Non-Polio Enteroviruses from Acute Flaccid Paralysis Surveillance in Korea, 2012–2019
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Virus Detection
2.3. Ethical Statement
2.4. Statistical Analysis
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Al Awaidy, S.T.; Khamis, F. Wild Poliovirus Type 1 in Oman: A re-emerging threat that requires urgent, targeted and strategic preparedness. Sultan Qaboos Univ. Med. J. 2020, 20, 1–4. [Google Scholar] [CrossRef]
- Leke, R.G.F.; King, A.; Pallansch, M.A.; Tangermann, R.H.; Mkanda, P.; Chunsuttiwat, S.; Jack, A.; Kaboré, B.J.; Kane, I.; Khomo, N.E.; et al. Certifying the interruption of wild poliovirus transmission in the WHO African region on the turbulent journey to a polio-free world. Lancet Glob. Health 2020, 8, e1345–e1351. [Google Scholar] [CrossRef]
- Song, K.M.; Choe, Y.J.; Cho, H.; Bae, G.-R.; Lee, J.-K. National Action Plan for Response to Poliovirus Importation. Osong Public Health Res. Perspect. 2011, 2, 65–71. [Google Scholar] [CrossRef][Green Version]
- Kim, H.-J.; Hwang, S.; Lee, S.; Kwon, Y.; Park, K.; Park, Y.J.; Bae, G.-R.; Lee, S.W.; Jeong, Y.-S.; Hyeon, J.-Y. A national cross-sectional study for poliovirus seroprevalence in the Republic of Korea in 2012: Implication for deficiency in immunity to polio among middle-aged people. BMC Infect. Dis. 2015, 15, 164. [Google Scholar] [CrossRef]
- Kim, H.; Kang, B.; Hwang, S.; Lee, S.W.; Cheon, D.-S.; Kim, K.; Jeong, Y.-S.; Hyeon, J.-Y. Clinical and enterovirus findings associated with acute flaccid paralysis in the republic of Korea during the recent decade. J. Med. Virol. 2013, 86, 1584–1589. [Google Scholar] [CrossRef] [PubMed]
- Tangermann, R.H.; Lamoureux, C.; Tallis, G.; Goel, A. The critical role of acute flaccid paralysis surveillance in the Global Polio Eradication Initiative. Int. Health 2017, 9, 156–163. [Google Scholar] [CrossRef]
- World Health Organization. Polio Laboratory Manual, 4th ed.; World Health Organization: Geneva, Switzerland, 2004. [Google Scholar]
