Abstract
Antiretroviral therapy (ART) has dramatically suppressed human immunodeficiency virus (HIV) replication and become undetectable viremia. However, a small number of residual replication-competent HIV proviruses can still persist in a latent state even with lifelong ART, fueling viral rebound in HIV-infected patient subjects after treatment interruption. Therefore, the proviral reservoirs distributed in tissues in the body represent a major obstacle to a cure for HIV infection. Given unavailable HIV vaccine and a failure to eradicate HIV proviral reservoirs by current treatment, it is crucial to develop new therapeutic strategies to eliminate proviral reservoirs for ART-free HIV remission (functional cure), including a sterilizing cure (eradication of HIV reservoirs). This review highlights recent advances in the establishment and persistence of HIV proviral reservoirs, their detection, and potential eradication strategies.
1. Introduction
In human immunodeficiency virus (HIV) life cycle, HIV RNA genome post-viral entry is reverse-transcribed into a double-stranded DNA, followed by transportation into the nucleus. Such a viral DNA/integrase complex preferentially integrates into the transcriptionally active sites of host chromosomes [1]. Notably, HIV DNA integration is an essential hallmark of viral production as integrated proviral DNA serves as a dominant template in the process of HIV replication, compared with unintegrated viral DNA forms [1,2,3]. By contrast, nonintegrated linear viral DNAs are unstable and accompanied by limited transcription to transiently express early viral elements [4,5,6,7], while nonintegrated long terminal repeat (LTR) circles, representing extrachromosomal bystander products that failed upon viral integration [8], basically lose the capacity of viral replication and are ultimately diluted due to cell proliferation [9]. However, the sporadic emergence of linear viral DNA and 2-LTR circles observed under ART is perhaps indicative of ongoing viral replication at low levels [6]. In the context of HIV replication from provirus transcription to virion assembling, unspliced RNA (~9-Kb) and more than 100 differentially spliced transcripts (predominant two-class sizes of early ~2-Kb and late ~4-Kb RNA) are generated [10,11]: the unspliced transcript is a template for gag/pol translation and viral RNA genome packaging; 4Kb incompletely spliced transcripts encode viral proteins env, vif, vpr, and vpu following export into the cytoplasm; and multiply spliced viral RNAs (~2 Kb) express regulatory tat, rev, and nef for transactivation and nuclear export of the viral RNAs [12,13,14,15,16,17,18,19,20] (Figure 1). Therefore, various cell-associated HIV RNA/DNA forms may reflect the different viral replication status and clinical significance [8,21,22,23,24,25,26]. For example, HIV gag RNA transcripts represent bona fide genomic HIV RNA or gag and gag-pol polyproteins in viral replication [27,28]; spliced HIV tat/rev RNAs are functional for viral replication and production [29]; and integrated proviral DNA is a marker to estimate the proviral reservoirs [22,24,30,31,32,33,34]. A small number of HIV-infected cells harboring proviral DNA may replenish proviral reservoirs through clonal expansion or cell division, maintaining viral persistence [9,35,36,37,38,39].
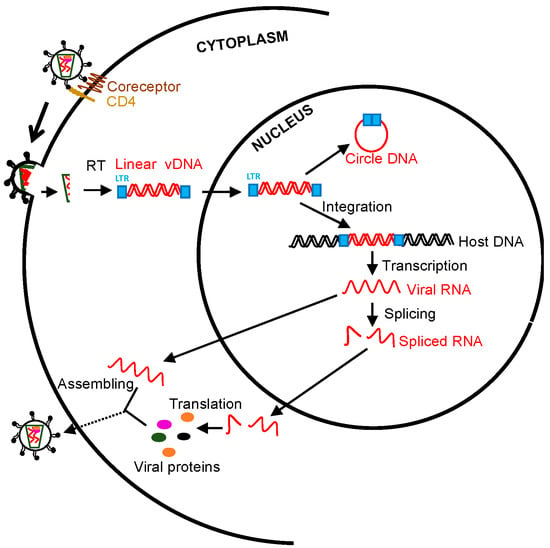
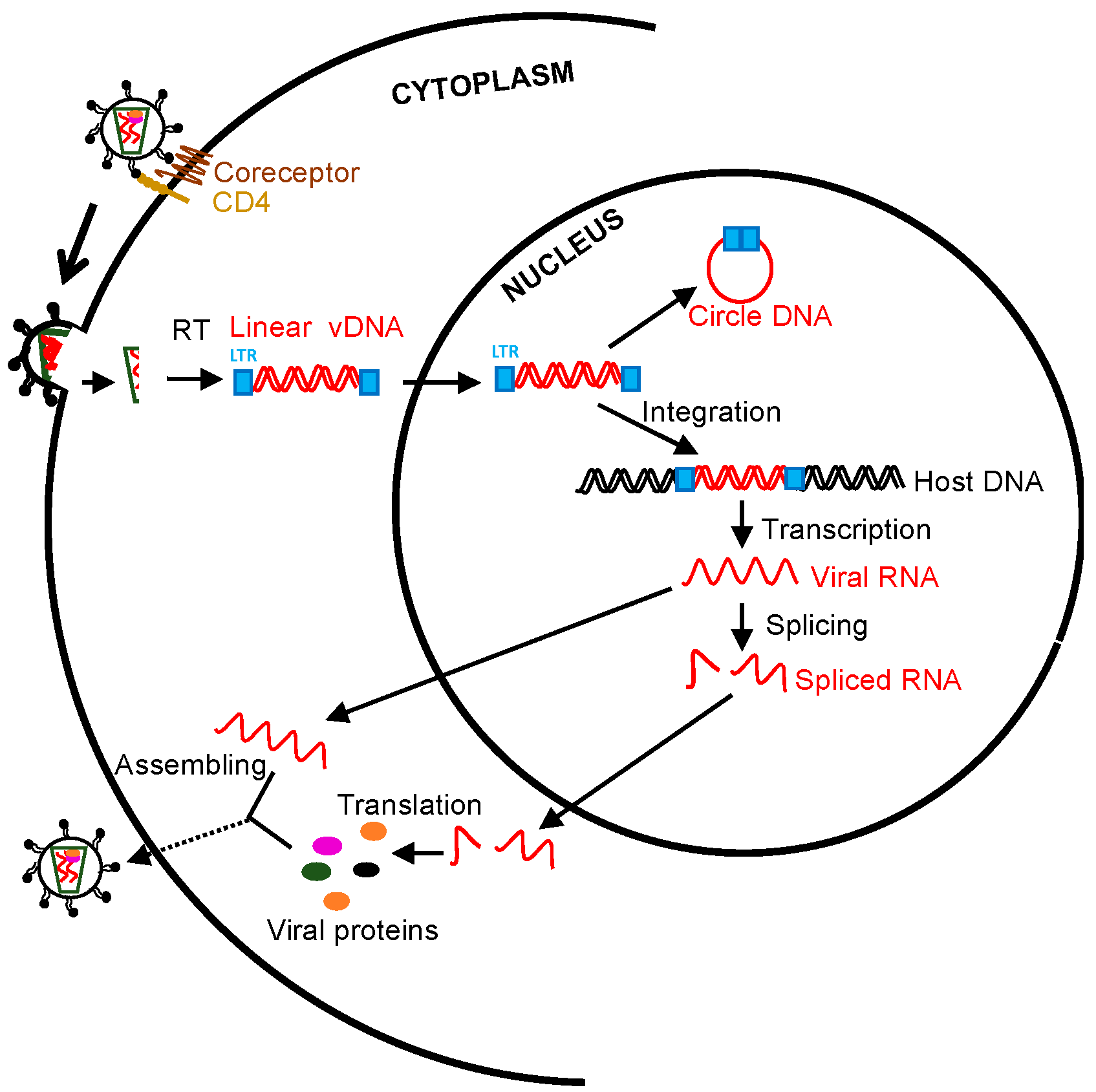
Figure 1.
Schematic overview of human immunodeficiency virus (HIV)/ simian immunodeficiency virus (SIV) life cycle and measurable viral parameters. Viral particles enter target cells, followed by reverse transcription, integration, transcription, splicing, translation, and virion packaging. The unspliced viral RNAs are transcribed from the integrated provirus. The transported single 5′ capped genomic viral RNAs (~9 Kb) are assembled to the nascent virions. Two or three guanosines 5′ full-length viral RNAs directly translate viral proteins (gag and pol). Rev-dependent export of incompletely spliced RNAs (~4 Kb) to cytoplasm contributes to env, vif, vpr, and vpu expression, whereas rev-independent multiply spliced species (~2 Kb) constitutively express accessory and regulatory proteins (tat, rev, and nef). Cell-associated viral RNA transcripts and viral DNA can be directly measured [18,19,20].
4. Measurement of HIV Reservoirs
As described in HIV life cycles, the existence and abundance of HIV RNA/DNA represent different infectious status and clinical significance [8,21,22,23,24,25,26]. The proviral reservoir could be a potential indicator to produce infectious progeny for the viral rebound after ATI. The advantages and disadvantages of the HIV reservoir measurements have been recently reviewed and discussed [139]. Current scalable assays to measure viable proviral reservoirs may underestimate the bona fide replication-competent reservoir [31,140,141,142,143,144,145,146,147], including Quantitative Viral Outgrowth Assays (QVOA) that assess the size of the replication-competent HIV latency in resting CD4+ T cells under conditions with latency reactivation [26,144,148,149], and Tat/rev-Induced limiting Dilution Assays (TILDA) that measure the frequency of productively HIV-infected cells with inducible multiply-spliced HIV transcripts [140,142,150], in which a proportion of intact proviral DNA may be non-reactivable in the host for a lifetime [144,151,152]. They may even maintaining repressive silence when intact proviral DNA integrates into the “gene desert” sites of chromosomes enriched in repressive chromatin marks [153]. In comparison, quantitative PCR [31,140,141,142,143,144,145,146] and near-Full-Length Individual Proviral sequencing (FLIPS) [154] may overestimate the proviral reservoir. More than 90% of proviruses in ART-treated patients might be replicated defective because of internal deletions, mutations, premature stop codons, or defects in splicing and packaging signals [146,155,156,157]. However, these defective proviruses may partially express viral proteins, eliciting host responses [145,158,159]. Proviral “quasispecies” may be distributed on distinct chromosomal sites in single cells of various tissues [160], and intact proviruses may not necessarily produce replication-competent virions at both transcription and translation levels [146,161,162,163], weakening the interpretation of the results from assays above. Although sensitive and practical assays are well developed, there is generally no single “gold-standard” approach to reliably evaluate proviral reservoirs in systemic and lymphoid tissues [140,150,163,164]. Biomarkers are still desperately needed to assess viral persistence, effective therapy, and treatment resumption.
