Characterization of Signal Sequences Determining the Nuclear/Nucleolar Import and Nuclear Export of the Caprine Arthritis-Encephalitis Virus Rev Protein
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Cultures and Transfection
2.2. Plasmid Constructs
2.3. Microscopy Analysis
2.4. CAT Assay
2.5. Immunoblotting
2.6. Rev(1.4)-EGFP Nuclear Export Assay
2.7. Statistics
3. Results
3.1. The CAEV Rev Protein Localizes to the Cytoplasm, Nucleus and Nucleoli of Transfected Cells
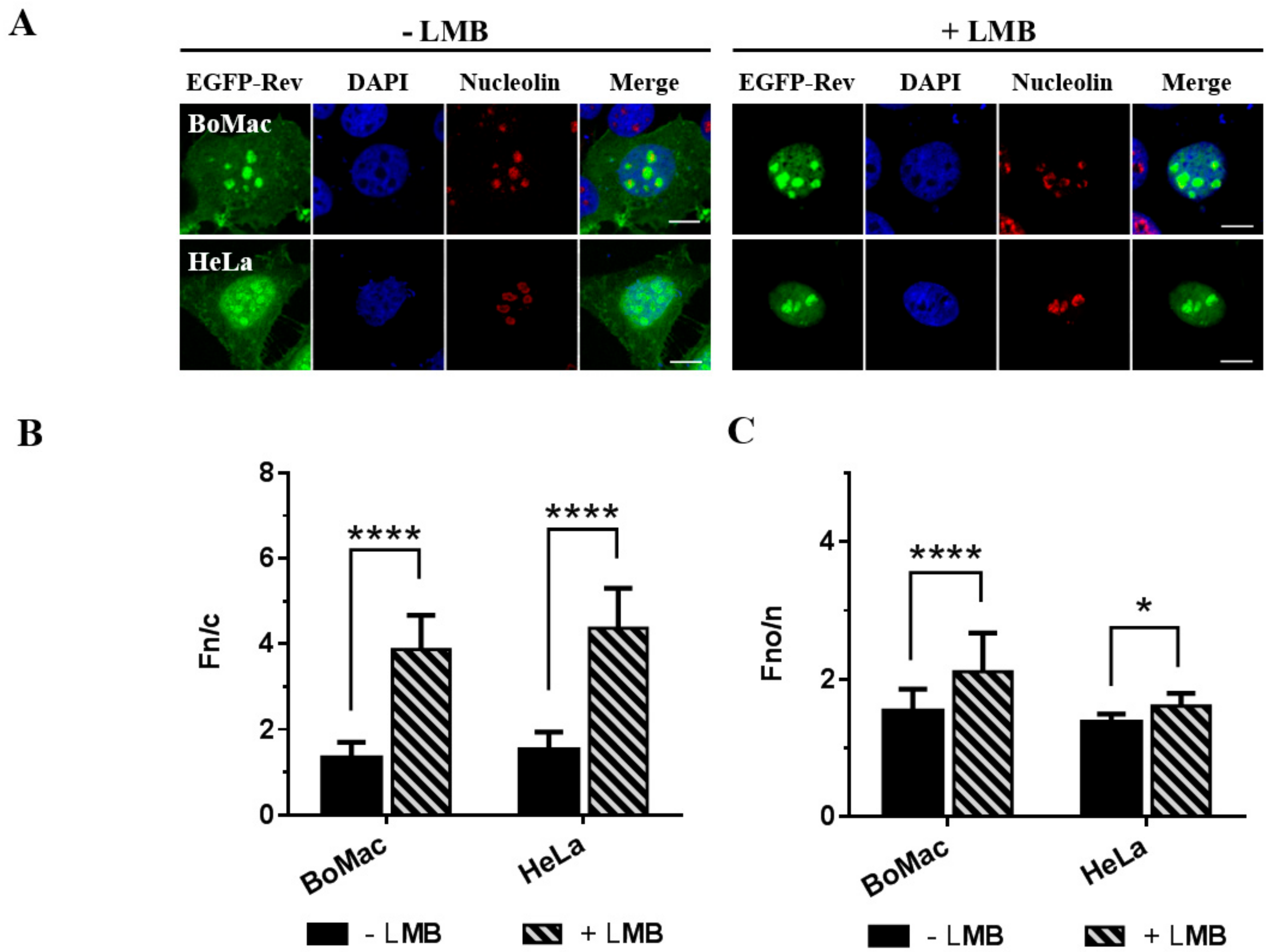
3.2. Leptomycin B Blocks the Nuclear Export of the CAEV Rev Protein
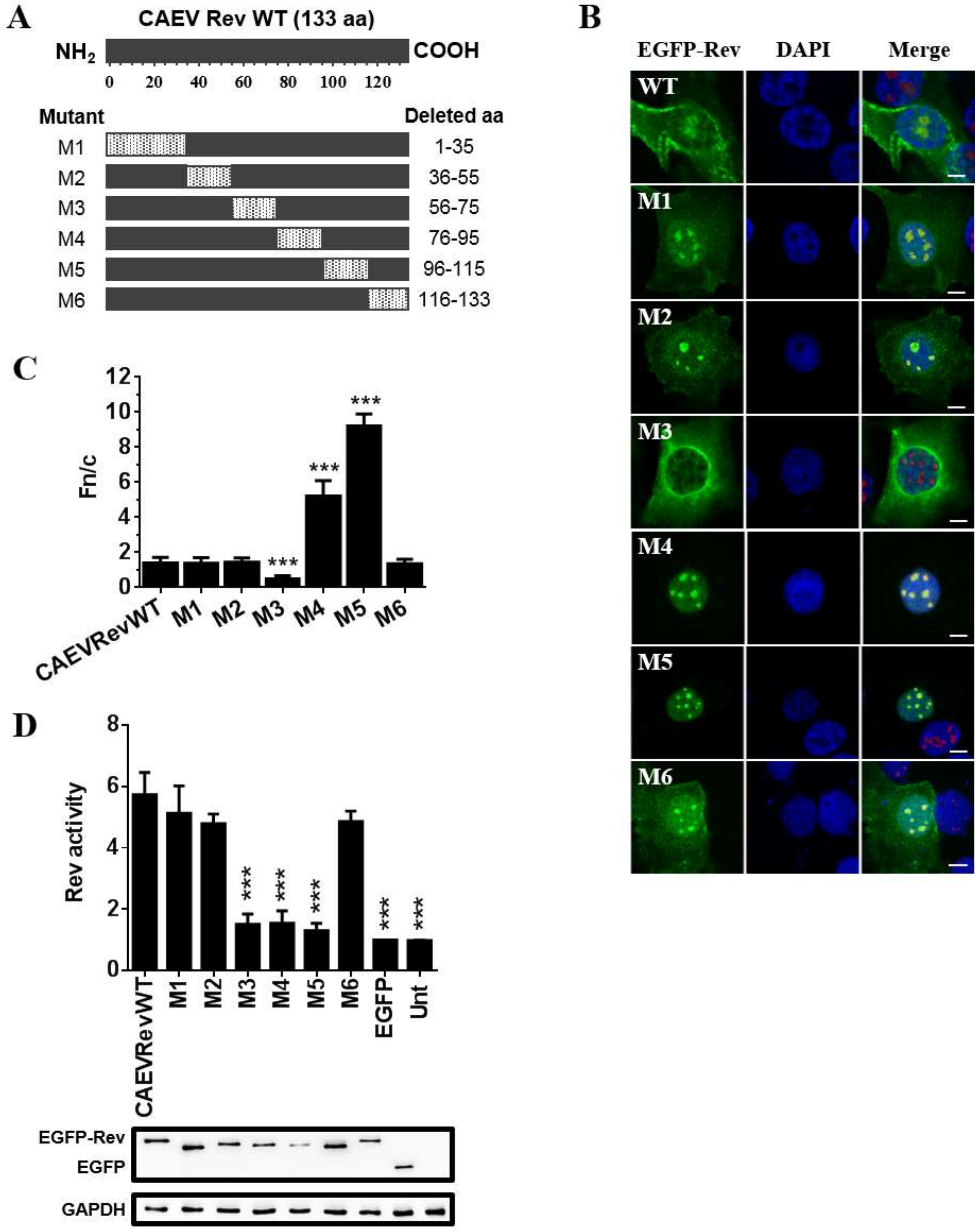
3.3. The Subcellular Localization Varies between CAEV Rev Deletion Mutant Proteins
3.4. Nuclear Export Activity of EGFP-CAEV Rev Deletion Mutant Proteins
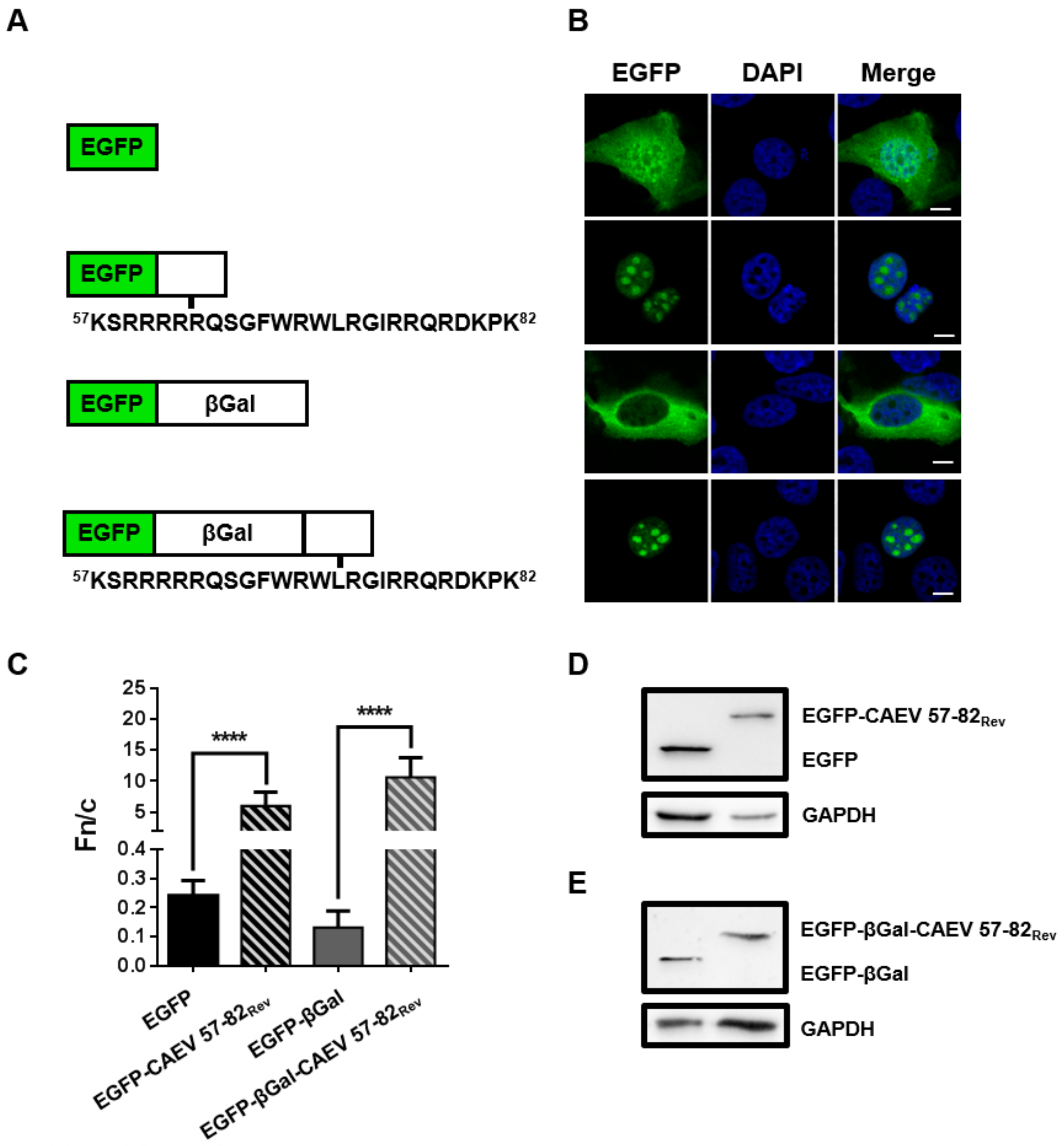
3.5. The 57 to 82 aa Sequence of the CAEV Rev Protein is Sufficient to Translocate Heterologous Proteins to the Nucleus
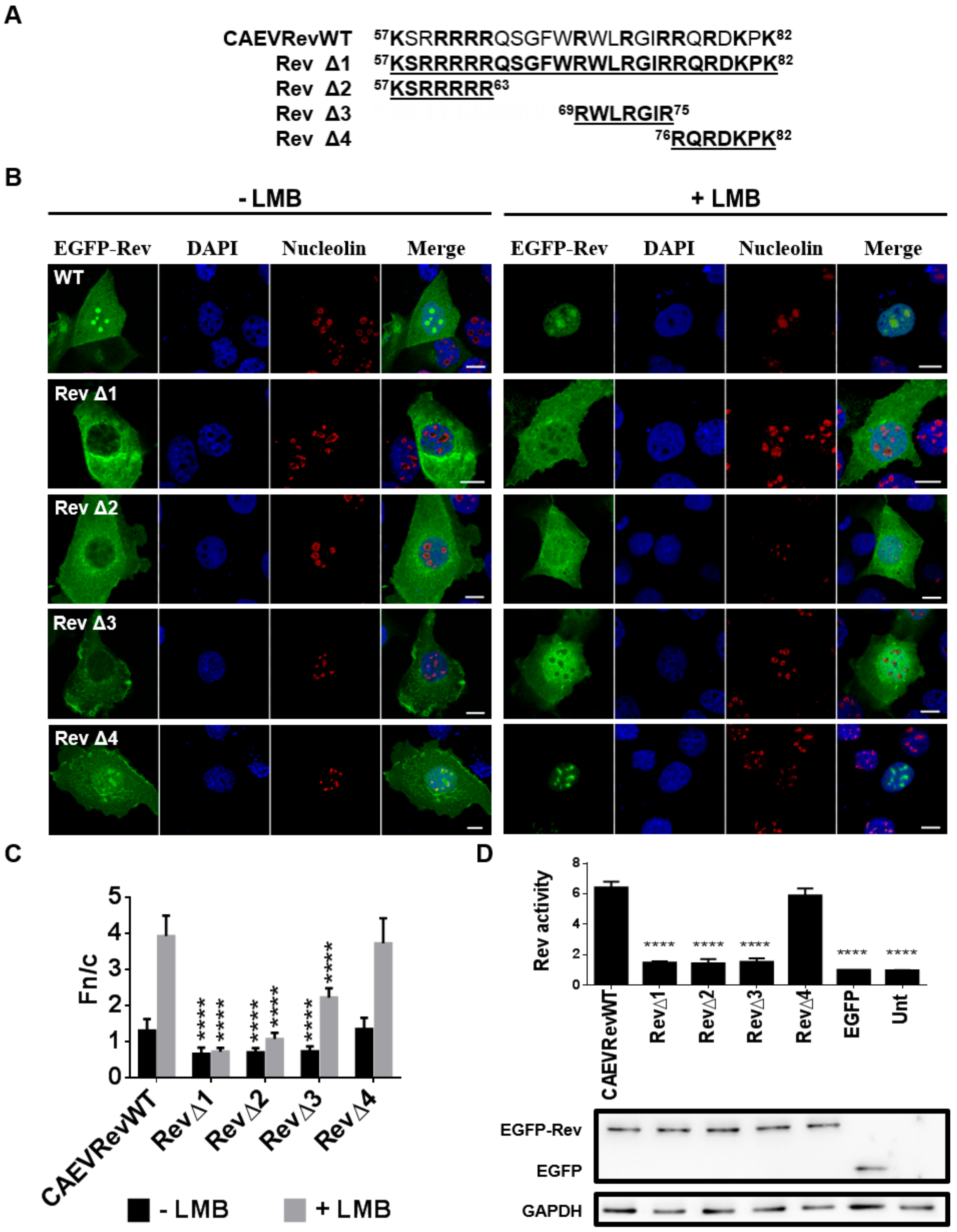
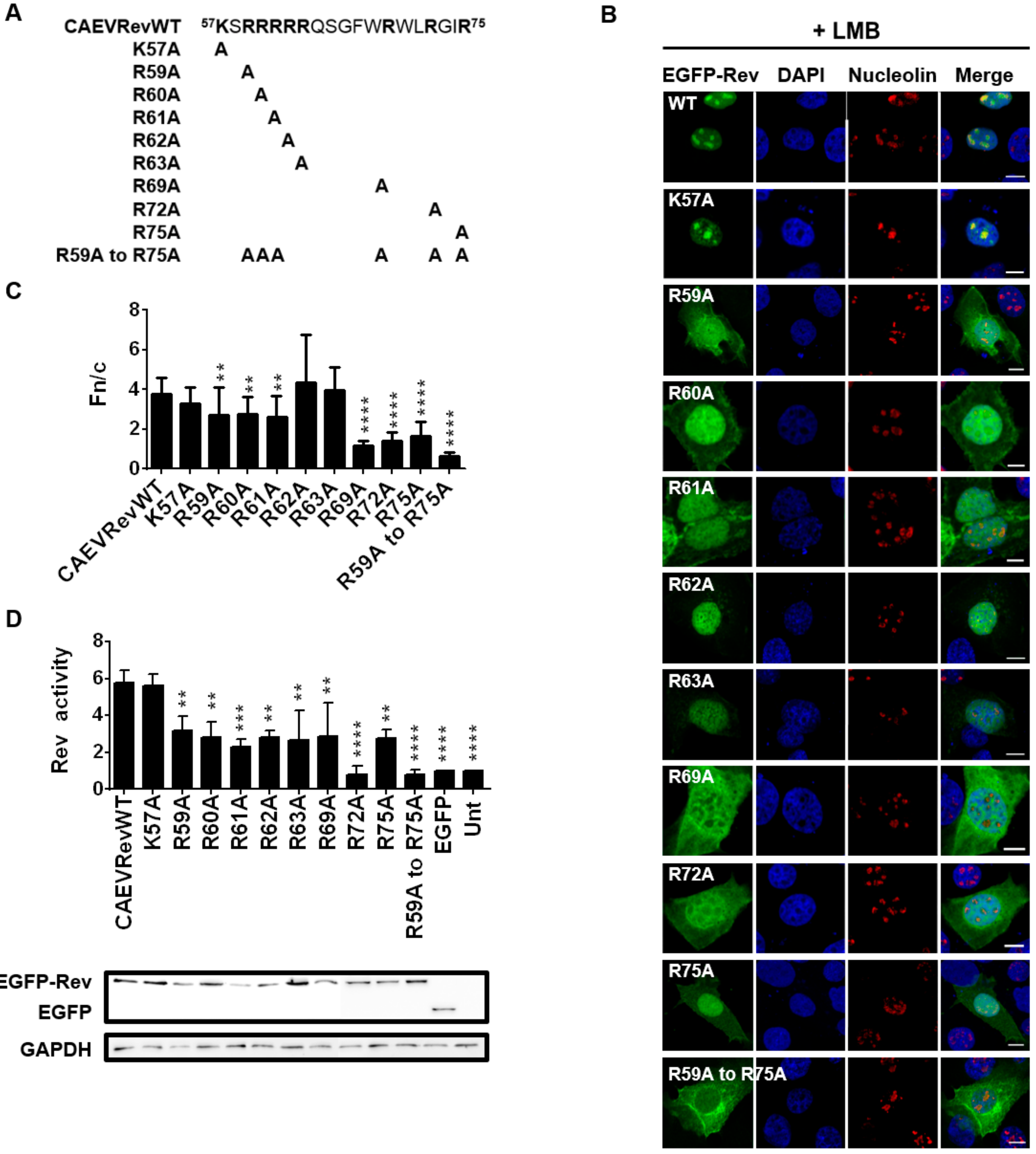
3.6. CAEV Rev NLS Mapped between aa 57 and 75
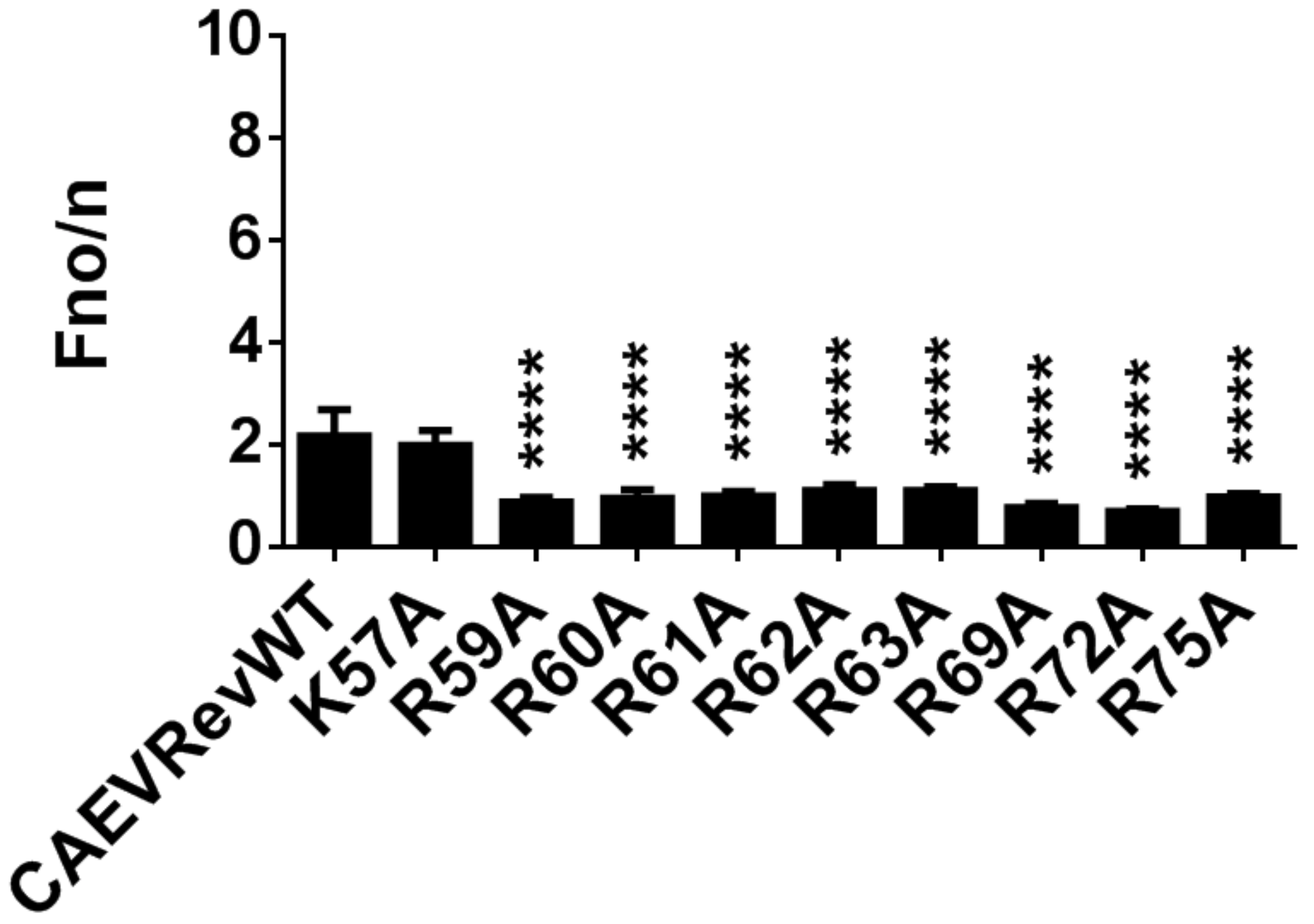
3.7. Identification of the Residues Composing the NLS and NoLS of the CAEV Rev Protein
3.8. The CAEV Rev NES
4. Discussion
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Larruskain, A.; Jugo, B.M. Retroviral infections in sheep and goats: Small ruminant lentiviruses and host interaction. Viruses 2013, 5, 2043–2061. [Google Scholar] [CrossRef] [PubMed]
- Santry, L.A.; de Jong, J.; Gold, A.C.; Walsh, S.R.; Menzies, P.I.; Wootton, S.K. Genetic characterization of small ruminant lentiviruses circulating in naturally infected sheep and goats in Ontario, Canada. Virus Res. 2013, 175, 30–44. [Google Scholar] [CrossRef] [PubMed]
