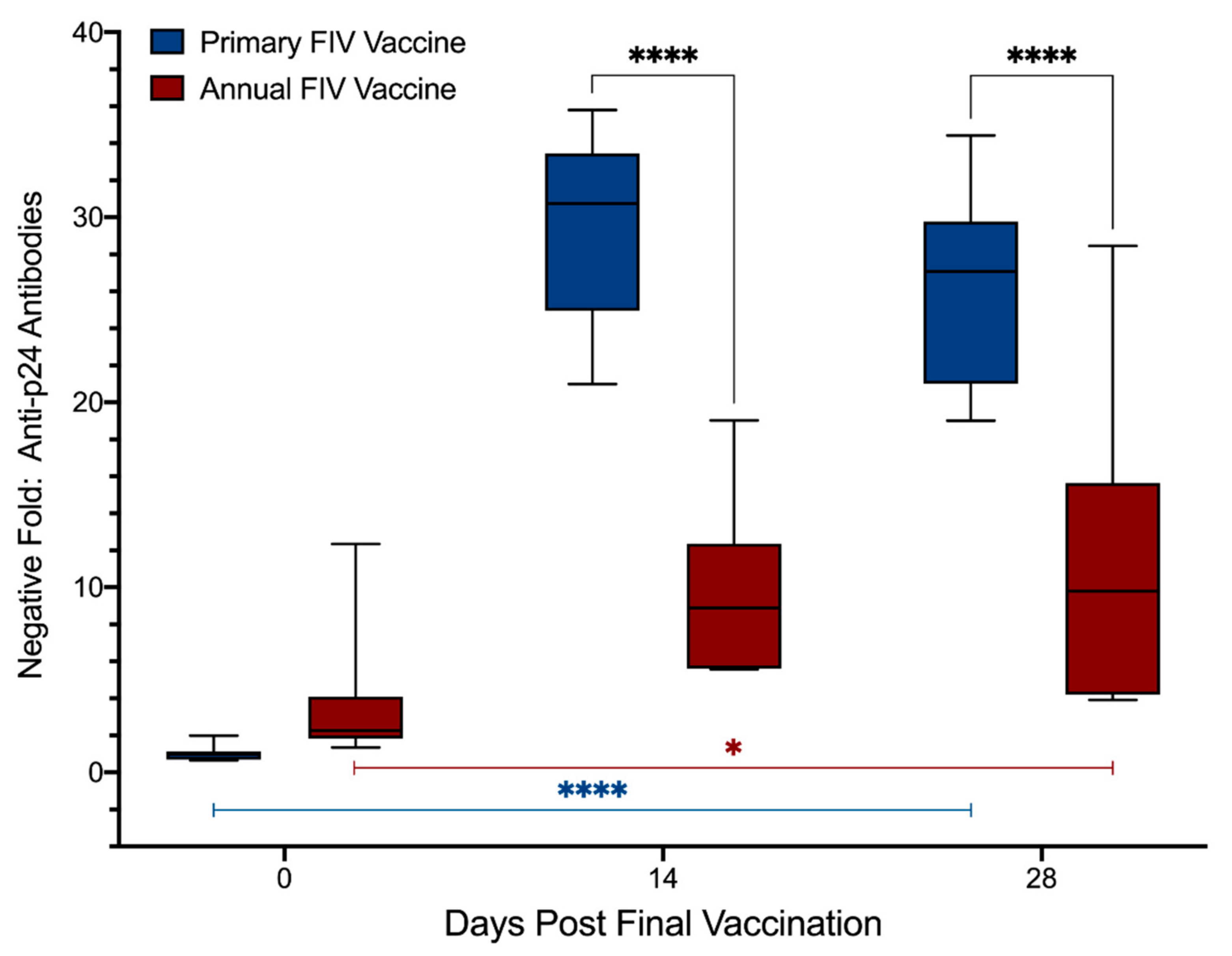
3.4. Antibodies to FIV p24 with MIA Testing
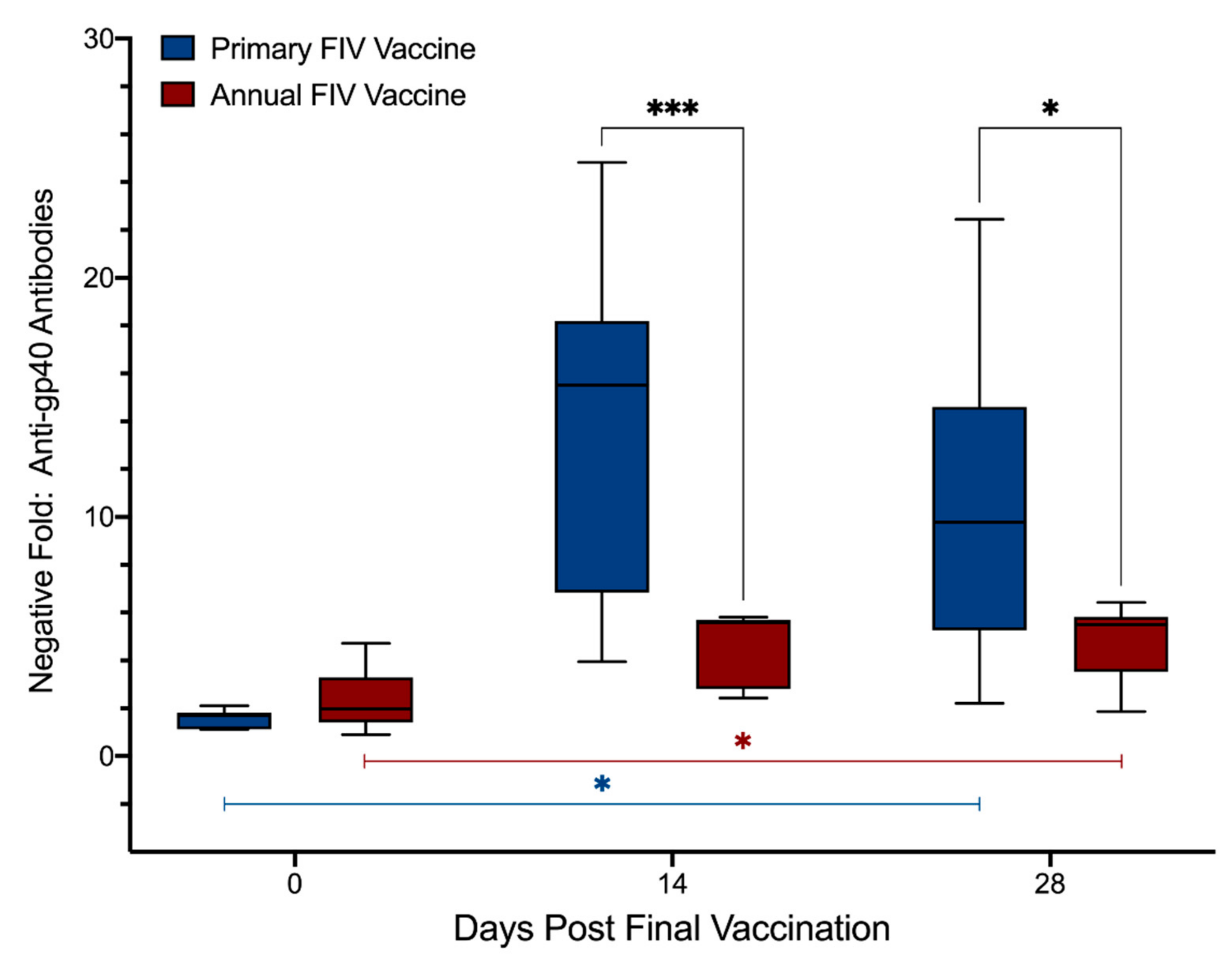
3.5. Antibodies to FIV gp40 with ELISA Testing
3.6. Comparing anti-p24 and anti-gp40 Antibody Responses to Number of Annual FIV Vaccinations
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yamamoto, J.K.; Pu, R.Y.; Sato, E.; Hohdatsu, T. Feline immunodeficiency virus pathogenesis and development of a dual-subtype feline-immunodeficiency-virus vaccine. AIDS 2007, 21, 547–563. [Google Scholar] [CrossRef] [PubMed]
- VandeWoude, S.; Apetrei, C. Going wild: Lessons from naturally occurring T-lymphotropic lentiviruses. Clin. Microbiol. Rev. 2006, 19, 728–762. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, N.; Ho, E.; Brown, M.; Yamamoto, J. Isolation of a T-lymphotropic virus from domestic cats with an immunodeficiency-like syndrome. Science 1987, 235, 790–793. [Google Scholar] [CrossRef] [PubMed]
- Bienzle, D. FIV in cats—A useful model of HIV in people? Vet. Immunol. Immunopathol. 2014, 159, 171–179. [Google Scholar] [CrossRef]
- White, J.; Stickney, A.; Norris, J.M. Feline immunodeficiency virus: Disease association versus causation in domestic and nondomestic felids. Vet. Clin. North Am. Small Anim. Pract. 2011, 41, 1197–1208. [Google Scholar] [CrossRef] [PubMed]
- Sahay, B.; Yamamoto, J.K. Lessons learned in developing a commercial FIV vaccine: The immunity required for an effective HIV-1 vaccine. Viruses 2018, 10, 277. [Google Scholar] [CrossRef] [PubMed]
- Dunham, S.P. Lessons from the cat: Development of vaccines against lentiviruses. Vet. Immunol. Immunopathol. 2006, 112, 67–77. [Google Scholar] [CrossRef] [PubMed]