5. Strategies to Eradicate Viral Reservoirs
The persistence of latent HIV-infected cellular proviral reservoirs represents the major hurdle to virus eradication in patients treated with ART. Since the transcription of HIV genes depends on cell activation state, integrated HIV DNA is transcriptionally silent in these cells and therefore unaffected by ART [165]. Therefore, various cure strategies are proposed toward HIV cure (Table 1) [114,116,117,166,167,168,169,170,171,172,173,174,175]. Of these, “shock and kill” and “block and lock” strategies are attempted to reactivate HIV-1 latency or to create a deep latent state. In “shock and kill”, combined with ART, cells harboring latent HIV provirus are activated by cytokines (e.g., IL-2), lipopolysaccharides, bacterial superantigens, anti-T cell antibodies (OKT3), histone deacetylase inhibitors/HDACi (SAHA), or protein kinase agonists. Once activated, these cells could be eliminated through viral cytopathic effects or host cytolytic T lymphocytes (CTL) responses [176,177]. However, most, if not all of these agents, are not effective in fully reactivating HIV latency in cells from patients on ART or reducing the size of latent reservoirs [178,179,180,181,182]. It remains uncertain whether ART combined with immune activation strategies could eventually (or ever) eliminate all productively infected cells through viral cytopathic effects or other immune mechanisms [166,179,183,184,185]. In the alternative “block and lock” approach, latency-promoting agents are applied to permanently prevent latency reactivation and replenishment [172,186]. However, this approach essentially lacks specificity of cells containing residual viral genome and efficiency in deep tissues, likely leading to lifelong treatment-induced adverse outcomes and complications. Given that extracellular HIV envelope glycoprotein (Env) could be presented on the productively HIV-infected cells, these cells could be recognized by anti-HIV antibody drug (e.g., toxin or radionuclides) conjugates [187,188] or broadly neutralizing antibodies (bnAbs) [173,189,190], likely leading to specifically and selectively killing of residual HIV-infected cells. However, current passive therapy by bnAbs does not fully eradicate proviral reservoir with potential in selecting escape variants and eventual viral rebound in ART-treated subjects after treatment cessation [191,192,193,194,195]. Meanwhile, although novel genome-editing technology is promising to disrupt or ablate proviral genome, it requires more advances in vivo delivery and specificity of target cells [196,197,198].

Table 1.
General HIV curative strategies.
6. Perspective
HIV cure remains the greatest challenge for therapeutic strategies. The existence of small cellular reservoirs containing an integrated intact viral genome is a major obstacle in finding a cure for HIV infection. Lifelong treatment by antiretroviral drugs or latency-reversing or promoting agents predisposes one to a high risk of physiological function and complications. The other challenges may also lie in discrepant latent status in individual HIV+ subjects and full evaluation of the proviral reservoir, not only in peripheral blood, owing to limitations in human sample collection. Among cure strategies, novel gene-editing applications in vivo might be able to potentially ablate HIV genome from cellular proviral reservoirs, albeit far more studies are still needed.
Author Contributions
X.W. and H.X. wrote the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by NIH grants R01 DE025432, HD099857, and AI147372. The funders had no role in study design, data collection and analysis, decision to publish, or manuscript preparation.
Institutional Review Board Statement
All animals in this study were housed at the Tulane National Primate Research Center in accordance with the Association for Assessment and Accreditation of Laboratory Animal Care International standards. All studies were reviewed and approved by the Tulane University Institutional Animal Care and Use Committee under protocol number P0401. Animal housing and studies were carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health (NIH, AAALAC #000594) and with the recommendations of the Weather all report; “The use of non-human primates in research”. All clinical procedures were carried out under the direction of a laboratory animal veterinarian. All procedures were performed under anesthesia using ketamine, and all efforts were made to minimize stress, improve housing conditions, and to provide enrichment opportunities (e.g., objects to manipulate in cage, varied food supplements, foraging and task-oriented feeding methods, interaction with caregivers and research staff).
Informed Consent Statement
Not applicable.
Data Availability Statement
All data generated during this study are included in this article.
Conflicts of Interest
The authors declare no competing financial interest.
Patient Consent
Patient consent was not required in this review.
References
- Craigie, R.; Bushman, F.D. HIV DNA integration. Cold Spring Harb. Perspect. Med. 2012, 2, a006890. [Google Scholar] [CrossRef] [PubMed]
- Schroder, A.R.; Shinn, P.; Chen, H.; Berry, C.; Ecker, J.R.; Bushman, F. HIV-1 integration in the human genome favors active genes and local hotspots. Cell 2002, 110, 521–529. [Google Scholar] [CrossRef]
- Maldarelli, F. The role of HIV integration in viral persistence: No more whistling past the proviral graveyard. J. Clin. Investig. 2016, 126, 438–447. [Google Scholar] [CrossRef] [PubMed]
- Koelsch, K.K.; Liu, L.; Haubrich, R.; May, S.; Havlir, D.; Gunthard, H.F.; Ignacio, C.C.; Campos-Soto, P.; Little, S.J.; Shafer, R.; et al. Dynamics of total, linear nonintegrated, and integrated HIV-1 DNA in vivo and in vitro. J. Infect. Dis. 2008, 197, 411–419. [Google Scholar] [CrossRef]
- Wu, Y.; Marsh, J.W. Early transcription from nonintegrated DNA in human immunodeficiency virus infection. J. Virol. 2003, 77, 10376–10382. [Google Scholar] [CrossRef]
- Sloan, R.D.; Wainberg, M.A. The role of unintegrated DNA in HIV infection. Retrovirology 2011, 8, 52. [Google Scholar] [CrossRef]
- Hamid, F.B.; Kim, J.; Shin, C.G. Distribution and fate of HIV-1 unintegrated DNA species: A comprehensive update. AIDS Res. Ther. 2017, 14, 9. [Google Scholar] [CrossRef]
- Policicchio, B.B.; Cardozo, E.F.; Sette, P.; Xu, C.; Haret-Richter, G.; Dunsmore, T.; Apetrei, C.; Pandrea, I.; Ribeiro, R.M. Dynamics of Simian Immunodeficiency Virus Two-Long-Terminal-Repeat Circles in the Presence and Absence of CD8+ Cells. J. Virol. 2018, 92. [Google Scholar] [CrossRef]
- Anderson, E.M.; Maldarelli, F. The role of integration and clonal expansion in HIV infection: Live long and prosper. Retrovirology 2018, 15, 71. [Google Scholar] [CrossRef] [PubMed]
- Ocwieja, K.E.; Sherrill-Mix, S.; Mukherjee, R.; Custers-Allen, R.; David, P.; Brown, M.; Wang, S.; Link, D.R.; Olson, J.; Travers, K.; et al. Dynamic regulation of HIV-1 mRNA populations analyzed by single-molecule enrichment and long-read sequencing. Nucleic Acids Res. 2012, 40, 10345–10355. [Google Scholar] [CrossRef]
- Purcell, D.F.; Martin, M.A. Alternative splicing of human immunodeficiency virus type 1 mRNA modulates viral protein expression, replication, and infectivity. J. Virol. 1993, 67, 6365–6378. [Google Scholar] [CrossRef]
- Karn, J.; Stoltzfus, C.M. Transcriptional and posttranscriptional regulation of HIV-1 gene expression. Cold Spring Harb. Perspect. Med. 2012, 2, a006916. [Google Scholar] [CrossRef] [PubMed]
- Jayaraman, B.; Crosby, D.C.; Homer, C.; Ribeiro, I.; Mavor, D.; Frankel, A.D. RNA-directed remodeling of the HIV-1 protein Rev orchestrates assembly of the Rev-Rev response element complex. eLife 2014, 3, e04120. [Google Scholar] [CrossRef] [PubMed]
- Williamson, J.R. Really exasperating viral protein from HIV. eLife 2015, 4, e05169. [Google Scholar] [CrossRef] [PubMed]