- Peterhans, E.; Greenland, T.; Badiola, J.; Harkiss, G.; Bertoni, G.; Amorena, B.; Eliaszewicz, M.; Juste, R.A.; Krassnig, R.; Lafont, J.P.; et al. Routes of transmission and consequences of small ruminant lentiviruses (SRLVs) infection and eradication schemes. Vet. Res. 2004, 35, 257–274. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, H.; Reina, R.; Amorena, B.; de Andres, D.; Martinez, H.A. Small ruminant lentiviruses: Genetic variability, tropism and diagnosis. Viruses 2013, 5, 1175–1207. [Google Scholar] [CrossRef] [PubMed]
- Da Cruz, J.C.M.; Singh, D.K.; Lamara, A.; Chebloune, Y. Small ruminant lentiviruses (SRLVs) break the species barrier to acquire new host range. Viruses 2013, 5, 1867–1884. [Google Scholar] [CrossRef] [PubMed]
- Villet, S.; Bouzar, B.A.; Morin, T.; Verdier, G.; Legras, C.; Chebloune, Y. Maedi-visna virus and caprine arthritis encephalitis virus genomes encode a Vpr-like but no Tat protein. J. Virol. 2003, 77, 9632–9638. [Google Scholar] [CrossRef]
- Valas, S.; Rolland, M.; Perrin, C.; Perrin, G.; Mamoun, R.Z. Characterization of a new 5′ splice site within the caprine arthritis encephalitis virus genome: Evidence for a novel auxiliary protein. Retrovirology 2008, 5, 22. [Google Scholar] [CrossRef]
- Pollard, V.W.; Malim, M.H. The HIV-1 Rev protein. Annu. Rev. Microbiol. 1998, 52, 491–532. [Google Scholar] [CrossRef]
- Corredor, A.G.; Archambault, D. The bovine immunodeficiency virus Rev protein: Identification of a novel nuclear import pathway and nuclear export signal among retroviral Rev/Rev-like proteins. J. Virol. 2012, 86, 4892–4905. [Google Scholar] [CrossRef]
- Arnold, M.; Nath, A.; Hauber, J.; Kehlenbach, R.H. Multiple importins function as nuclear transport receptors for the Rev protein of human immunodeficiency virus type 1. J. Biol. Chem. 2006, 281, 20883–20890. [Google Scholar] [CrossRef]
- Kosugi, S.; Hasebe, M.; Matsumura, N.; Takashima, H.; Miyamoto-Sato, E.; Tomita, M.; Yanagawa, H. Six classes of nuclear localization signals specific to different binding grooves of importin alpha. J. Biol. Chem. 2009, 284, 478–485. [Google Scholar] [CrossRef] [PubMed]