- Kang, H.J.; Yoon, Y.; Lee, Y.P.; Kim, H.J.; Lee, D.Y.; Lee, J.W.; Hyeon, J.Y.; Yoo, J.S.; Lee, S.; Kang, C.; et al. A Different Epi-demiology of Enterovirus A and Enterovirus B Co-circulating in Korea, 2012–2019. J. Pediatr. Infect. Dis. Soc. 2020, piaa111. [Google Scholar] [CrossRef]
- Thornton, J. Polio returns to the Philippines. Lancet 2019, 394, 1217. [Google Scholar] [CrossRef]
- Ming, L.C.; Hussain, Z.; Yeoh, S.F.; Koh, D.; Lee, K.S. Circulating vaccine-derived poliovirus: A menace to the end game of polio eradication. Glob. Health 2020, 16, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Choe, Y.J.; Yang, J.J.; Park, S.K.; Choi, E.H.; Lee, H.J. Comparative estimation of coverage between national immunization program vaccines and non-NIP vaccines in Korea. J. Korean Med. Sci. 2013, 28, 1283. [Google Scholar] [CrossRef] [PubMed]
- Masa-Calles, J.; Torner, N.; López-Perea, N.; de Mier, M.D.V.T.; Fernández-Martínez, B.; Cabrerizo, M.; Gallardo-García, V.; Malo, C.; Margolles-Martins, M.J.; Portell, M.; et al. Acute flaccid paralysis (AFP) surveillance: Challenges and opportunities from 18 years’ experience, Spain, 1998 to 2015. Eurosurveillance 2018, 23, 1700423. [Google Scholar] [CrossRef]
- Bitnun, A.; Yeh, E.A. Acute Flaccid Paralysis and Enteroviral Infections. Curr. Infect. Dis. Rep. 2018, 20, 34. [Google Scholar] [CrossRef]
- Momen, A.A.; Shakurnia, A.; Momen, M. Eleven-year surveillance of acute flaccid paralysis in southwestern Iran. Turk. J. Pediatr. 2019, 61, 544–551. [Google Scholar] [CrossRef] [PubMed]
- Tesfaye, B.; Sowe, A.; Kisangau, N.; Ogange, J.; Ntoburi, S.; Nekar, I.; Muitherero, C.; Camara, Y.; Gathenji, C.; Langat, D.; et al. An epidemiological analysis of Acute Flaccid Paralysis (AFP) surveillance in Kenya, 2016 to 2018. BMC Infect. Dis. 2020, 20, 1–11. [Google Scholar] [CrossRef]
- Suresh, S.; Forgie, S.; Robinson, J. Non-polio Enterovirus detection with acute flaccid paralysis: A systematic review. J. Med. Virol. 2018, 90, 3–7. [Google Scholar] [CrossRef] [PubMed]
- Mandary, M.B.; Poh, C.L. Changes in the EV-A71 Genome through Recombination and Spontaneous Mutations: Impact on Virulence. Viruses 2018, 10, 320. [Google Scholar] [CrossRef]