- Spector, C.; Mele, A.R.; Wigdahl, B.; Nonnemacher, M.R. Genetic variation and function of the HIV-1 Tat protein. Med. Microbiol. Immunol. 2019, 208, 131–169. [Google Scholar] [CrossRef]
- Van der Velden, G.J.; Klaver, B.; Das, A.T.; Berkhout, B. Upstream AUG codons in the simian immunodeficiency virus SIVmac239 genome regulate Rev and Env protein translation. J. Virol. 2012, 86, 12362–12371. [Google Scholar] [CrossRef] [PubMed]
- Erkelenz, S.; Poschmann, G.; Theiss, S.; Stefanski, A.; Hillebrand, F.; Otte, M.; Stuhler, K.; Schaal, H. Tra2-mediated recognition of HIV-1 5’ splice site D3 as a key factor in the processing of vpr mRNA. J. Virol. 2013, 87, 2721–2734. [Google Scholar] [CrossRef]
- Pasternak, A.O.; Lukashov, V.V.; Berkhout, B. Cell-associated HIV RNA: A dynamic biomarker of viral persistence. Retrovirology 2013, 10, 41. [Google Scholar] [CrossRef]
- Kuzembayeva, M.; Dilley, K.; Sardo, L.; Hu, W.S. Life of psi: How full-length HIV-1 RNAs become packaged genomes in the viral particles. Virology 2014, 454–455, 362–370. [Google Scholar] [CrossRef] [PubMed]
- Dubois, N.; Marquet, R.; Paillart, J.C.; Bernacchi, S. Retroviral RNA Dimerization: From Structure to Functions. Front. Microbiol. 2018, 9, 527. [Google Scholar] [CrossRef]
- Fischer, M.; Joos, B.; Hirschel, B.; Bleiber, G.; Weber, R.; Gunthard, H.F. Swiss HIV Cohort Study. Cellular viral rebound after cessation of potent antiretroviral therapy predicted by levels of multiply spliced HIV-1 RNA encoding nef. J. Infect. Dis. 2004, 190, 1979–1988. [Google Scholar] [CrossRef]
- Williams, J.P.; Hurst, J.; Stohr, W.; Robinson, N.; Brown, H.; Fisher, M.; Kinloch, S.; Cooper, D.; Schechter, M.; Tambussi, G.; et al. HIV-1 DNA predicts disease progression and post-treatment virological control. eLife 2014, 3, e03821. [Google Scholar] [CrossRef] [PubMed]
- Hong, F.; Aga, E.; Cillo, A.R.; Yates, A.L.; Besson, G.; Fyne, E.; Koontz, D.L.; Jennings, C.; Zheng, L.; Mellors, J.W. Novel Assays for Measurement of Total Cell-Associated HIV-1 DNA and RNA. J. Clin. Microbiol. 2016, 54, 902–911. [Google Scholar] [CrossRef] [PubMed]
- Kiselinova, M.; De Spiegelaere, W.; Buzon, M.J.; Malatinkova, E.; Lichterfeld, M.; Vandekerckhove, L. Integrated and Total HIV-1 DNA Predict Ex Vivo Viral Outgrowth. PLoS Pathog. 2016, 12, e1005472. [Google Scholar]
- Pasternak, A.O.; Berkhout, B. What do we measure when we measure cell-associated HIV RNA. Retrovirology 2018, 15, 13. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.L.; Pai, J.A.; Nogueira, L.; Mendoza, P.; Gruell, H.; Oliveira, T.Y.; Barton, J.; Lorenzi, J.C.C.; Cohen, Y.Z.; Cohn, L.B.; et al. Relationship between intact HIV-1 proviruses in circulating CD4+ T cells and rebound viruses emerging during treatment interruption. Proc. Natl. Acad. Sci. USA 2018, 115, E11341–E11348. [Google Scholar] [CrossRef]
- Pasternak, A.O.; DeMaster, L.K.; Kootstra, N.A.; Reiss, P.; O’Doherty, U.; Berkhout, B. Minor Contribution of Chimeric Host-HIV Readthrough Transcripts to the Level of HIV Cell-Associated gag RNA. J. Virol. 2016, 90, 1148–1151. [Google Scholar] [CrossRef] [PubMed]
- Baxter, A.E.; Niessl, J.; Fromentin, R.; Richard, J.; Porichis, F.; Charlebois, R.; Massanella, M.; Brassard, N.; Alsahafi, N.; Delgado, G.G.; et al. Single-Cell Characterization of Viral Translation-Competent Reservoirs in HIV-Infected Individuals. Cell Host Microbe 2016, 20, 368–380. [Google Scholar] [CrossRef]
- Procopio, F.A.; Fromentin, R.; Kulpa, D.A.; Brehm, J.H.; Bebin, A.G.; Strain, M.C.; Richman, D.D.; O’Doherty, U.; Palmer, S.; Hecht, F.M.; et al. A Novel Assay to Measure the Magnitude of the Inducible Viral Reservoir in HIV-infected Individuals. EBioMedicine 2015, 2, 874–883. [Google Scholar] [CrossRef]
- Yerly, S.; Gunthard, H.F.; Fagard, C.; Joos, B.; Perneger, T.V.; Hirschel, B.; Perrin, L.; Swiss HIV Cohort Study. Proviral HIV-DNA predicts viral rebound and viral setpoint after structured treatment interruptions. AIDS 2004, 18, 1951–1953. [Google Scholar] [CrossRef]
- Avettand-Fenoel, V.; Hocqueloux, L.; Ghosn, J.; Cheret, A.; Frange, P.; Melard, A.; Viard, J.P.; Rouzioux, C. Total HIV-1 DNA, a Marker of Viral Reservoir Dynamics with Clinical Implications. Clin. Microbiol. Rev. 2016, 29, 859–880. [Google Scholar] [CrossRef] [PubMed]
- Rouzioux, C.; Avettand-Fenoel, V. Total HIV DNA: A global marker of HIV persistence. Retrovirology 2018, 15, 30. [Google Scholar] [CrossRef] [PubMed]
- Cillo, A.R.; Hong, F.; Tsai, A.; Irrinki, A.; Kaur, J.; Sloan, D.D.; Follen, M.; Geleziunas, R.; Cihlar, T.; Win, S.S.; et al. Blood biomarkers of expressed and inducible HIV-1. AIDS 2018, 32, 699–708. [Google Scholar] [CrossRef] [PubMed]
- Bachmann, N.; von Siebenthal, C.; Vongrad, V.; Turk, T.; Neumann, K.; Beerenwinkel, N.; Bogojeska, J.; Fellay, J.; Roth, V.; Swiss HIV Cohort Study. Determinants of HIV-1 reservoir size and long-term dynamics during suppressive ART. Nat. Commun. 2019, 10, 3193. [Google Scholar] [CrossRef] [PubMed]
- Maldarelli, F.; Wu, X.; Su, L.; Simonetti, F.R.; Shao, W.; Hill, S.; Spindler, J.; Ferris, A.L.; Mellors, J.W.; Kearney, M.F.; et al. HIV latency. Specific HIV integration sites are linked to clonal expansion and persistence of infected cells. Science 2014, 345, 179–183. [Google Scholar] [CrossRef] [PubMed]
- Chomont, N.; El-Far, M.; Ancuta, P.; Trautmann, L.; Procopio, F.A.; Yassine-Diab, B.; Boucher, G.; Boulassel, M.R.; Ghattas, G.; Brenchley, J.M.; et al. HIV reservoir size and persistence are driven by T cell survival and homeostatic proliferation. Nat. Med. 2009, 15, 893–900. [Google Scholar] [CrossRef]
- Maldarelli, F. HIV-infected cells are frequently clonally expanded after prolonged antiretroviral therapy: Implications for HIV persistence. J. Virus Erad. 2015, 1, 237–244. [Google Scholar] [CrossRef]
- Kwon, K.J.; Siliciano, R.F. HIV persistence: Clonal expansion of cells in the latent reservoir. J. Clin. Investig. 2017, 127, 2536–2538. [Google Scholar] [CrossRef]
- Ferris, A.L.; Wells, D.W.; Guo, S.; Del Prete, G.Q.; Swanstrom, A.E.; Coffin, J.M.; Wu, X.; Lifson, J.D.; Hughes, S.H. Clonal expansion of SIV-infected cells in macaques on antiretroviral therapy is similar to that of HIV-infected cells in humans. PLoS Pathog. 2019, 15, e1007869. [Google Scholar] [CrossRef]
- Mestas, J.; Hughes, C.C. Of mice and not men: Differences between mouse and human immunology. J. Immunol. 2004, 172, 2731–2738. [Google Scholar] [CrossRef]
- Hirsch, V.M.; Lifson, J.D. Simian immunodeficiency virus infection of monkeys as a model system for the study of AIDS pathogenesis, treatment, and prevention. Adv. Pharmacol. 2000, 49, 437–477. [Google Scholar] [PubMed]
- Staprans, S.I.; Feinberg, M.B.; Shiver, J.W.; Casimiro, D.R. Role of nonhuman primates in the evaluation of candidate AIDS vaccines: An industry perspective. Curr. Opin. HIV AIDS 2010, 5, 377–385. [Google Scholar] [CrossRef] [PubMed]
- Deere, J.D.; Schinazi, R.F.; North, T.W. Simian immunodeficiency virus macaque models of HIV latency. Curr. Opin. HIV AIDS 2011, 6, 57–61. [Google Scholar] [CrossRef] [PubMed]
- Ananworanich, J.; Puthanakit, T.; Suntarattiwong, P.; Chokephaibulkit, K.; Kerr, S.J.; Fromentin, R.; Bakeman, W.; Intasan, J.; Mahanontharit, A.; Sirivichayakul, S.; et al. Reduced markers of HIV persistence and restricted HIV-specific immune responses after early antiretroviral therapy in children. AIDS 2014, 28, 1015–1020. [Google Scholar] [CrossRef]
- Del Prete, G.Q.; Lifson, J.D.; Keele, B.F. Nonhuman primate models for the evaluation of HIV-1 preventive vaccine strategies: Model parameter considerations and consequences. Curr. Opin. HIV AIDS 2016, 11, 546–554. [Google Scholar] [CrossRef] [PubMed]
- Parsons, M.S.; Le Grand, R.; Kent, S.J. Neutralizing Antibody-Based Prevention of Cell-Associated HIV-1 Infection. Viruses 2018, 10, 333. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z. Monkey Models and HIV Vaccine Research. Adv. Exp. Med. Biol. 2018, 1075, 97–124. [Google Scholar]