- Romanelli, M.G.; Morandi, C. Importin alpha binds to an unusual bipartite nuclear localization signal in the heterogeneous ribonucleoprotein type I. Eur. J. Biochem. 2002, 269, 2727–2734. [Google Scholar] [CrossRef] [PubMed]
- Suhasini, M.; Reddy, T.R. Cellular proteins and HIV-1 Rev function. Curr. HIV Res. 2009, 7, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Rausch, J.W.; Le Grice, S.F. HIV Rev assembly on the Rev Response Element (RRE): A structural perspective. Viruses 2015, 7, 3053–3075. [Google Scholar] [CrossRef] [PubMed]
- Tiley, L.S.; Brown, P.H.; Le, S.Y.; Maizel, J.V.; Clements, J.E.; Cullen, B.R. Visna virus encodes a post-transcriptional regulator of viral structural gene expression. Proc. Natl. Acad. Sci. USA 1990, 87, 7497–7501. [Google Scholar] [CrossRef] [PubMed]
- Schoborg, R.V.; Saltarelli, M.J.; Clements, J.E. A Rev protein is expressed in caprine arthritis encephalitis virus (CAEV)-infected cells and is required for efficient viral replication. Virology 1994, 202, 1–15. [Google Scholar] [CrossRef]
- Saltarelli, M.; Querat, G.; Konings, D.A.; Vigne, R.; Clements, J.E. Nucleotide sequence and transcriptional analysis of molecular clones of CAEV which generate infectious virus. Virology 1990, 179, 347–364. [Google Scholar] [CrossRef]
- Schoborg, R.V.; Clements, J.E. Definition of the RRE binding and activation domains of the caprine arthritis encephalitis virus Rev protein. Virology 1996, 226, 113–121. [Google Scholar] [CrossRef][Green Version]
- Saltarelli, M.J.; Schoborg, R.; Pavlakis, G.N.; Clements, J.E. Identification of the caprine arthritis encephalitis virus Rev protein and its cis-acting Rev-responsive element. Virology 1994, 199, 47–55. [Google Scholar] [CrossRef]
- Stabel, J.R.; Stabel, T.J. Immortalization and characterization of bovine peritoneal macrophages transfected with SV40 plasmid DNA. Vet. Immunol. Immunopathol. 1995, 45, 211–220. [Google Scholar] [CrossRef]