- Huang, S.-W.; Cheng, D.; Wang, J.-R. Enterovirus A71: Virulence, antigenicity, and genetic evolution over the years. J. Biomed. Sci. 2019, 26, 81–89. [Google Scholar] [CrossRef]
- Liu, A.-P.; Tan, H.; Xie, Q.; Chen, B.-T.; Liu, X.-F.; Zhang, Y. Genetic evidence for recombination and mutation in the emergence of human enterovirus 71. Chin. J. Virol. 2014, 30, 572–578. [Google Scholar]
- Yee, P.T.I.; Poh, C.L. Impact of genetic changes, pathogenicity and antigenicity on Enterovirus-A71 vaccine development. Virology 2017, 506, 121–129. [Google Scholar] [CrossRef]
- Feng, M.; Liao, Y.; Gao, Y.; Jiang, G.; Wang, L.; Zhang, Y.; Fan, S.; Xu, X.; Li, Q. Mechanism for the lethal effect of enterovirus A71 intracerebral injection in neonatal mice. Lab. Investig. 2019, 100, 596–605. [Google Scholar] [CrossRef]
- Uprety, P.; Curtis, D.; Elkan, M.; Fink, J.; Rajagopalan, R.; Zhao, C.; Bittinger, K.; Mitchell, S.; Ulloa, E.R.; Hopkins, S.; et al. Association of Enterovirus D68 with Acute Flaccid Myelitis, Philadelphia, Pennsylvania, USA, 2009–2018. Emerg. Infect. Dis. 2019, 25, 1676–1682. [Google Scholar] [CrossRef]
- Dyda, A.; Stelzer-Braid, S.; Adam, D.; Chughtai, A.A.; MacIntyre, C.R. The association between acute flaccid myelitis (AFM) and Enterovirus D68 (EV-D68)—What is the evidence for causation? Eurosurveillance 2018, 23, 17–00310. [Google Scholar] [CrossRef]
- Cassidy, H.; Poelman, R.; Knoester, M.; Van Leer-Buter, C.C.; Niesters, H.G.M. Enterovirus D68—The New Polio? Front. Microbiol. 2018, 9, 2677. [Google Scholar] [CrossRef]
- Zhang, Y.; Cao, J.; Zhang, S.; Lee, A.J.; Sun, G.; Larsen, C.N.; Zhao, H.; Gu, Z.; He, S.; Klem, E.B.; et al. Genetic changes found in a distinct clade of Enterovirus D68 associated with paralysis during the 2014 outbreak. Virus Evol. 2016, 2, vew015. [Google Scholar] [CrossRef]
- Landry, M.L.; Madore, H.P.; Fong, C.K.; Hsiung, G.D. Use of guinea pig embryo cell cultures for isolation and propagation of group A coxsackieviruses. J. Clin. Microbiol. 1981, 13, 588–593. [Google Scholar] [CrossRef]
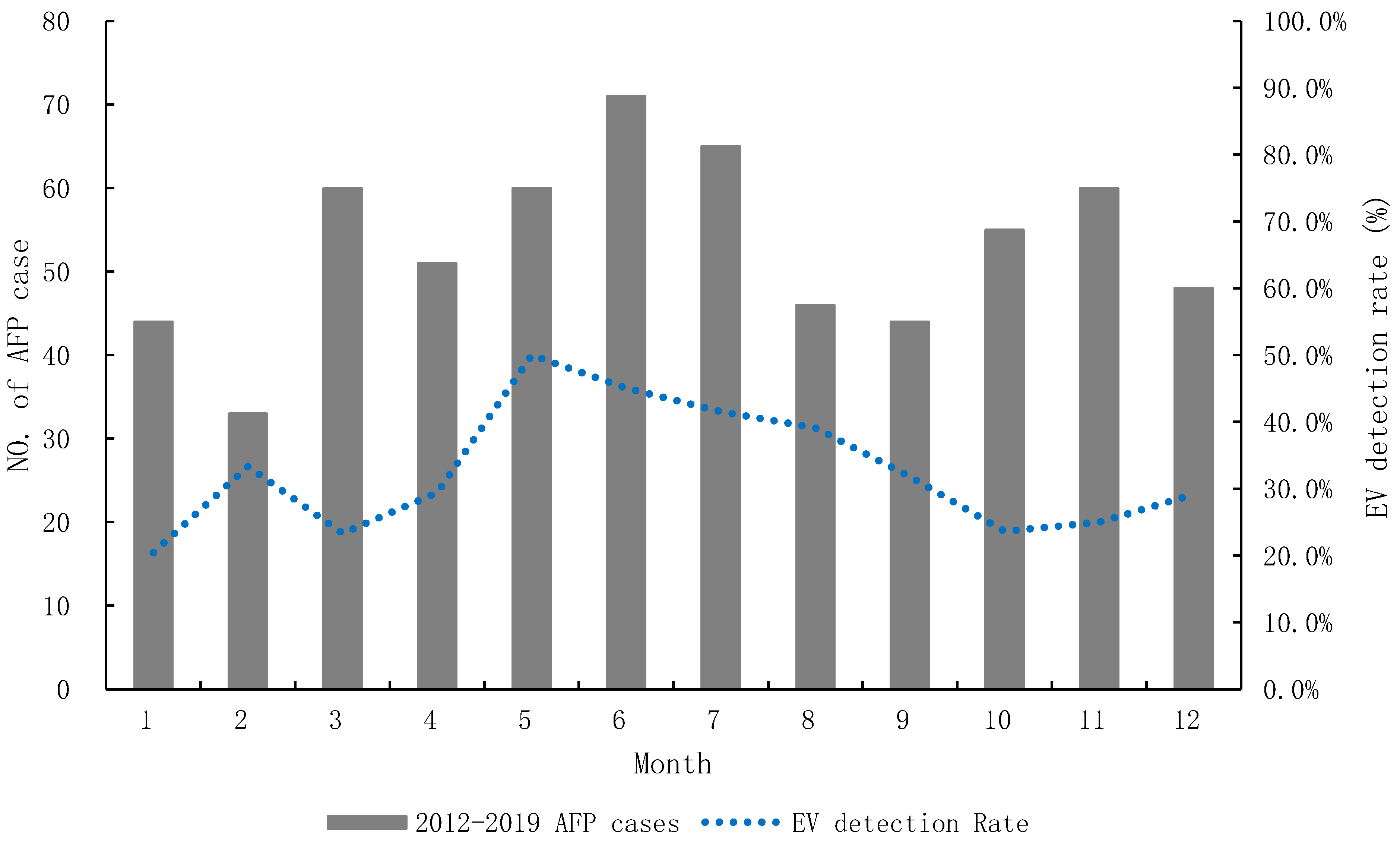
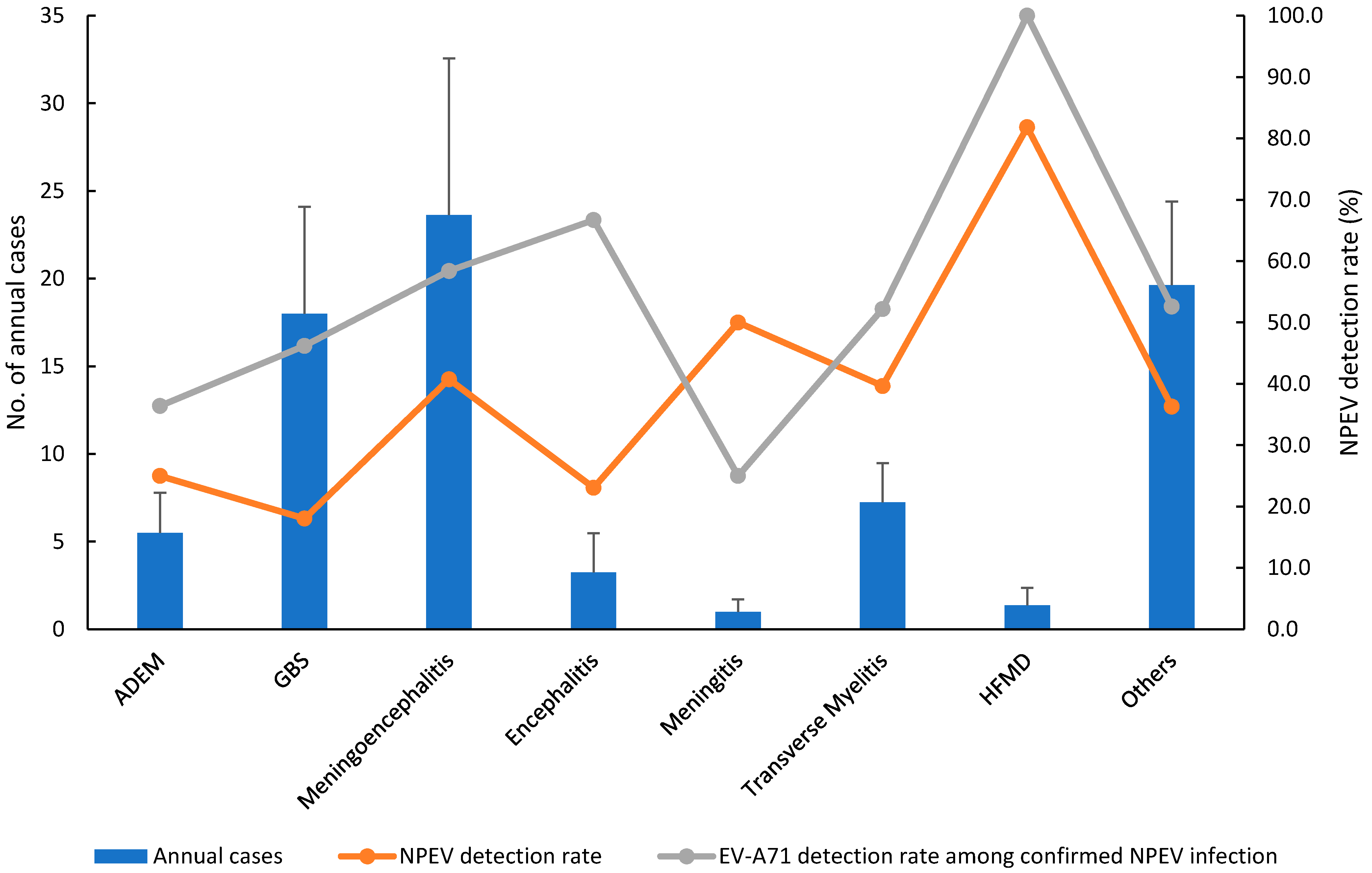
| 2012 | 2013 | 2014 | 2015 | 2016 | 2017 | 2018 | 2019 | |