- Naranjo-Gomez, M.; Pelegrin, M. Vaccinal effect of HIV-1 antibody therapy. Curr. Opin. HIV AIDS 2019, 14, 325–333. [Google Scholar] [CrossRef]
- Horsburgh, B.A.; Palmer, S. For Viral Reservoir Studies, Timing Matters. Trends Microbiol. 2019, 27, 809–810. [Google Scholar] [CrossRef]
- Whitney, J.B.; Lim, S.Y.; Osuna, C.E.; Kublin, J.L.; Chen, E.; Yoon, G.; Liu, P.T.; Abbink, P.; Borducci, E.N.; Hill, A.; et al. Prevention of SIVmac251 reservoir seeding in rhesus monkeys by early antiretroviral therapy. Nat. Commun. 2018, 9, 5429. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, C.V.; Staskus, K.; Wietgrefe, S.W.; Rothenberger, M.; Reilly, C.; Chipman, J.G.; Beilman, G.J.; Khoruts, A.; Thorkelson, A.; Schmidt, T.E.; et al. Persistent HIV-1 replication is associated with lower antiretroviral drug concentrations in lymphatic tissues. Proc. Natl. Acad. Sci. USA 2014, 111, 2307–2312. [Google Scholar] [CrossRef] [PubMed]
- Fukazawa, Y.; Lum, R.; Okoye, A.A.; Park, H.; Matsuda, K.; Bae, J.Y.; Hagen, S.I.; Shoemaker, R.; Deleage, C.; Lucero, C.; et al. B cell follicle sanctuary permits persistent productive simian immunodeficiency virus infection in elite controllers. Nat. Med. 2015, 21, 132–139. [Google Scholar] [CrossRef]
- Xu, H.; Wang, X.; Malam, N.; Aye, P.P.; Alvarez, X.; Lackner, A.A.; Veazey, R.S. Persistent Simian Immunodeficiency Virus Infection Drives Differentiation, Aberrant Accumulation, and Latent Infection of Germinal Center Follicular T Helper Cells. J. Virol. 2015, 90, 1578–1587. [Google Scholar] [CrossRef]
- Wong, J.K.; Yukl, S.A. Tissue reservoirs of HIV. Curr. Opin. HIV AIDS 2016, 11, 362–370. [Google Scholar] [CrossRef]
- Collins, D.R.; Gaiha, G.D.; Walker, B.D. CD8+ T cells in HIV control, cure and prevention. Nat. Rev. Immunol. 2020, 20, 471–482. [Google Scholar] [CrossRef] [PubMed]
- Yukl, S.A.; Shergill, A.K.; Ho, T.; Killian, M.; Girling, V.; Epling, L.; Li, P.; Wong, L.K.; Crouch, P.; Deeks, S.G.; et al. The distribution of HIV DNA and RNA in cell subsets differs in gut and blood of HIV-positive patients on ART: Implications for viral persistence. J. Infect. Dis. 2013, 208, 1212–1220. [Google Scholar] [CrossRef]
- North, T.W.; Higgins, J.; Deere, J.D.; Hayes, T.L.; Villalobos, A.; Adamson, L.; Shacklett, B.L.; Schinazi, R.F.; Luciw, P.A. Viral sanctuaries during highly active antiretroviral therapy in a nonhuman primate model for AIDS. J. Virol. 2010, 84, 2913–2922. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sigal, A.; Kim, J.T.; Balazs, A.B.; Dekel, E.; Mayo, A.; Milo, R.; Baltimore, D. Cell-to-cell spread of HIV permits ongoing replication despite antiretroviral therapy. Nature 2011, 477, 95–98. [Google Scholar] [CrossRef]
- Ziani, W.; Shao, J.; Wang, X.; Russell-Lodrigue, K.; Liu, Y.Z.; Montaner, L.J.; Veazey, R.S.; Xu, H. Increased proviral DNA in circulating cells correlates with plasma viral rebound in SIV-infected rhesus macaques after antiretroviral therapy interruption. J. Virol. 2021. [Google Scholar] [CrossRef] [PubMed]
- Couturier, J.; Lewis, D.E. HIV Persistence in Adipose Tissue Reservoirs. Curr. HIV/AIDS Rep. 2018, 15, 60–71. [Google Scholar] [CrossRef] [PubMed]
- Denton, P.W.; Sogaard, O.S.; Tolstrup, M. Impacts of HIV Cure Interventions on Viral Reservoirs in Tissues. Front. Microbiol. 2019, 10, 1956. [Google Scholar] [CrossRef]
- Estes, J.D.; Kityo, C.; Ssali, F.; Swainson, L.; Makamdop, K.N.; Del Prete, G.Q.; Deeks, S.G.; Luciw, P.A.; Chipman, J.G.; Beilman, G.J.; et al. Defining total-body AIDS-virus burden with implications for curative strategies. Nat. Med. 2017, 23, 1271–1276. [Google Scholar] [CrossRef]
- Chaillon, A.; Gianella, S.; Dellicour, S.; Rawlings, S.A.; Schlub, T.E.; De Oliveira, M.F.; Ignacio, C.; Porrachia, M.; Vrancken, B.; Smith, D.M. HIV persists throughout deep tissues with repopulation from multiple anatomical sources. J. Clin. Investig. 2020, 130, 1699–1712. [Google Scholar] [CrossRef] [PubMed]
- Buzon, M.J.; Sun, H.; Li, C.; Shaw, A.; Seiss, K.; Ouyang, Z.; Martin-Gayo, E.; Leng, J.; Henrich, T.J.; Li, J.Z.; et al. HIV-1 persistence in CD4+ T cells with stem cell-like properties. Nat. Med. 2014, 20, 139–142. [Google Scholar] [CrossRef]
- Horiike, M.; Iwami, S.; Kodama, M.; Sato, A.; Watanabe, Y.; Yasui, M.; Ishida, Y.; Kobayashi, T.; Miura, T.; Igarashi, T. Lymph nodes harbor viral reservoirs that cause rebound of plasma viremia in SIV-infected macaques upon cessation of combined antiretroviral therapy. Virology 2012, 423, 107–118. [Google Scholar] [CrossRef]
- Finzi, D.; Hermankova, M.; Pierson, T.; Carruth, L.M.; Buck, C.; Chaisson, R.E.; Quinn, T.C.; Chadwick, K.; Margolick, J.; Brookmeyer, R.; et al. Identification of a reservoir for HIV-1 in patients on highly active antiretroviral therapy. Science 1997, 278, 1295–1300. [Google Scholar] [CrossRef]
- Koppensteiner, H.; Brack-Werner, R.; Schindler, M. Macrophages and their relevance in Human Immunodeficiency Virus Type I infection. Retrovirology 2012, 9, 82. [Google Scholar] [CrossRef]
- Watters, S.A.; Mlcochova, P.; Gupta, R.K. Macrophages: The neglected barrier to eradication. Curr. Opin. Infect. Dis. 2013, 26, 561–566. [Google Scholar] [CrossRef] [PubMed]
- Spiegel, H.; Herbst, H.; Niedobitek, G.; Foss, H.D.; Stein, H. Follicular dendritic cells are a major reservoir for human immunodeficiency virus type 1 in lymphoid tissues facilitating infection of CD4+ T-helper cells. Am. J. Pathol. 1992, 140, 15–22. [Google Scholar]
- Wang, X.; Ziani, W.; Xu, H. Changes in Follicular CD4+ T Helper Cells as a Marker for Evaluating Disease Progression in the Competition between HIV and Host Immunity. Front. Immunol. 2016, 7, 474. [Google Scholar] [CrossRef] [PubMed]
- Deenick, E.K.; Chan, A.; Ma, C.S.; Gatto, D.; Schwartzberg, P.L.; Brink, R.; Tangye, S.G. Follicular helper T cell differentiation requires continuous antigen presentation that is independent of unique B cell signaling. Immunity 2010, 33, 241–253. [Google Scholar] [CrossRef]
- Baumjohann, D.; Preite, S.; Reboldi, A.; Ronchi, F.; Ansel, K.M.; Lanzavecchia, A.; Sallusto, F. Persistent antigen and germinal center B cells sustain T follicular helper cell responses and phenotype. Immunity 2013, 38, 596–605. [Google Scholar] [CrossRef]
- Barnett, L.G.; Simkins, H.M.; Barnett, B.E.; Korn, L.L.; Johnson, A.L.; Wherry, E.J.; Wu, G.F.; Laufer, T.M. B cell antigen presentation in the initiation of follicular helper T cell and germinal center differentiation. J. Immunol. 2014, 192, 3607–3617. [Google Scholar] [CrossRef]
- Stieh, D.J.; Matias, E.; Xu, H.; Fought, A.J.; Blanchard, J.L.; Marx, P.A.; Veazey, R.S.; Hope, T.J. Th17 Cells Are Preferentially Infected Very Early after Vaginal Transmission of SIV in Macaques. Cell Host Microbe 2016, 19, 529–540. [Google Scholar] [CrossRef]
- Ganor, Y.; Real, F.; Sennepin, A.; Dutertre, C.A.; Prevedel, L.; Xu, L.; Tudor, D.; Charmeteau, B.; Couedel-Courteille, A.; Marion, S.; et al. HIV-1 reservoirs in urethral macrophages of patients under suppressive antiretroviral therapy. Nat. Microbiol. 2019, 4, 633–644. [Google Scholar] [CrossRef]
- Abreu, C.M.; Veenhuis, R.T.; Avalos, C.R.; Graham, S.; Parrilla, D.R.; Ferreira, E.A.; Queen, S.E.; Shirk, E.N.; Bullock, B.T.; Li, M.; et al. Myeloid and CD4 T Cells Comprise the Latent Reservoir in Antiretroviral Therapy-Suppressed SIVmac251-Infected Macaques. mBio 2019, 10. [Google Scholar] [CrossRef] [PubMed]
- Wong, M.E.; Jaworowski, A.; Hearps, A.C. The HIV Reservoir in Monocytes and Macrophages. Front. Immunol. 2019, 10, 1435. [Google Scholar] [CrossRef] [PubMed]
- DiNapoli, S.R.; Ortiz, A.M.; Wu, F.; Matsuda, K.; Twigg, H.L., 3rd; Hirsch, V.M.; Knox, K.; Brenchley, J.M. Tissue-resident macrophages can contain replication-competent virus in antiretroviral-naive, SIV-infected Asian macaques. JCI Insight 2017, 2, e91214. [Google Scholar] [CrossRef]