- Scherer, W.F.; Syverton, J.T.; Gey, G.O. Studies on the propagation in vitro of poliomyelitis viruses. IV. Viral multiplication in a stable strain of human malignant epithelial cells (strain HeLa) derived from an epidermoid carcinoma of the cervix. J. Exp. Med. 1953, 97, 695–710. [Google Scholar] [CrossRef] [PubMed]
- McLinton, E.C.; Wagstaff, K.M.; Lee, A.; Moseley, G.W.; Marsh, G.A.; Wang, L.-F.; Jans, D.A.; Lieu, K.G.; Netter, H.J. Nuclear localization and secretion competence are conserved among henipavirus matrix proteins. J. Gen. Virol. 2017, 98, 563–576. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.J.; Hope, T.J.; Bond, B.L.; McDonald, D.; Grahl, K.; Parslow, T.G. Minimal Rev-response element for type 1 human immunodeficiency virus. J. Virol. 1991, 65, 2131–2134. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Gorelik, R.; Nanda, V.; Law, P.B.; Lear, J.D.; DeGrado, W.F.; Bennett, J.S. Dimerization of the transmembrane domain of Integrin alphaIIb subunit in cell membranes. J. Biol. Chem. 2004, 279, 26666–26673. [Google Scholar] [CrossRef]
- Bogerd, H.P.; Echarri, A.; Ross, T.M.; Cullen, B.R. Inhibition of human immunodeficiency virus Rev and human T-cell leukemia virus Rex function, but not Mason-Pfizer monkey virus constitutive transport element activity, by a mutant human nucleoporin targeted to Crm1. J. Virol. 1998, 72, 8627–8635. [Google Scholar] [CrossRef]
- Henderson, B.R.; Eleftheriou, A. A comparison of the activity, sequence specificity, and CRM1-dependence of different nuclear export signals. Exp. Cell Res. 2000, 256, 213–224. [Google Scholar] [CrossRef]
- Indik, S.; Günzburg, W.H.; Salmons, B.; Rouault, F. A novel, mouse mammary tumor virus encoded protein with Rev-like properties. Virology 2005, 337, 1–6. [Google Scholar] [CrossRef]
- Marchand, C.; Lemay, G.; Archambault, D. The Jembrana disease virus Rev protein: Identification of nuclear and novel lentiviral nucleolar localization and nuclear export signals. PLoS ONE 2019, 14, e0221505. [Google Scholar] [CrossRef]