- Uhl, E.W.; Martin, M.; Coleman, J.K.; Yamamoto, J.K. Advances in FIV vaccine technology. Vet. Immunol. Immunopathol. 2008, 123, 65–80. [Google Scholar] [CrossRef] [PubMed]
- Levy, J.K.; Crawford, P.C.; Kusuhara, H.; Motokawa, K.; Gemma, T.; Watanabe, R.; Arai, S.; Bienzle, D.; Hohdatsu, T. Differentiation of feline immunodeficiency virus vaccination, infection, or vaccination and infection in cats. J. Vet. Intern. Med. 2008, 22, 330–334. [Google Scholar] [CrossRef] [PubMed]
- Litster, A.; Lin, J.-M.; Nichols, J.; Weng, H.-Y. Diagnostic utility of CD4%:CD8(low)% T-lymphocyte ratio to differentiate feline immunodeficiency virus (FIV)-infected from FIV-vaccinated cats. Vet. Microbiol. 2014, 170, 197–205. [Google Scholar] [CrossRef]
- Westman, M.E.; Malik, R.; Hall, E.; Sheehy, P.A.; Norris, J.M. Determining the feline immunodeficiency virus (FIV) status of FIV-vaccinated cats using point-of-care antibody kits. Comp. Immun. Microbiol. Infect. Dis. 2015, 42, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Arora, D.R.; Maheshwari, M.; Arora, B. Rapid point-of-care testing for detection of HIV and clinical monitoring. ISRN AIDS 2013, 2013, 287269. [Google Scholar] [CrossRef]
- Dewsnap, C.H.; Mcowan, A. A review of HIV point-of-care tests. Int. J. STD AIDS 2006, 17, 357–359. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, J.K.; Sanou, M.P.; Abbott, J.R.; Coleman, J.K. Feline immunodeficiency virus model for designing HIV/AIDS vaccines. Curr. HIV Res. 2010, 8, 14–25. [Google Scholar] [CrossRef] [PubMed]