- Avalos, C.R.; Price, S.L.; Forsyth, E.R.; Pin, J.N.; Shirk, E.N.; Bullock, B.T.; Queen, S.E.; Li, M.; Gellerup, D.; O’Connor, S.L.; et al. Quantitation of Productively Infected Monocytes and Macrophages of Simian Immunodeficiency Virus-Infected Macaques. J. Virol. 2016, 90, 5643–5656. [Google Scholar] [CrossRef]
- Cattin, A.; Wiche Salinas, T.R.; Gosselin, A.; Planas, D.; Shacklett, B.; Cohen, E.A.; Ghali, M.P.; Routy, J.P.; Ancuta, P. HIV-1 is rarely detected in blood and colon myeloid cells during viral-suppressive antiretroviral therapy. AIDS 2019, 33, 1293–1306. [Google Scholar] [CrossRef] [PubMed]
- Massanella, M.; Bakeman, W.; Sithinamsuwan, P.; Fletcher, J.L.K.; Chomchey, N.; Tipsuk, S.; Chalermchai, T.; Routy, J.P.; Ananworanich, J.; Valcour, V.G.; et al. Infrequent HIV Infection of Circulating Monocytes during Antiretroviral Therapy. J. Virol. 2019, 94. [Google Scholar] [CrossRef] [PubMed]
- Abreu, C.M.; Veenhuis, R.T.; Avalos, C.R.; Graham, S.; Queen, S.E.; Shirk, E.N.; Bullock, B.T.; Li, M.; Metcalf Pate, K.A.; Beck, S.E.; et al. Infectious Virus Persists in CD4+ T Cells and Macrophages in Antiretroviral Therapy-Suppressed Simian Immunodeficiency Virus-Infected Macaques. J. Virol. 2019, 93. [Google Scholar] [CrossRef]
- Jambo, K.C.; Banda, D.H.; Kankwatira, A.M.; Sukumar, N.; Allain, T.J.; Heyderman, R.S.; Russell, D.G.; Mwandumba, H.C. Small alveolar macrophages are infected preferentially by HIV and exhibit impaired phagocytic function. Mucosal Immunol. 2014, 7, 1116–1126. [Google Scholar] [CrossRef]
- Damouche, A.; Lazure, T.; Avettand-Fenoel, V.; Huot, N.; Dejucq-Rainsford, N.; Satie, A.P.; Melard, A.; David, L.; Gommet, C.; Ghosn, J.; et al. Adipose Tissue Is a Neglected Viral Reservoir and an Inflammatory Site during Chronic HIV and SIV Infection. PLoS Pathog. 2015, 11, e1005153. [Google Scholar] [CrossRef]
- Zalar, A.; Figueroa, M.I.; Ruibal-Ares, B.; Bare, P.; Cahn, P.; de Bracco, M.M.; Belmonte, L. Macrophage HIV-1 infection in duodenal tissue of patients on long term HAART. Antivir. Res. 2010, 87, 269–271. [Google Scholar] [CrossRef] [PubMed]
- Bernard-Stoecklin, S.; Gommet, C.; Corneau, A.B.; Guenounou, S.; Torres, C.; Dejucq-Rainsford, N.; Cosma, A.; Dereuddre-Bosquet, N.; Le Grand, R. Semen CD4+ T cells and macrophages are productively infected at all stages of SIV infection in macaques. PLoS Pathog. 2013, 9, e1003810. [Google Scholar] [CrossRef]
- Cenker, J.J.; Stultz, R.D.; McDonald, D. Brain Microglial Cells Are Highly Susceptible to HIV-1 Infection and Spread. AIDS Res. Hum. Retrovir. 2017, 33, 1155–1165. [Google Scholar] [CrossRef]
- Andrade, V.M.; Mavian, C.; Babic, D.; Cordeiro, T.; Sharkey, M.; Barrios, L.; Brander, C.; Martinez-Picado, J.; Dalmau, J.; Llano, A.; et al. A minor population of macrophage-tropic HIV-1 variants is identified in recrudescing viremia following analytic treatment interruption. Proc. Natl. Acad. Sci. USA 2020, 117, 9981–9990. [Google Scholar] [CrossRef] [PubMed]
- Bruel, T.; Schwartz, O. Markers of the HIV-1 reservoir: Facts and controversies. Curr. Opin. HIV AIDS 2018, 13, 383–388. [Google Scholar] [CrossRef] [PubMed]
- Darcis, G.; Berkhout, B.; Pasternak, A.O. The Quest for Cellular Markers of HIV Reservoirs: Any Color You Like. Front. Immunol. 2019, 10, 2251. [Google Scholar] [CrossRef]
- Hurst, J.; Hoffmann, M.; Pace, M.; Williams, J.P.; Thornhill, J.; Hamlyn, E.; Meyerowitz, J.; Willberg, C.; Koelsch, K.K.; Robinson, N.; et al. Immunological biomarkers predict HIV-1 viral rebound after treatment interruption. Nat. Commun. 2015, 6, 8495. [Google Scholar] [CrossRef] [PubMed]
- Fenwick, C.; Joo, V.; Jacquier, P.; Noto, A.; Banga, R.; Perreau, M.; Pantaleo, G. T-cell exhaustion in HIV infection. Immunol. Rev. 2019, 292, 149–163. [Google Scholar] [CrossRef]
- Iglesias-Ussel, M.; Vandergeeten, C.; Marchionni, L.; Chomont, N.; Romerio, F. High levels of CD2 expression identify HIV-1 latently infected resting memory CD4+ T cells in virally suppressed subjects. J. Virol. 2013, 87, 9148–9158. [Google Scholar] [CrossRef] [PubMed]
- Serra-Peinado, C.; Grau-Exposito, J.; Luque-Ballesteros, L.; Astorga-Gamaza, A.; Navarro, J.; Gallego-Rodriguez, J.; Martin, M.; Curran, A.; Burgos, J.; Ribera, E.; et al. Expression of CD20 after viral reactivation renders HIV-reservoir cells susceptible to Rituximab. Nat. Commun. 2019, 10, 3705. [Google Scholar] [CrossRef] [PubMed]
- Biswas, P.; Mantelli, B.; Delfanti, F.; Ferrarini, M.; Poli, G.; Lazzarin, A. CD30 ligation differentially affects CXCR4-dependent HIV-1 replication and soluble CD30 secretion in non-Hodgkin cell lines and in gamma delta T lymphocytes. Eur. J. Immunol. 2003, 33, 3136–3145. [Google Scholar] [CrossRef] [PubMed]
- Descours, B.; Petitjean, G.; Lopez-Zaragoza, J.L.; Bruel, T.; Raffel, R.; Psomas, C.; Reynes, J.; Lacabaratz, C.; Levy, Y.; Schwartz, O.; et al. CD32a is a marker of a CD4 T-cell HIV reservoir harbouring replication-competent proviruses. Nature 2017, 543, 564–567. [Google Scholar] [CrossRef]
- Hogan, L.E.; Vasquez, J.; Hobbs, K.S.; Hanhauser, E.; Aguilar-Rodriguez, B.; Hussien, R.; Thanh, C.; Gibson, E.A.; Carvidi, A.B.; Smith, L.C.B.; et al. Increased HIV-1 transcriptional activity and infectious burden in peripheral blood and gut-associated CD4+ T cells expressing CD30. PLoS Pathog. 2018, 14, e1006856. [Google Scholar] [CrossRef]
- Garcia, M.; Navarrete-Munoz, M.A.; Ligos, J.M.; Cabello, A.; Restrepo, C.; Lopez-Bernaldo, J.C.; de la Hera, F.J.; Barros, C.; Montoya, M.; Fernandez-Guerrero, M.; et al. CD32 Expression is not Associated to HIV-DNA content in CD4 cell subsets of individuals with Different Levels of HIV Control. Sci. Rep. 2018, 8, 15541. [Google Scholar] [CrossRef]
- Abdel-Mohsen, M.; Kuri-Cervantes, L.; Grau-Exposito, J.; Spivak, A.M.; Nell, R.A.; Tomescu, C.; Vadrevu, S.K.; Giron, L.B.; Serra-Peinado, C.; Genesca, M.; et al. CD32 is expressed on cells with transcriptionally active HIV but does not enrich for HIV DNA in resting T cells. Sci. Transl. Med. 2018, 10, eaar6759. [Google Scholar] [CrossRef]
- Osuna, C.E.; Lim, S.Y.; Kublin, J.L.; Apps, R.; Chen, E.; Mota, T.M.; Huang, S.H.; Ren, Y.; Bachtel, N.D.; Tsibris, A.M.; et al. Evidence that CD32a does not mark the HIV-1 latent reservoir. Nature 2018, 561, E20–E28. [Google Scholar] [CrossRef]
- Goncalves, J.; Moreira, E.; Sequeira, I.J.; Rodrigues, A.S.; Rueff, J.; Bras, A. Integration of HIV in the Human Genome: Which Sites Are Preferential? A Genetic and Statistical Assessment. Int. J. Genom. 2016, 2016, 2168590. [Google Scholar] [CrossRef]
- Lee, S.Y.; Choi, B.S.; Yoon, C.H.; Kang, C.; Kim, K.; Kim, K.C. Selection of biomarkers for HIV-1 latency by integrated analysis. Genomics 2019, 111, 327–333. [Google Scholar] [CrossRef]
- Calin, R.; Hamimi, C.; Lambert-Niclot, S.; Carcelain, G.; Bellet, J.; Assoumou, L.; Tubiana, R.; Calvez, V.; Dudoit, Y.; Costagliola, D.; et al. Treatment interruption in chronically HIV-infected patients with an ultralow HIV reservoir. AIDS 2016, 30, 761–769. [Google Scholar] [CrossRef] [PubMed]
- Ananworanich, J.; Chomont, N.; Eller, L.A.; Kroon, E.; Tovanabutra, S.; Bose, M.; Nau, M.; Fletcher, J.L.K.; Tipsuk, S.; Vandergeeten, C.; et al. HIV DNA Set Point is Rapidly Established in Acute HIV Infection and Dramatically Reduced by Early ART. EBioMedicine 2016, 11, 68–72. [Google Scholar] [CrossRef] [PubMed]
- Henrich, T.J.; Hatano, H.; Bacon, O.; Hogan, L.E.; Rutishauser, R.; Hill, A.; Kearney, M.F.; Anderson, E.M.; Buchbinder, S.P.; Cohen, S.E.; et al. HIV-1 persistence following extremely early initiation of antiretroviral therapy (ART) during acute HIV-1 infection: An observational study. PLoS Med. 2017, 14, e1002417. [Google Scholar] [CrossRef]
- Colby, D.J.; Trautmann, L.; Pinyakorn, S.; Leyre, L.; Pagliuzza, A.; Kroon, E.; Rolland, M.; Takata, H.; Buranapraditkun, S.; Intasan, J.; et al. Rapid HIV RNA rebound after antiretroviral treatment interruption in persons durably suppressed in Fiebig I acute HIV infection. Nat. Med. 2018, 24, 923–926. [Google Scholar] [CrossRef] [PubMed]