- Mayer, J.; Ehlhardt, S.; Seifert, M.; Sauter, M.; Müller-Lantzsch, N.; Mehraein, Y.; Zang, K.-D.; Meese, E. Human endogenous retrovirus HERV-K(HML-2) proviruses with Rec protein coding capacity and transcriptional activity. Virology 2004, 322, 190–198. [Google Scholar] [CrossRef]
- Otero, G.C.; Harris, M.E.; Donello, J.E.; Hope, T.J. Leptomycin B inhibits equine infectious anemia virus Rev and feline immunodeficiency virus rev function but not the function of the hepatitis B virus posttranscriptional regulatory element. J. Virol. 1998, 72, 7593–7597. [Google Scholar] [CrossRef]
- Kudo, N.; Wolff, B.; Sekimoto, T.; Schreiner, E.P.; Yoneda, Y.; Yanagida, M.; Horinouchi, S.; Yoshida, M. Leptomycin B inhibition of signal-mediated nuclear export by direct binding to CRM1. Exp. Cell Res. 1998, 242, 540–547. [Google Scholar] [CrossRef]
- Guttler, T.; Madl, T.; Neumann, P.; Deichsel, D.; Corsini, L.; Monecke, T.; Ficner, R.; Sattler, M.; Gorlich, D. NES consensus redefined by structures of PKI-type and Rev-type nuclear export signals bound to CRM1. Nat. Struct. Mol. Biol. 2010, 17, 1367–1376. [Google Scholar] [CrossRef] [PubMed]
- Fung, H.Y.; Fu, S.C.; Chook, Y.M. Nuclear export receptor CRM1 recognizes diverse conformations in nuclear export signals. eLife 2017, 6, e23961. [Google Scholar] [CrossRef] [PubMed]
- La Cour, T.; Kiemer, L.; Mølgaard, A.; Gupta, R.; Skriver, K.; Brunak, S. Analysis and prediction of leucine-rich nuclear export signals. Protein Eng. Des. Sel. 2004, 17, 527–536. [Google Scholar] [CrossRef] [PubMed]
- Christie, M.; Chang, C.W.; Róna, G.; Smith, K.M.; Stewart, A.G.; Takeda, A.A.; Fontes, M.R.; Stewart, M.; Vértessy, B.G.; Forwood, J.K.; et al. Structural biology and regulation of protein import into the nucleus. J. Mol. Biol. 2016, 428, 2060–2090. [Google Scholar] [CrossRef] [PubMed]
- Knockenhauer, K.E.; Schwartz, T.U. The nuclear pore complex as a flexible and dynamic gate. Cell 2016, 164, 1162–1171. [Google Scholar] [CrossRef]