- Uhl, E.W.; Heaton-Jones, T.G.; Pu, R.; Yamamoto, J.K. FIV vaccine development and its importance to veterinary and human medicine: FIV vaccine 2002 update and review. Vet. Immunol. Immunopathol. 2002, 90, 113–132. [Google Scholar] [CrossRef]
- Crawford, P.C.; Levy, J.K. New challenges for the diagnosis of feline immunodeficiency virus infection. Vet. Clin. North Am. Small Anim. Pract. 2007, 37, 335–350. [Google Scholar] [CrossRef] [PubMed]
- Levy, J.; Crawford, C.; Hartmann, K.; Hofmann-Lehmann, R.; Little, S.; Sundahl, E.; Thayer, V. 2008 American Association of Feline Practitioners’ feline retrovirus management guidelines. J. Feline Med. Surg. 2008, 10, 300–316. [Google Scholar] [CrossRef]
- Available online: http://www.wsava.org/sites/default/files/VaccinationGuidelines2010.pdf (accessed on 11 February 2016).
- Westman, M.E.; Malik, R.; Hall, E.; Norris, J.M. Diagnosing feline immunodeficiency virus (FIV) infection in FIV-vaccinated and FIV-unvaccinated cats using saliva. Comp. Immun. Microbiol. Infect. Dis. 2016, 46, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Crawford, C. Does a DIVA test exist for differentiating FIV infection from FIV vaccination? (2016 ACVIM Forum Research Abstract Program). J. Vet. Intern. Med. 2016, 30, 1475. [Google Scholar] [CrossRef]
- Westman, M.E.; Malik, R.; Hall, E.; Harris, M.; Hosie, M.J.; Sheehy, P.A.; Norris, J.M. Duration of antibody response following vaccination against feline immunodeficiency virus. J. Feline Med. Surg. 2017, 19, 1055–1064. [Google Scholar] [CrossRef]
- Available online: http://www.wsava.org/sites/default/files/WSAVA%20Vaccination%20Guidelines%202015%20Full%20Version.pdf (accessed on 25 April 2016).