- Prendergast, A.; Mphatswe, W.; Tudor-Williams, G.; Rakgotho, M.; Pillay, V.; Thobakgale, C.; McCarthy, N.; Morris, L.; Walker, B.D.; Goulder, P. Early virological suppression with three-class antiretroviral therapy in HIV-infected African infants. AIDS 2008, 22, 1333–1343. [Google Scholar] [CrossRef]
- Persaud, D.; Gay, H.; Ziemniak, C.; Chen, Y.H.; Piatak, M., Jr.; Chun, T.W.; Strain, M.; Richman, D.; Luzuriaga, K. Absence of detectable HIV-1 viremia after treatment cessation in an infant. N. Engl. J. Med. 2013, 369, 1828–1835. [Google Scholar] [CrossRef]
- Klein, N.; Palma, P.; Luzuriaga, K.; Pahwa, S.; Nastouli, E.; Gibb, D.M.; Rojo, P.; Borkowsky, W.; Bernardi, S.; Zangari, P.; et al. Early antiretroviral therapy in children perinatally infected with HIV: A unique opportunity to implement immunotherapeutic approaches to prolong viral remission. Lancet Infect. Dis. 2015, 15, 1108–1114. [Google Scholar] [CrossRef]
- Rainwater-Lovett, K.; Luzuriaga, K.; Persaud, D. Very early combination antiretroviral therapy in infants: Prospects for cure. Curr. Opin. HIV AIDS 2015, 10, 4–11. [Google Scholar] [CrossRef]
- Pankau, M.D.; Wamalwa, D.; Benki-Nugent, S.; Tapia, K.; Ngugi, E.; Langat, A.; Otieno, V.; Moraa, H.; Maleche-Obimbo, E.; Overbaugh, J.; et al. Decay of HIV DNA in the Reservoir and the Impact of Short Treatment Interruption in Kenyan Infants. Open Forum Infect. Dis. 2018, 5, ofx268. [Google Scholar] [CrossRef]
- Katusiime, M.G.; Halvas, E.K.; Wright, I.; Joseph, K.; Bale, M.J.; Kirby-McCullough, B.; Engelbrecht, S.; Shao, W.; Hu, W.S.; Cotton, M.F.; et al. Intact HIV Proviruses Persist in Children Seven to Nine Years after Initiation of Antiretroviral Therapy in the First Year of Life. J. Virol. 2020, 94. [Google Scholar] [CrossRef]
- Palma, P.; McManus, M.; Cotugno, N.; Rocca, S.; Rossi, P.; Luzuriaga, K. The HIV-1 antibody response: A footprint of the viral reservoir in children vertically infected with HIV. Lancet HIV 2020, 7, e359–e365. [Google Scholar] [CrossRef]
- Hutter, G.; Nowak, D.; Mossner, M.; Ganepola, S.; Mussig, A.; Allers, K.; Schneider, T.; Hofmann, J.; Kucherer, C.; Blau, O.; et al. Long-term control of HIV by CCR5 Delta32/Delta32 stem-cell transplantation. N. Engl. J. Med. 2009, 360, 692–698. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.K.; Abdul-Jawad, S.; McCoy, L.E.; Mok, H.P.; Peppa, D.; Salgado, M.; Martinez-Picado, J.; Nijhuis, M.; Wensing, A.M.J.; Lee, H.; et al. HIV-1 remission following CCR5Delta32/Delta32 haematopoietic stem-cell transplantation. Nature 2019, 568, 244–248. [Google Scholar] [CrossRef]
- Scarborough, R.J.; Goguen, R.P.; Gatignol, A. A second patient cured of HIV infection: Hopes and limitations. Virologie 2019, 23, 1–4. [Google Scholar]
- Gupta, R.K.; Peppa, D.; Hill, A.L.; Galvez, C.; Salgado, M.; Pace, M.; McCoy, L.E.; Griffith, S.A.; Thornhill, J.; Alrubayyi, A.; et al. Evidence for HIV-1 cure after CCR5Delta32/Delta32 allogeneic haemopoietic stem-cell transplantation 30 months post analytical treatment interruption: A case report. Lancet HIV 2020, 7, e340–e347. [Google Scholar] [CrossRef]
- Whitney, J.B.; Hill, A.L.; Sanisetty, S.; Penaloza-MacMaster, P.; Liu, J.; Shetty, M.; Parenteau, L.; Cabral, C.; Shields, J.; Blackmore, S.; et al. Rapid seeding of the viral reservoir prior to SIV viraemia in rhesus monkeys. Nature 2014, 512, 74–77. [Google Scholar] [CrossRef]
- Okoye, A.A.; Hansen, S.G.; Vaidya, M.; Fukazawa, Y.; Park, H.; Duell, D.M.; Lum, R.; Hughes, C.M.; Ventura, A.B.; Ainslie, E.; et al. Early antiretroviral therapy limits SIV reservoir establishment to delay or prevent post-treatment viral rebound. Nat. Med. 2018, 24, 1430–1440. [Google Scholar] [CrossRef] [PubMed]
- Pinkevych, M.; Fennessey, C.M.; Cromer, D.; Reid, C.; Trubey, C.M.; Lifson, J.D.; Keele, B.F.; Davenport, M.P. Predictors of SIV recrudescence following antiretroviral treatment interruption. eLife 2019, 8, e49022. [Google Scholar] [CrossRef]
- Wang, X.; Rasmussen, T.; Pahar, B.; Poonia, B.; Alvarez, X.; Lackner, A.A.; Veazey, R.S. Massive infection and loss of CD4+ T cells occurs in the intestinal tract of neonatal rhesus macaques in acute SIV infection. Blood 2007, 109, 1174–1181. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Xu, H.; Pahar, B.; Alvarez, X.; Green, L.C.; Dufour, J.; Moroney-Rasmussen, T.; Lackner, A.A.; Veazey, R.S. Simian immunodeficiency virus selectively infects proliferating CD4+ T cells in neonatal rhesus macaques. Blood 2010, 116, 4168–4174. [Google Scholar] [CrossRef]
- Muenchhoff, M.; Prendergast, A.J.; Goulder, P.J. Immunity to HIV in Early Life. Front. Immunol. 2014, 5, 391. [Google Scholar] [CrossRef]
- Goulder, P.J.; Lewin, S.R.; Leitman, E.M. Paediatric HIV infection: The potential for cure. Nat. Rev. Immunol. 2016, 16, 259–271. [Google Scholar] [CrossRef]
- Violari, A.; Cotton, M.F.; Gibb, D.M.; Babiker, A.G.; Steyn, J.; Madhi, S.A.; Jean-Philippe, P.; McIntyre, J.A.; CHER Study Team. Early antiretroviral therapy and mortality among HIV-infected infants. N. Engl. J. Med. 2008, 359, 2233–2244. [Google Scholar] [CrossRef]
- Cotton, M.F.; Violari, A.; Otwombe, K.; Panchia, R.; Dobbels, E.; Rabie, H.; Josipovic, D.; Liberty, A.; Lazarus, E.; Innes, S.; et al. Early time-limited antiretroviral therapy versus deferred therapy in South African infants infected with HIV: Results from the children with HIV early antiretroviral (CHER) randomised trial. Lancet 2013, 382, 1555–1563. [Google Scholar] [CrossRef]
- Luzuriaga, K.; Tabak, B.; Garber, M.; Chen, Y.H.; Ziemniak, C.; McManus, M.M.; Murray, D.; Strain, M.C.; Richman, D.D.; Chun, T.W.; et al. HIV type 1 (HIV-1) proviral reservoirs decay continuously under sustained virologic control in HIV-1-infected children who received early treatment. J. Infect. Dis. 2014, 210, 1529–1538. [Google Scholar] [CrossRef] [PubMed]
- Van Zyl, G.U.; Bedison, M.A.; van Rensburg, A.J.; Laughton, B.; Cotton, M.F.; Mellors, J.W. Early Antiretroviral Therapy in South African Children Reduces HIV-1-Infected Cells and Cell-Associated HIV-1 RNA in Blood Mononuclear Cells. J. Infect. Dis. 2015, 212, 39–43. [Google Scholar] [CrossRef]
- Martinez-Bonet, M.; Puertas, M.C.; Fortuny, C.; Ouchi, D.; Mellado, M.J.; Rojo, P.; Noguera-Julian, A.; Munoz-Fernandez, M.A.; Martinez-Picado, J. Establishment and Replenishment of the Viral Reservoir in Perinatally HIV-1-infected Children Initiating Very Early Antiretroviral Therapy. Clin. Infect. Dis. 2015, 61, 1169–1178. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Broncano, P.; Maddali, S.; Einkauf, K.B.; Jiang, C.; Gao, C.; Chevalier, J.; Chowdhury, F.Z.; Maswabi, K.; Ajibola, G.; Moyo, S.; et al. Early antiretroviral therapy in neonates with HIV-1 infection restricts viral reservoir size and induces a distinct innate immune profile. Sci. Transl. Med. 2019, 11, eaax7350. [Google Scholar] [CrossRef] [PubMed]
- Giacomet, V.; Trabattoni, D.; Zanchetta, N.; Biasin, M.; Gismondo, M.; Clerici, M.; Zuccotti, G. No cure of HIV infection in a child despite early treatment and apparent viral clearance. Lancet 2014, 384, 1320. [Google Scholar] [CrossRef]
- Luzuriaga, K.; Gay, H.; Ziemniak, C.; Sanborn, K.B.; Somasundaran, M.; Rainwater-Lovett, K.; Mellors, J.W.; Rosenbloom, D.; Persaud, D. Viremic relapse after HIV-1 remission in a perinatally infected child. N. Engl. J. Med. 2015, 372, 786–788. [Google Scholar] [CrossRef]
- Butler, K.M.; Gavin, P.; Coughlan, S.; Rochford, A.; Mc Donagh, S.; Cunningham, O.; Poulsom, H.; Watters, S.A.; Klein, N. Rapid viral rebound after 4 years of suppressive therapy in a seronegative HIV-1 infected infant treated from birth. Pediatr. Infect. Dis. J. 2015, 34, e48–e51. [Google Scholar] [CrossRef] [PubMed]