- Musser, S.M.; Grünwald, D. Deciphering the structure and function of nuclear pores using single-molecule fluorescence approaches. J. Mol. Biol. 2016, 428, 2091–2119. [Google Scholar] [CrossRef]
- Fu, X.; Liang, C.; Li, F.; Wang, L.; Wu, X.; Lu, A.; Xiao, G.; Zhang, G. The rules and functions of nucleocytoplasmic shuttling proteins. Int. J. Mol. Sci. 2018, 19, 1445. [Google Scholar] [CrossRef]
- Wente, S.R.; Rout, M.P. The nuclear pore complex and nuclear transport. Cold Spring Harb. Perspect. Biol. 2010, 2, a000562. [Google Scholar] [CrossRef]
- Kiyomasu, T.; Miyazawa, T.; Furuya, T.; Shibata, R.; Sakai, H.; Sakuragi, J.; Fukasawa, M.; Maki, N.; Hasegawa, A.; Mikami, T.; et al. Identification of feline immunodeficiency virus rev gene activity. J. Virol. 1991, 65, 4539–4542. [Google Scholar] [CrossRef]
- Lee, J.H.; Murphy, S.C.; Belshan, M.; Sparks, W.O.; Wannemuehler, Y.; Liu, S.; Hope, T.J.; Dobbs, D.; Carpenter, S. Characterization of functional domains of equine infectious anemia virus Rev suggests a bipartite RNA-binding domain. J. Virol. 2006, 80, 3844–3852. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Malim, M.H.; Böhnlein, S.; Fenrick, R.; Le, S.Y.; Maizel, J.V.; Cullen, B.R. Functional comparison of the Rev trans-activators encoded by different primate immunodeficiency virus species. Proc. Natl. Acad. Sci. USA 1989, 86, 8222–8226. [Google Scholar] [CrossRef] [PubMed]
- Narayan, M.; Younis, I.; D’Agostino, D.M.; Green, P.L. Functional domain structure of human T-cell leukemia virus type 2 rex. J. Virol. 2003, 77, 12829–12840. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yang, J.; Bogerd, H.P.; Peng, S.; Wiegand, H.; Truant, R.; Cullen, B.R. An ancient family of human endogenous retroviruses encodes a functional homolog of the HIV-1 Rev protein. Proc. Natl. Acad. Sci. USA 1999, 96, 13404–13408. [Google Scholar] [CrossRef]
- Shida, H. Role of nucleocytoplasmic RNA transport during the life cycle of retroviruses. Front. Microbiol. 2012, 3, 179. [Google Scholar] [CrossRef]