- Hartmann, K.; Griessmayr, P.; Schulz, B.; Greene, C.E.; Vidyashankar, A.N.; Jarrett, O.; Egberink, H.F. Quality of different in-clinic test systems for feline immunodeficiency virus and feline leukaemia virus infection. J. Feline Med. Surg. 2007, 9, 439–445. [Google Scholar] [CrossRef] [PubMed]
- Levy, J.K.; Crawford, P.C.; Tucker, S.J. Performance of 4 point-of-care screening tests for feline leukemia virus and feline immunodeficiency virus. J. Vet. Intern. Med. 2017, 31, 521–526. [Google Scholar] [CrossRef] [PubMed]
- Wood, B.A.; Carver, S.; Troyer, R.M.; Elder, J.H.; VandeWoude, S. Domestic cat microsphere immunoassays: Detection of antibodies during feline immunodeficiency virus infection. J. Immunol. Methods 2013, 396, 74–86. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Miller, C.; Emanuelli, M.; Fink, E.; Musselman, E.; Mackie, R.; Troyer, R.; Elder, J.; VandeWoude, S. FIV vaccine with receptor epitopes results in neutralizing antibodies but does not confer resistance to challenge. NPJ Vaccines 2018, 3. [Google Scholar] [CrossRef] [PubMed]
- Wood, B.A.; O’Halloran, K.P.; VandeWoude, S. Development and validation of a multiplex microsphere-based assay for detection of domestic cat (Felis catus) cytokines. Clin. Vaccine Immunol. 2011, 18, 387–392. [Google Scholar] [CrossRef][Green Version]
- Harris, M. Studies towards the Development of an FIV DIVA Vaccine. Ph.D. Thesis, University of Glasgow, Scotland, UK, 2018. [Google Scholar]
- Classen, D.C.; Morningstar, J.M.; Shanley, J.D. Detection of antibody to murine cytomegalovirus by enzyme-linked immunosorbent and indirect immunofluorescence assays. J. Clin. Microbiol. 1987, 25, 600–604. [Google Scholar] [CrossRef]
- Avrameas, A.; Strosberg, A.; Moraillon, A.; Sonigo, P.; Pancino, G. Serological diagnosis of feline immunodeficiency virus infection based on synthetic peptides from Env glycoproteins. Res. Virol. 1993, 144, 209–218. [Google Scholar] [CrossRef]
- Lecollinet, S.; Richardson, J. Vaccination against the feline immunodeficiency virus: The road not taken. Comp. Immun. Microbiol. Infect. Dis. 2008, 31, 167–190. [Google Scholar] [CrossRef]
- Kusuhara, H.; Hohdatsu, T.; Seta, T.; Nemoto, K.; Motokawa, K.; Gemma, T.; Watanabe, R.; Huang, C.; Arai, S.; Koyama, H. Serological differentiation of FIV-infected cats from dual-subtype feline immunodeficiency virus vaccine (Fel-O-Vax FIV) inoculated cats. Vet. Microbiol. 2007, 120, 217–225. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Conlee, D.; Loop, J.; Champ, D.; Gill, M.; Chu, H.-J. Efficacy and safety of a feline immunodeficiency virus vaccine. An. Health Res. Rev. 2004, 5, 295–300. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Conlee, D.; Gill, M.; Chu, H.-J. Dual-subtype feline immunodeficiency virus vaccine provides 12 months of protective immunity against heterologous challenge. J. Feline Med. Surg. 2010, 12, 451–457. [Google Scholar] [CrossRef] [PubMed]
- Kusuhara, H.; Hohdatsu, T.; Okumura, M.; Sato, K.; Suzuki, Y.; Motokawa, K.; Gemma, T.; Watanabe, R.; Huang, C.J.; Arai, S.; et al. Dual-subtype vaccine (Fel-O-Vax FIV) protects cats against contact challenge with heterologous subtype B FIV infected cats. Vet. Microbiol. 2005, 108, 155–165. [Google Scholar] [CrossRef]