- Ateba Ndongo, F.; Texier, G.; Ida Penda, C.; Tejiokem, M.C.; Tetang Ndiang, S.; Ndongo, J.A.; Guemkam, G.; Sofeu, C.L.; Kfutwah, A.; Faye, A.; et al. Virologic Response to Early Antiretroviral Therapy in HIV-infected Infants: Evaluation After 2 Years of Treatment in the Pediacam Study, Cameroon. Pediatr. Infect. Dis. J. 2018, 37, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, L.; Strehlau, R.; Shiau, S.; Patel, F.; Shen, Y.; Technau, K.G.; Burke, M.; Sherman, G.; Coovadia, A.; Aldrovandi, G.M.; et al. Early antiretroviral treatment of infants to attain HIV remission. EClinicalMedicine 2020, 18, 100241. [Google Scholar] [CrossRef] [PubMed]
- Luzuriaga, K. Early Combination Antiretroviral Therapy Limits HIV-1 Persistence in Children. Annu. Rev. Med. 2016, 67, 201–213. [Google Scholar] [CrossRef] [PubMed]
- Simonetti, F.R.; White, J.A.; Tumiotto, C.; Ritter, K.D.; Cai, M.; Gandhi, R.T.; Deeks, S.G.; Howell, B.J.; Montaner, L.J.; Blankson, J.N.; et al. Intact proviral DNA assay analysis of large cohorts of people with HIV provides a benchmark for the frequency and composition of persistent proviral DNA. Proc. Natl. Acad. Sci. USA 2020, 117, 18692–18700. [Google Scholar] [CrossRef]
- Clarridge, K.E.; Blazkova, J.; Einkauf, K.; Petrone, M.; Refsland, E.W.; Justement, J.S.; Shi, V.; Huiting, E.D.; Seamon, C.A.; Lee, G.Q.; et al. Effect of analytical treatment interruption and reinitiation of antiretroviral therapy on HIV reservoirs and immunologic parameters in infected individuals. PLoS Pathog. 2018, 14, e1006792. [Google Scholar] [CrossRef]
- Abdel-Mohsen, M.; Richman, D.; Siliciano, R.F.; Nussenzweig, M.C.; Howell, B.J.; Martinez-Picado, J.; Chomont, N.; Bar, K.J.; Yu, X.G.; Lichterfeld, M.; et al. Recommendations for measuring HIV reservoir size in cure-directed clinical trials. Nat. Med. 2020, 26, 1339–1350. [Google Scholar] [CrossRef]
- Hodel, F.; Patxot, M.; Snaka, T.; Ciuffi, A. HIV-1 latent reservoir: Size matters. Future Virol. 2016, 11, 785–794. [Google Scholar] [CrossRef]
- Mexas, A.M.; Graf, E.H.; Pace, M.J.; Yu, J.J.; Papasavvas, E.; Azzoni, L.; Busch, M.P.; Di Mascio, M.; Foulkes, A.S.; Migueles, S.A.; et al. Concurrent measures of total and integrated HIV DNA monitor reservoirs and ongoing replication in eradication trials. AIDS 2012, 26, 2295–2306. [Google Scholar] [CrossRef] [PubMed]
- Yukl, S.A.; Kaiser, P.; Kim, P.; Telwatte, S.; Joshi, S.K.; Vu, M.; Lampiris, H.; Wong, J.K. HIV latency in isolated patient CD4+ T cells may be due to blocks in HIV transcriptional elongation, completion, and splicing. Sci. Transl. Med. 2018, 10, eaap9927. [Google Scholar] [CrossRef]
- Rutsaert, S.; Bosman, K.; Trypsteen, W.; Nijhuis, M.; Vandekerckhove, L. Digital PCR as a tool to measure HIV persistence. Retrovirology 2018, 15, 16. [Google Scholar] [CrossRef]
- Ho, Y.C.; Shan, L.; Hosmane, N.N.; Wang, J.; Laskey, S.B.; Rosenbloom, D.I.; Lai, J.; Blankson, J.N.; Siliciano, J.D.; Siliciano, R.F. Replication-competent noninduced proviruses in the latent reservoir increase barrier to HIV-1 cure. Cell 2013, 155, 540–551. [Google Scholar] [CrossRef] [PubMed]
- Imamichi, H.; Dewar, R.L.; Adelsberger, J.W.; Rehm, C.A.; O’Doherty, U.; Paxinos, E.E.; Fauci, A.S.; Lane, H.C. Defective HIV-1 proviruses produce novel protein-coding RNA species in HIV-infected patients on combination antiretroviral therapy. Proc. Natl. Acad. Sci. USA 2016, 113, 8783–8788. [Google Scholar] [CrossRef] [PubMed]
- Bruner, K.M.; Murray, A.J.; Pollack, R.A.; Soliman, M.G.; Laskey, S.B.; Capoferri, A.A.; Lai, J.; Strain, M.C.; Lada, S.M.; Hoh, R.; et al. Defective proviruses rapidly accumulate during acute HIV-1 infection. Nat. Med. 2016, 22, 1043–1049. [Google Scholar] [CrossRef]
- Siliciano, J.D.; Siliciano, R.F. Assays to Measure Latency, Reservoirs, and Reactivation. Curr. Top. Microbiol. Immunol. 2018, 417, 23–41. [Google Scholar] [PubMed]
- Massanella, M.; Richman, D.D. Measuring the latent reservoir in vivo. J. Clin. Investig. 2016, 126, 464–472. [Google Scholar] [CrossRef] [PubMed]
- Plantin, J.; Massanella, M.; Chomont, N. Inducible HIV RNA transcription assays to measure HIV persistence: Pros and cons of a compromise. Retrovirology 2018, 15, 9. [Google Scholar] [CrossRef]
- Falcinelli, S.D.; Ceriani, C.; Margolis, D.M.; Archin, N.M. New Frontiers in Measuring and Characterizing the HIV Reservoir. Front. Microbiol. 2019, 10, 2878. [Google Scholar] [CrossRef]
- Battivelli, E.; Dahabieh, M.S.; Abdel-Mohsen, M.; Svensson, J.P.; Tojal Da Silva, I.; Cohn, L.B.; Gramatica, A.; Deeks, S.; Greene, W.C.; Pillai, S.K.; et al. Distinct chromatin functional states correlate with HIV latency reactivation in infected primary CD4+ T cells. eLife 2018, 7, e34655. [Google Scholar] [CrossRef] [PubMed]
- Kwon, K.J.; Timmons, A.E.; Sengupta, S.; Simonetti, F.R.; Zhang, H.; Hoh, R.; Deeks, S.G.; Siliciano, J.D.; Siliciano, R.F. Different human resting memory CD4+ T cell subsets show similar low inducibility of latent HIV-1 proviruses. Sci. Transl. Med. 2020, 12, eaax6795. [Google Scholar] [CrossRef]
- Jiang, C.; Lian, X.; Gao, C.; Sun, X.; Einkauf, K.B.; Chevalier, J.M.; Chen, S.M.Y.; Hua, S.; Rhee, B.; Chang, K.; et al. Distinct viral reservoirs in individuals with spontaneous control of HIV-1. Nature 2020, 585, 261–267. [Google Scholar] [CrossRef]
- Hiener, B.; Horsburgh, B.A.; Eden, J.S.; Barton, K.; Schlub, T.E.; Lee, E.; von Stockenstrom, S.; Odevall, L.; Milush, J.M.; Liegler, T.; et al. Identification of Genetically Intact HIV-1 Proviruses in Specific CD4+ T Cells from Effectively Treated Participants. Cell Rep. 2017, 21, 813–822. [Google Scholar] [CrossRef] [PubMed]
- Vandergeeten, C.; Fromentin, R.; DaFonseca, S.; Lawani, M.B.; Sereti, I.; Lederman, M.M.; Ramgopal, M.; Routy, J.P.; Sekaly, R.P.; Chomont, N. Interleukin-7 promotes HIV persistence during antiretroviral therapy. Blood 2013, 121, 4321–4329. [Google Scholar] [CrossRef] [PubMed]
- Hughes, S.H.; Coffin, J.M. What Integration Sites Tell Us about HIV Persistence. Cell Host Microbe 2016, 19, 588–598. [Google Scholar] [CrossRef]
- Antar, A.A.R.; Jenike, K.M.; Jang, S.; Rigau, D.N.; Reeves, D.B.; Hoh, R.; Krone, M.R.; Keruly, J.C.; Moore, R.D.; Schiffer, J.T.; et al. Longitudinal study reveals HIV-1-infected CD4+ T cell dynamics during long-term antiretroviral therapy. J. Clin. Investig. 2020, 30, 3543–3559. [Google Scholar] [CrossRef]
- Pollack, R.A.; Jones, R.B.; Pertea, M.; Bruner, K.M.; Martin, A.R.; Thomas, A.S.; Capoferri, A.A.; Beg, S.A.; Huang, S.H.; Karandish, S.; et al. Defective HIV-1 Proviruses Are Expressed and Can Be Recognized by Cytotoxic T Lymphocytes, which Shape the Proviral Landscape. Cell Host Microbe 2017, 21, 494–506. [Google Scholar] [CrossRef] [PubMed]
- Sharaf, R.; Lee, G.Q.; Sun, X.; Etemad, B.; Aboukhater, L.M.; Hu, Z.; Brumme, Z.L.; Aga, E.; Bosch, R.J.; Wen, Y.; et al. HIV-1 proviral landscapes distinguish posttreatment controllers from noncontrollers. J. Clin. Investig. 2018, 128, 4074–4085. [Google Scholar] [CrossRef]
- Einkauf, K.B.; Lee, G.Q.; Gao, C.; Sharaf, R.; Sun, X.; Hua, S.; Chen, S.M.; Jiang, C.; Lian, X.; Chowdhury, F.Z.; et al. Intact HIV-1 proviruses accumulate at distinct chromosomal positions during prolonged antiretroviral therapy. J. Clin. Investig. 2019, 129, 988–998. [Google Scholar] [CrossRef]
- Bui, J.K.; Sobolewski, M.D.; Keele, B.F.; Spindler, J.; Musick, A.; Wiegand, A.; Luke, B.T.; Shao, W.; Hughes, S.H.; Coffin, J.M.; et al. Proviruses with identical sequences comprise a large fraction of the replication-competent HIV reservoir. PLoS Pathog. 2017, 13, e1006283. [Google Scholar] [CrossRef]
- Wiegand, A.; Spindler, J.; Hong, F.F.; Shao, W.; Cyktor, J.C.; Cillo, A.R.; Halvas, E.K.; Coffin, J.M.; Mellors, J.W.; Kearney, M.F. Single-cell analysis of HIV-1 transcriptional activity reveals expression of proviruses in expanded clones during ART. Proc. Natl. Acad. Sci. USA 2017, 114, E3659–E3668. [Google Scholar] [CrossRef]