- Schoborg, R.V.; Clements, J.E. The Rev protein of visna virus is localized to the nucleus of infected cells. Virology 1994, 202, 485–490. [Google Scholar] [CrossRef]
- Cochrane, A.W.; Perkins, A.; Rosen, C.A. Identification of sequences important in the nucleolar localization of human immunodeficiency virus Rev: Relevance of nucleolar localization to function. J. Virol. 1990, 64, 881–885. [Google Scholar] [CrossRef]
- Corredor, A.G.; Archambault, D. The bovine immunodeficiency virus Rev protein: Identification of a novel lentiviral bipartite nuclear localization signal harboring an atypical spacer sequence. J. Virol. 2009, 83, 12842–12853. [Google Scholar] [CrossRef]
- Phillips, T.R.; Lamont, C.; Konings, D.A.; Shacklett, B.L.; Hamson, C.A.; Luciw, P.A.; Elder, J.H. Identification of the Rev transactivation and Rev-responsive elements of feline immunodeficiency virus. J. Virol. 1992, 66, 5464–5471. [Google Scholar] [CrossRef]
- Kubota, S.; Siomi, H.; Satoh, T.; Endo, S.; Maki, M.; Hatanaka, M. Functional similarity of HIV-I rev and HTLV-I rex proteins: Identification of a new nucleolar-targeting signal in rev protein. Biochem. Biophys. Res. Commun. 1989, 162, 963–970. [Google Scholar] [CrossRef]
- Mathew, C.; Ghildyal, R. CRM1 inhibitors for antiviral therapy. Front. Microbiol. 2017, 8, 1171. [Google Scholar] [CrossRef] [PubMed]
- Kutay, U.; Güttinger, S. Leucine-rich nuclear-export signals: Born to be weak. Trends Cell Biol. 2005, 15, 121–124. [Google Scholar] [CrossRef] [PubMed]
- Meyer, B.E.; Meinkoth, J.L.; Malim, M.H. Nuclear transport of human immunodeficiency virus type 1, visna virus, and equine infectious anemia virus Rev proteins: Identification of a family of transferable nuclear export signals. J. Virol. 1996, 70, 2350–2359. [Google Scholar] [CrossRef] [PubMed]
- Hiscox, J.A.; Whitehouse, A.; Matthews, D.A. Nucleolar proteomics and viral infection. Proteomics 2010, 10, 4077–4086. [Google Scholar] [CrossRef] [PubMed]