- Hosie, M.J.; Pajek, D.; Samman, A.; Willett, B.J. Feline immunodeficiency virus (FIV) neutralization: A review. Viruses 2011, 3, 1870–1890. [Google Scholar] [CrossRef]
- Coleman, J.K.; Pu, R.; Martin, M.M.; Noon-Song, E.N.; Zwijnenberg, R.; Yamamoto, J.K. Feline immunodeficiency virus (FIV) vaccine efficacy and FIV neutralizing antibodies. Vaccine 2014, 32, 746–754. [Google Scholar] [CrossRef] [PubMed]
- Omori, M.; Pu, R.; Tanabe, T.; Hou, W.; Coleman, J.K.; Arai, M.; Yamamoto, J.K. Cellular immune responses to feline immunodeficiency virus (FIV) induced by dual-subtype FIV vaccine. Vaccine 2004, 23, 386–398. [Google Scholar] [CrossRef]
- Heaton, P.R.; Blount, D.G.; Mann, S.J.; Devlin, P.; Koelsch, S.; Smith, B.H.E.; Stevenson, J.; Harper, E.J.; Rawlings, J.M. Assessing age-related changes in peripheral blood leukocyte phenotypes in domestic shorthaired cats using flow cytometry. J. Nutr. 2002, 132, 1607S–1609S. [Google Scholar] [CrossRef]
- Campbell, D.J.; Rawlings, J.M.; Koelsch, S.; Wallace, J.; Strain, J.J.; Hannigan, B.M. Age-related differences in parameters of feline immune status. Vet. Immunol. Immunopathol. 2004, 100, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Roos-van Eijndhoven, D.G.; Cools, H.J.M.; Westendorp, R.G.J.; Ten Cate-Hoek, A.J.; Knook, D.L.; Remarque, E.J. Randomized controlled trial of seroresponses to double dose and booster influenza vaccination in frail elderly subjects. J. Med. Virol. 2001, 63, 293–298. [Google Scholar] [CrossRef]
- Boenzli, E.; Hadorn, M.; Hartnack, S.; Huder, J.; Hofmann-Lehmann, R.; Lutz, H. Detection of antibodies to the feline leukemia virus (FeLV) transmembrane protein p15E: An alternative approach for serological FeLV detection based on antibodies to p15E. J. Clin. Microbiol. 2014, 52, 2046–2052. [Google Scholar] [CrossRef] [PubMed]
- Lappin, M.R.; Veir, J.; Hawley, J. Feline panleukopenia virus, feline herpesvirus-1, and feline calicivirus antibody responses in seronegative specific pathogen-free cats after a single administration of two different modified live FVRCP vaccines. J. Feline Med. Surg. 2009, 14, 161–164. [Google Scholar] [CrossRef]
- Sebring, R.; Chu, H.; Chavez, L.; Sandblom, D.; Hustead, D.; Dale, B.; Wolf, D.; Acree, W. Feline leukemia virus vaccine development. J. Am. Vet. Med. Assoc. 1991, 199, 1413–1419. [Google Scholar]
- McElhaney, J.E.; Hooton, J.W.; Hooton, N.; Bleackley, R.C. Comparison of single versus booster dose of influenza vaccination on humoral and cellular immune responses in older adults. Vaccine 2005, 23, 3294–3300. [Google Scholar] [CrossRef] [PubMed]
- Bergmann, M.; Schwertler, S.; Reese, S.; Speck, S.; Truyen, U.; Hartmann, K. Antibody response to feline panleukopenia virus vaccination in healthy adult cats. J. Feline Med. Surg. 2018, 20, 1087–1093. [Google Scholar] [CrossRef] [PubMed]
- DiGangi, B.A.; Levy, J.K.; Griffin, B.; Reese, M.J.; Dingman, P.A.; Tucker, S.J.; Dubovi, E.J. Effects of maternally-derived antibodies on serologic responses to vaccination in kittens. J. Feline Med. Surg. 2012, 14, 118–123. [Google Scholar] [CrossRef]
| Day 0 | Day 14 | Day 28 | Day 42 | Day 56 | Day 70 * | Day 84 † | Day 238 | |
| Primary vaccination study (n = 11) | ||||||||
| FIV vaccination | × | × | × | |||||
| PoC antibody testing | × | × | × | × | × | × | × | × |