- Baxter, A.E.; O’Doherty, U.; Kaufmann, D.E. Beyond the replication-competent HIV reservoir: Transcription and translation-competent reservoirs. Retrovirology 2018, 15, 18. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Simonetti, F.R.; Siliciano, R.F.; Laird, G.M. Measuring replication competent HIV-1: Advances and challenges in defining the latent reservoir. Retrovirology 2018, 15, 21. [Google Scholar] [CrossRef] [PubMed]
- Blankson, J.N.; Persaud, D.; Siliciano, R.F. The challenge of viral reservoirs in HIV-1 infection. Annu. Rev. Med. 2002, 53, 557–593. [Google Scholar] [CrossRef]
- Elsheikh, M.M.; Tang, Y.; Li, D.; Jiang, G. Deep latency: A new insight into a functional HIV cure. EBioMedicine 2019, 45, 624–629. [Google Scholar] [CrossRef]
- Sgadari, C.; Monini, P.; Tripiciano, A.; Picconi, O.; Casabianca, A.; Orlandi, C.; Moretti, S.; Francavilla, V.; Arancio, A.; Paniccia, G.; et al. Continued Decay of HIV Proviral DNA Upon Vaccination With HIV-1 Tat of Subjects on Long-Term ART: An 8-Year Follow-Up Study. Front. Immunol. 2019, 10, 233. [Google Scholar] [CrossRef] [PubMed]
- Pitisuttithum, P.; Nitayaphan, S.; Chariyalertsak, S.; Kaewkungwal, J.; Dawson, P.; Dhitavat, J.; Phonrat, B.; Akapirat, S.; Karasavvas, N.; Wieczorek, L.; et al. Late boosting of the RV144 regimen with AIDSVAX B/E and ALVAC-HIV in HIV-uninfected Thai volunteers: A double-blind, randomised controlled trial. Lancet HIV 2020, 7, e238–e248. [Google Scholar] [CrossRef]
- Spivak, A.M.; Planelles, V. Novel Latency Reversal Agents for HIV-1 Cure. Annu. Rev. Med. 2018, 69, 421–436. [Google Scholar] [CrossRef]
- Bashiri, K.; Rezaei, N.; Nasi, M.; Cossarizza, A. The role of latency reversal agents in the cure of HIV: A review of current data. Immunol. Lett. 2018, 196, 135–139. [Google Scholar] [CrossRef]
- Thomas, J.; Ruggiero, A.; Paxton, W.A.; Pollakis, G. Measuring the Success of HIV-1 Cure Strategies. Front. Cell. Infect. Microbiol. 2020, 10, 134. [Google Scholar] [CrossRef] [PubMed]
- Darcis, G.; Van Driessche, B.; Van Lint, C. HIV Latency: Should We Shock or Lock? Trends Immunol. 2017, 38, 217–228. [Google Scholar] [CrossRef] [PubMed]
- Caskey, M. Broadly neutralizing antibodies for the treatment and prevention of HIV infection. Curr. Opin. HIV AIDS 2020, 15, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Jacobson, J.M.; Khalili, K. Toward the Cure of HIV-1 Infection: Lessons Learned and Yet to be Learned as New Strategies are Developed. AIDS Rev. 2018, 20, 220–225. [Google Scholar] [CrossRef] [PubMed]
- Atkins, A.J.; Allen, A.G.; Dampier, W.; Haddad, E.K.; Nonnemacher, M.R.; Wigdahl, B. HIV-1 cure strategies: Why CRISPR? Expert Opin. Biol. Ther. 2020. [Google Scholar] [CrossRef]
- Deeks, S.G. HIV: Shock and kill. Nature 2012, 487, 439–440. [Google Scholar] [CrossRef]
- Archin, N.M.; Margolis, D.M. Emerging strategies to deplete the HIV reservoir. Curr. Opin. Infect. Dis. 2014, 27, 29–35. [Google Scholar] [CrossRef]
- Cillo, A.R.; Sobolewski, M.D.; Bosch, R.J.; Fyne, E.; Piatak, M., Jr.; Coffin, J.M.; Mellors, J.W. Quantification of HIV-1 latency reversal in resting CD4+ T cells from patients on suppressive antiretroviral therapy. Proc. Natl. Acad. Sci. USA 2014, 111, 7078–7083. [Google Scholar] [CrossRef]
- Del Prete, G.Q.; Shoemaker, R.; Oswald, K.; Lara, A.; Trubey, C.M.; Fast, R.; Schneider, D.K.; Kiser, R.; Coalter, V.; Wiles, A.; et al. Effect of SAHA administration on the residual virus pool in a model of combination antiretroviral therapy-mediated suppression in SIVmac239-infected Indian rhesus macaques. Antimicrob. Agents Chemother. 2014, 58, 6790–6806. [Google Scholar] [CrossRef]
- Mohammadi, P.; di Iulio, J.; Munoz, M.; Martinez, R.; Bartha, I.; Cavassini, M.; Thorball, C.; Fellay, J.; Beerenwinkel, N.; Ciuffi, A.; et al. Dynamics of HIV latency and reactivation in a primary CD4+ T cell model. PLoS Pathog. 2014, 10, e1004156. [Google Scholar] [CrossRef] [PubMed]
- Lucera, M.B.; Tilton, C.A.; Mao, H.; Dobrowolski, C.; Tabler, C.O.; Haqqani, A.A.; Karn, J.; Tilton, J.C. The histone deacetylase inhibitor vorinostat (SAHA) increases the susceptibility of uninfected CD4+ T cells to HIV by increasing the kinetics and efficiency of postentry viral events. J. Virol. 2014, 88, 10803–10812. [Google Scholar] [CrossRef]
- Jones, R.B.; O’Connor, R.; Mueller, S.; Foley, M.; Szeto, G.L.; Karel, D.; Lichterfeld, M.; Kovacs, C.; Ostrowski, M.A.; Trocha, A.; et al. Histone deacetylase inhibitors impair the elimination of HIV-infected cells by cytotoxic T-lymphocytes. PLoS Pathog. 2014, 10, e1004287. [Google Scholar] [CrossRef]
- Richman, D.D.; Margolis, D.M.; Delaney, M.; Greene, W.C.; Hazuda, D.; Pomerantz, R.J. The challenge of finding a cure for HIV infection. Science 2009, 323, 1304–1307. [Google Scholar] [CrossRef]
- Marsden, M.D.; Zack, J.A. Eradication of HIV: Current challenges and new directions. J. Antimicrob. Chemother. 2009, 63, 7–10. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Anderson, J.L.; Lewin, S.R. Getting the “Kill” into “Shock and Kill”: Strategies to Eliminate Latent HIV. Cell Host Microbe 2018, 23, 14–26. [Google Scholar] [CrossRef]
- Niu, Q.; Liu, Z.; Alamer, E.; Fan, X.; Chen, H.; Endsley, J.; Gelman, B.B.; Tian, B.; Kim, J.H.; Michael, N.L.; et al. Structure-guided drug design identifies a BRD4-selective small molecule that suppresses HIV. J. Clin. Investig. 2019, 129, 3361–3373. [Google Scholar] [CrossRef]
- Pincus, S.H.; Song, K.; Maresh, G.A.; Frank, A.; Worthylake, D.; Chung, H.K.; Polacino, P.; Hamer, D.H.; Coyne, C.P.; Rosenblum, M.G.; et al. Design and In Vivo Characterization of Immunoconjugates Targeting HIV gp160. J. Virol. 2017, 91. [Google Scholar] [CrossRef]
- Berger, E.A. Targeted cytotoxic therapy: Adapting a rapidly progressing anticancer paradigm for depletion of persistent HIV-infected cell reservoirs. Curr. Opin. HIV AIDS 2011, 6, 80–85. [Google Scholar] [CrossRef] [PubMed]
- Niessl, J.; Baxter, A.E.; Mendoza, P.; Jankovic, M.; Cohen, Y.Z.; Butler, A.L.; Lu, C.L.; Dube, M.; Shimeliovich, I.; Gruell, H.; et al. Combination anti-HIV-1 antibody therapy is associated with increased virus-specific T cell immunity. Nat. Med. 2020, 26, 222–227. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Cao, W.; Sun, M.; Li, T. Broadly neutralizing antibodies for HIV-1: Efficacies, challenges and opportunities. Emerg. Microbes Infect. 2020, 9, 194–206. [Google Scholar] [CrossRef] [PubMed]
- Cohen, Y.Z.; Caskey, M. Broadly neutralizing antibodies for treatment and prevention of HIV-1 infection. Curr. Opin. HIV AIDS 2018, 13, 366–373. [Google Scholar] [CrossRef]
- Haynes, B.F.; Burton, D.R.; Mascola, J.R. Multiple roles for HIV broadly neutralizing antibodies. Sci. Transl. Med. 2019, 11, eaaz2686. [Google Scholar] [CrossRef]
- Sok, D.; Burton, D.R. Recent progress in broadly neutralizing antibodies to HIV. Nat. Immunol. 2018, 19, 1179–1188. [Google Scholar] [CrossRef] [PubMed]
- Jaworski, J.P.; Cahn, P. Preventive and therapeutic features of broadly neutralising monoclonal antibodies against HIV-1. Lancet HIV 2018, 5, e723–e731. [Google Scholar] [CrossRef]
- Tuyishime, M.; Garrido, C.; Jha, S.; Moeser, M.; Mielke, D.; LaBranche, C.; Montefiori, D.; Haynes, B.F.; Joseph, S.; Margolis, D.M.; et al. Improved killing of HIV-infected cells using three neutralizing and non-neutralizing antibodies. J. Clin. Investig. 2020, 130, 5157–5170. [Google Scholar] [CrossRef] [PubMed]
- Panfil, A.R.; London, J.A.; Green, P.L.; Yoder, K.E. CRISPR/Cas9 Genome Editing to Disable the Latent HIV-1 Provirus. Front. Microbiol. 2018, 9, 3107. [Google Scholar] [CrossRef]
- Xiao, Q.; Guo, D.; Chen, S. Application of CRISPR/Cas9-Based Gene Editing in HIV-1/AIDS Therapy. Front. Cell. Infect. Microbiol. 2019, 9, 69. [Google Scholar] [CrossRef] [PubMed]
- Das, A.T.; Binda, C.S.; Berkhout, B. Elimination of infectious HIV DNA by CRISPR-Cas9. Curr. Opin. Virol. 2019, 38, 81–88. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).