- Rawlinson, S.M.; Moseley, G.W. The nucleolar interface of RNA viruses. Cell. Microbiol. 2015, 17, 1108–1120. [Google Scholar] [CrossRef]
- Passos-Castilho, A.M.; Marchand, C.; Archambault, D. B23/nucleophosmin interacts with bovine immunodeficiency virus Rev protein and facilitates viral replication. Virology 2018, 515, 158–164. [Google Scholar] [CrossRef]

| Protein | NES 1 | References |
|---|---|---|
| PKI-type NESs | ||
| Consensus | Φ0XXΦ1 XXXΦ2 XXΦ3XΦ4 | |
| PKI | INELALKLAGLDI | [32] |
| JDV Rev | MAELEERFEDLAL | [28] |
| BIV Rev | IQQLEDLVRHMSL | [9] |
| HIV-1 Rev-type NES | ||
| Consensus | 0Φ1 XΦ2 XXΦ3XΦ4 | |
| HIV-1 Rev | LQLPPLERLTL | [32] |
| CAEV Rev NES | ||
| Consensus | Φ0XXXΦ1 XXΦ2 XXΦ3XΦ4 | This study |
| CAEV Rev | LGSCVGALAELTL |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Labrecque, M.; Marchand, C.; Archambault, D. Characterization of Signal Sequences Determining the Nuclear/Nucleolar Import and Nuclear Export of the Caprine Arthritis-Encephalitis Virus Rev Protein. Viruses 2020, 12, 900. https://doi.org/10.3390/v12080900
Labrecque M, Marchand C, Archambault D. Characterization of Signal Sequences Determining the Nuclear/Nucleolar Import and Nuclear Export of the Caprine Arthritis-Encephalitis Virus Rev Protein. Viruses. 2020; 12(8):900. https://doi.org/10.3390/v12080900
Chicago/Turabian StyleLabrecque, Marlène, Claude Marchand, and Denis Archambault. 2020. "Characterization of Signal Sequences Determining the Nuclear/Nucleolar Import and Nuclear Export of the Caprine Arthritis-Encephalitis Virus Rev Protein" Viruses 12, no. 8: 900. https://doi.org/10.3390/v12080900
APA StyleLabrecque, M., Marchand, C., & Archambault, D. (2020). Characterization of Signal Sequences Determining the Nuclear/Nucleolar Import and Nuclear Export of the Caprine Arthritis-Encephalitis Virus Rev Protein. Viruses, 12(8), 900. https://doi.org/10.3390/v12080900