|---|---|---|---|---|---|---|---|---|
| No. of annual expected cases in children <15 years of age | 76 | 76 | 72 | 70 | 69 | 68 | 67 | 66 |
| Reported number of cases | 94 | 83 | 88 | 83 | 69 | 68 | 70 | 82 |
| Non-polio AFP rate | 1.24 | 1.11 | 1.22 | 1.19 | 1.00 | 1.00 | 1.04 | 1.21 |
| Adequate specimen (%) | 89 | 94 | 86 | 86 | 94 | 94 | 90 | 85 |
| 60-day follow-up (%) | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
| 2012 n = 94 | 2013 n = 83 | 2014 n = 88 | 2015 n = 83 | 2016 n = 69 | 2017 n = 68 | 2018 n = 70 | 2019 n = 82 | Total n = 637 | p-Value | |
|---|---|---|---|---|---|---|---|---|---|---|
| Sex | 0.0009 | |||||||||
| Male | 54 (57.4) | 48 (57.8) | 42 (47.7) | 49 (59.0) | 46 (66.7) | 41 (60.3) | 40 (57.1) | 51 (62.2) | 371 (58.2) | |
| Female | 40 (42.6) | 35 (42.2) | 46 (52.3) | 34 (41.0) | 23 (33.3) | 27 (39.7) | 30 (42.9) | 31 (37.8) | 266 (41.8) | |
| Age, years | <0.0001 | |||||||||
| <1 | 6 (6.4) | 7 (8.4) | 3 (3.4) | 6 (7.2) | 2 (2.9) | 3 (4.4) | 1 (1.4) | 3 (3.7) | 31 (4.9) | |
| 1–5 | 65 (69.1) | 44 (53.0) | 46 (52.3) | 50 (60.2) | 41 (59.4) | 24 (35.3) | 25 (35.7) | 38 (46.3) | 333 (52.3) | |
| 6–10 | 10 (10.6) | 17 (20.5) | 23 (26.1) | 14 (16.9) | 15 (21.7) | 26 (38.2) | 23 (32.9) | 24 (29.3) | 152 (23.9) | |
| 11–15 | 13 (13.8) | 13 (15.7) | 14 (15.9) | 13 (15.7) | 9 (13.0) | 15 (22.1) | 18 (25.7) | 17 (20.7) | 112 (17.6) | |
| >15 | 0 (0.0) | 3 (3.6) | 2 (2.3) | 0 (0.0) | 2 (2.9) | 0 (0.0) | 3 (4.3) | 0 (0.0) | 10 (1.6) | |
| Fever at onset | 0.0001 | |||||||||
| Yes | 65 (69.1) | 57 (68.7) | 58 (65.9) | 62 (74.7) | 42 (60.9) | 35 (51.5) | 37 (52.9) | 53 (64.6) | 409 (64.2) | |
| No | 29 (30.9) | 26 (31.1) | 30 (34.1) | 21 (25.3) | 27 (39.1) | 33 (48.5) | 33 (47.1) | 29 (35.4) | 228 (35.8) | |
| IPV dose | <0.0001 | |||||||||
| 0 | 2 (2.1) | 3 (3.6) | 5 (5.7) | 3 (3.6) | 0 (0.0) | 0 (0.0) | 1 (1.4) | 1 (1.2) | 15 (2.4) | |
| 1 | 1 (1.1) | 2 (2.4) | 3 (3.4) | 1 (1.2) | 2 (2.9) | 0 (0.0) | 0 (0.0) | 2 (2.4) | 11 (1.7) | |
| 2 | 3 (3.2) | 2 (2.4) | 2 (2.3) | 3 (3.6) | 0 (0.0) | 1 (1.5) | 1 (1.4) | 0 (0.0) | 12 (1.9) | |
| 3 | 25 (26.6) | 37 (44.6) | 30 (34.1) | 34 (41.0) | 20 (29.0) | 18 (26.5) | 16 (22.9) | 33 (40.2) | 213 (33.4) | |