| MIA p24/ELISA gp40 testing | × | (×) | (×) | (×) | (×) | × | × | (×) |
| PCR testing | × | × | ||||||
| Day 0 | Day 14 * | Day 28 † | Day 42 | |||||
| Annual vaccination study (n = 10) | ||||||||
| FIV vaccination | × | |||||||
| PoC antibody testing | × | × | × | × | ||||
| MIA p24/ELISA gp40 testing | × | × | × | (×) | ||||
| PCR testing | × | × |
| Group | Age (Years) | Sex | Breed | p Value |
|---|---|---|---|---|
| Primary Vaccination | 0.3–7.6 (median 1.5; IQR 0.5–2.6) | 6M, 5F | 1P, 10NP | p = 0.005 (age) p = 0.36 (sex) p = 1.00 (breed) |
| Annual Vaccination | 1.3–10.8 (median 6.0; IQR 2.6–8.9) | 8M, 2F | 10NP |
| Day 0 | Day 14 | Day 28 | Day 42 | Day 56 | Day 70 | Day 84 | Day 238 | |
| Primary vaccination study (n = 11) | VACC | VACC | VACC | |||||
| Witness® | 0/11 | 2/11 | 6/11 | 7/11 | 6/11 | 6/11 | 2/11 | 0/11 |
| Anigen Rapid® | 0/11 | 0/11 | 1/11 | 6/11 | 5/11 | 4/11 | 2/11 | 0/11 |
| SNAP® Combo | 0/11 | 7/11 | 11/11 | 11/11 | 11/11 | 11/11 | 11/11 | 11/11 |
| VETSCAN® Rapid | 0/11 | 0/11 | 3/11 | 11/11 | 11/11 | 11/11 | 11/11 | 9/11 |
| PCR testing (FIV RealPCRTM) | 0/11 | 0/11 | ||||||
| Day 0 | Day 14 | Day 28 | Day 42 | |||||
| Annual vaccination study (n = 10) | VACC | |||||||
| Witness® | 0/10 | 0/10 | 0/10 | 0/10 | ||||
| Anigen Rapid® | 0/10 | 0/10 | 0/10 | 0/10 | ||||
| SNAP® Combo | 10/10 | 7/7 | 6/6 | 7/7 | ||||
| VETSCAN® Rapid | NP | NP | NP | NP | ||||
| PCR testing (FIV RealPCRTM) | 0/10 | 0/10 |
| Anti-p24 Antibodies (MIA) 12 Months Post-Vaccination | Anti-gp40 Antibodies (ELISA) 12 Months Post-Vaccination | No. of Annual FIV Vaccinations (Prior to Enrollment) |
|---|---|---|
| − | − | 0 |
| − | + | 1 |
| + | − | 1 |
| − | − | 2 |
| + | − | 2 |
| + | − | 2 |
| − | + | 2 |
| + | + | 2 |
| + | − | 3 |
| + | + | 7 |
| 6/10 cats | 4/10 cats |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Westman, M.; Yang, D.; Green, J.; Norris, J.; Malik, R.; Parr, Y.A.; McDonald, M.; Hosie, M.J.; VandeWoude, S.; Miller, C. Antibody Responses in Cats Following Primary and Annual Vaccination against Feline Immunodeficiency Virus (FIV) with an Inactivated Whole-Virus Vaccine (Fel-O-Vax® FIV). Viruses 2021, 13, 470. https://doi.org/10.3390/v13030470
Westman M, Yang D, Green J, Norris J, Malik R, Parr YA, McDonald M, Hosie MJ, VandeWoude S, Miller C. Antibody Responses in Cats Following Primary and Annual Vaccination against Feline Immunodeficiency Virus (FIV) with an Inactivated Whole-Virus Vaccine (Fel-O-Vax® FIV). Viruses. 2021; 13(3):470. https://doi.org/10.3390/v13030470
Chicago/Turabian StyleWestman, Mark, Dennis Yang, Jennifer Green, Jacqueline Norris, Richard Malik, Yasmin A. Parr, Mike McDonald, Margaret J. Hosie, Sue VandeWoude, and Craig Miller. 2021. "Antibody Responses in Cats Following Primary and Annual Vaccination against Feline Immunodeficiency Virus (FIV) with an Inactivated Whole-Virus Vaccine (Fel-O-Vax® FIV)" Viruses 13, no. 3: 470. https://doi.org/10.3390/v13030470
APA StyleWestman, M., Yang, D., Green, J., Norris, J., Malik, R., Parr, Y. A., McDonald, M., Hosie, M. J., VandeWoude, S., & Miller, C. (2021). Antibody Responses in Cats Following Primary and Annual Vaccination against Feline Immunodeficiency Virus (FIV) with an Inactivated Whole-Virus Vaccine (Fel-O-Vax® FIV). Viruses, 13(3), 470. https://doi.org/10.3390/v13030470