| 4 | 29 (30.9) | 19 (22.9) | 32 (36.4) | 28 (33.7) | 33 (47.8) | 33 (48.5) | 26 (37.1) | 27 (32.9) | 227 (35.6) | |
| 5 | 1 (1.1) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (0.2) | |
| Unknown | 33 (35.1) | 20 (24.1) | 16 (18.2) | 14 (16.9) | 14 (20.3) | 16 (23.5) | 26 (37.1) | 19 (23.2) | 158 (24.8) |
| Year | Total no. of AFP Patientswith Specimens | Virus Isolation Results | Real-Time RT-PCR Positive | Negative | ||
|---|---|---|---|---|---|---|
| L20B Positive | L20B Positive + NPEV | RD-A Positive | ||||
| 2012 | 94 | 0 | 0 | 8 | 34 | 60 |
| 2013 | 83 | 0 | 0 | 2 | 47 | 36 |
| 2014 | 88 | 0 | 1 | 8 | 41 | 47 |
| 2015 | 83 | 0 | 0 | 8 | 30 | 53 |
| 2016 | 69 | 0 | 0 | 7 | 33 | 36 |
| 2017 | 68 | 0 | 0 | 4 | 6 | 62 |
| 2018 | 70 | 0 | 0 | 5 | 6 | 64 |
| 2019 | 82 | 0 | 1 | 7 | 16 | 66 |
| Total | 637 | 0 | 2 | 49 | 213 | 424 |
| NPEV-Types | No. of Viral RNA Detected | No. of Virus Isolation | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2012 (n = 94) | 2013 (n = 83) | 2014 (n = 88) | 2015 (n = 83) | 2016 (n = 69) | 2017 (n = 68) | 2018 (n = 70) | 2019 (n = 82) | Total (n = 637) | Rate (%) | |||
| Enterovirus A | 28 | 35 | 30 | 16 | 21 | 2 | 1 | 13 | 146 | 68.5 | 26 | |
| CA2 | 0 | 0 | 3 | 0 | 1 | 0 | 0 | 0 | 4 | 1.9 | 1 | |
| CA4 | 1 | 0 | 2 | 0 | 1 | 0 | 0 | 0 | 4 | 1.9 | 0 | |
| CA5 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 3 | 5 | 2.3 | 2 | |
| CA6 | 4 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 4 | 1.9 | 0 | |
| CA10 | 0 | 2 | 0 | 1 | 0 | 0 | 0 | 1 | 4 | 1.9 | 2 | |
| CA16 | 0 | 0 | 4 | 3 | 0 | 0 | 0 | 1 | 8 | 3.8 | 4 | |
| EV-A71 | 23 | 32 | 21 | 12 | 19 | 1 | 1 | 8 | 117 | 54.9 | 17 | |
| Enterovirus B | 6 | 5 | 6 | 2 | 6 | 3 | 5 | 2 | 35 | 16.4 | 18 | |
| CA9 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0.5 | 0 | |
| CB1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0.5 | 0 | |
| CB2 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0.5 | 1 | |
| CB3 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 3 | 1.4 | 2 | |
| CB5 | 0 | 1 | 6 | 0 | 0 | 0 | 0 | 1 | 8 | 3.8 | 6 | |
| E3 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 2 | 0.9 | 3 | |
| E6 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 2 | 0.9 | 0 | |
| E7 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0.9 | 2 | |
| E11 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0.5 | 0 | |
| E12 | 0 | 0 | 0 | 0 | 2 | 1 | 0 | 0 | 3 | 1.4 | 0 | |
| E13 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0.5 | 1 | |
| E18 | 0 | 0 | 0 | 0 | 3 | 0 | 0 | 0 | 3 | 1.4 | 0 | |
| E30 | 1 | 4 | 0 | 0 | 0 | 0 | 1 | 0 | 6 | 2.8 | 3 | |
| Enterovirus C | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 2 | 0.9 | 0 | |
| CA19 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0.5 | 0 | |
| CA24 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0.5 | 0 | |
| UT | 0 | 7 | 5 | 12 | 5 | 0 | 0 | 1 | 30 | 14.1 | 5 | |
| Total | 34 | 47 | 41 | 30 | 33 | 6 | 6 | 16 | 213 | 100 | 49 | |
| EV detection rate (%) | 36.2 | 56.6 | 46.6 | 36.1 | 47.8 | 8.8 | 8.6 | 19.5 | 33.4 | - | 7.7 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoon, Y.; Lee, Y.-P.; Lee, D.-Y.; Kim, H.-J.; Lee, J.-W.; Lee, S.; Kang, C.; Choi, W.; Bin, J.H.; Kim, Y.H.; et al. Non-Polio Enteroviruses from Acute Flaccid Paralysis Surveillance in Korea, 2012–2019. Viruses 2021, 13, 411. https://doi.org/10.3390/v13030411
Yoon Y, Lee Y-P, Lee D-Y, Kim H-J, Lee J-W, Lee S, Kang C, Choi W, Bin JH, Kim YH, et al. Non-Polio Enteroviruses from Acute Flaccid Paralysis Surveillance in Korea, 2012–2019. Viruses. 2021; 13(3):411. https://doi.org/10.3390/v13030411
Chicago/Turabian StyleYoon, Youngsil, Yong-Pyo Lee, Deog-Yong Lee, Hye-Jin Kim, June-Woo Lee, Sangwon Lee, Chun Kang, Wooyoung Choi, Joong Hyun Bin, Young Hoon Kim, and et al. 2021. "Non-Polio Enteroviruses from Acute Flaccid Paralysis Surveillance in Korea, 2012–2019" Viruses 13, no. 3: 411. https://doi.org/10.3390/v13030411
APA StyleYoon, Y., Lee, Y.-P., Lee, D.-Y., Kim, H.-J., Lee, J.-W., Lee, S., Kang, C., Choi, W., Bin, J. H., Kim, Y. H., Han, M.-G., & Kang, H. J. (2021). Non-Polio Enteroviruses from Acute Flaccid Paralysis Surveillance in Korea, 2012–2019. Viruses, 13(3), 411. https://doi.org/10.3390/v13030411






