HIV-1 Gag-Pol Sequences from Ugandan Early Infections Reveal Sequence Variants Associated with Elevated Replication Capacity
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Subjects
2.2. Amplification and Sequencing Of Transmitted Virus for Identification of Early Gag-Pol Sequences
2.3. Generation of Gag-Pol-NL4.3 Chimera Infectious Clones
2.4. In Vitro Assay for HIV-1 Replicative Capacity
2.5. Quantification of HIV-1 Reverse Transcriptase Using Radioactive and Colorimetric Assays
2.6. Protein Domain Methods
2.7. Additional
3. Results
3.1. Participant and Virus Characteristics
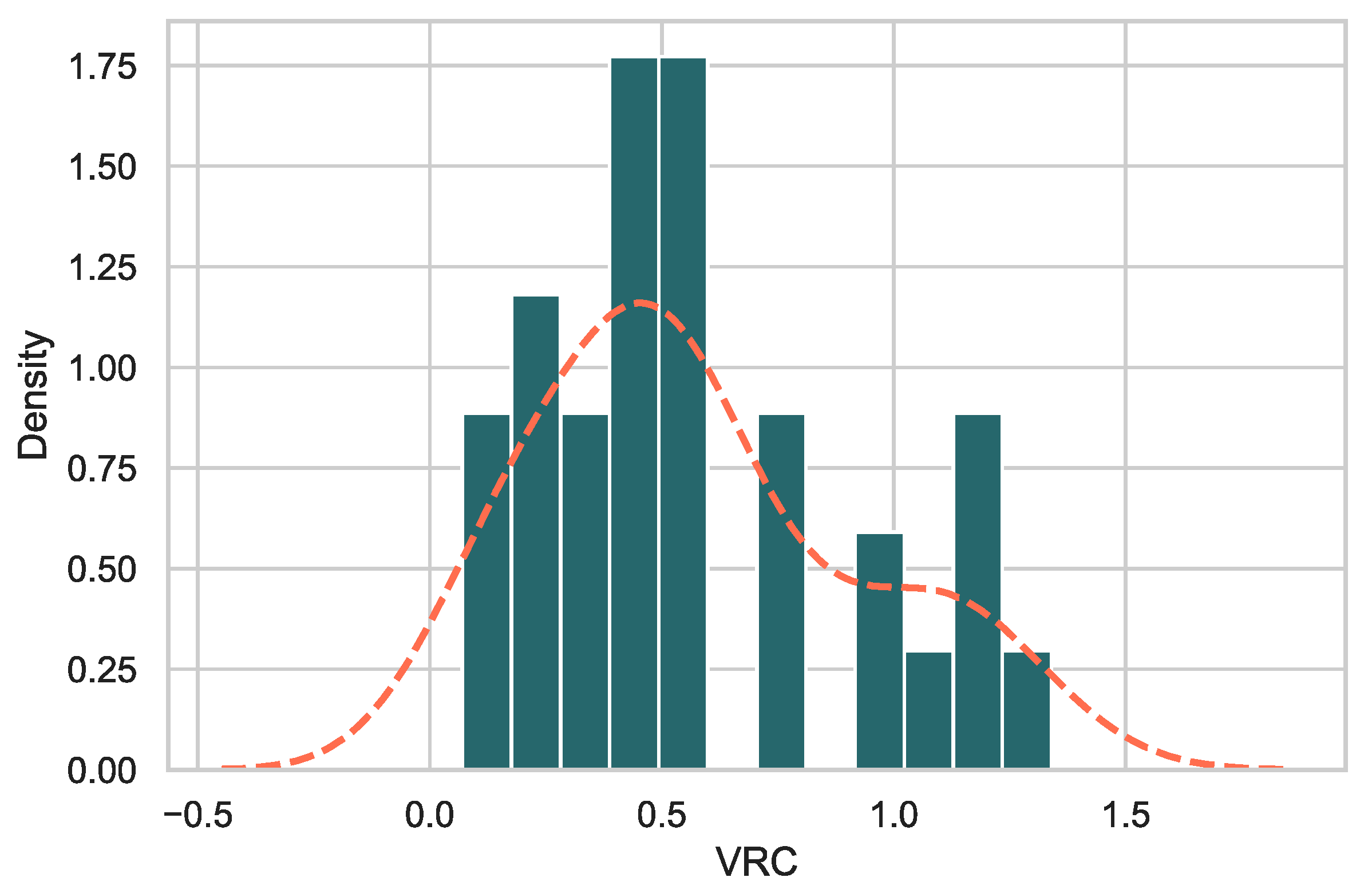
3.2. Gag-Pol-NL4.3 Chimeras Showed a Range of Replicative Capacities
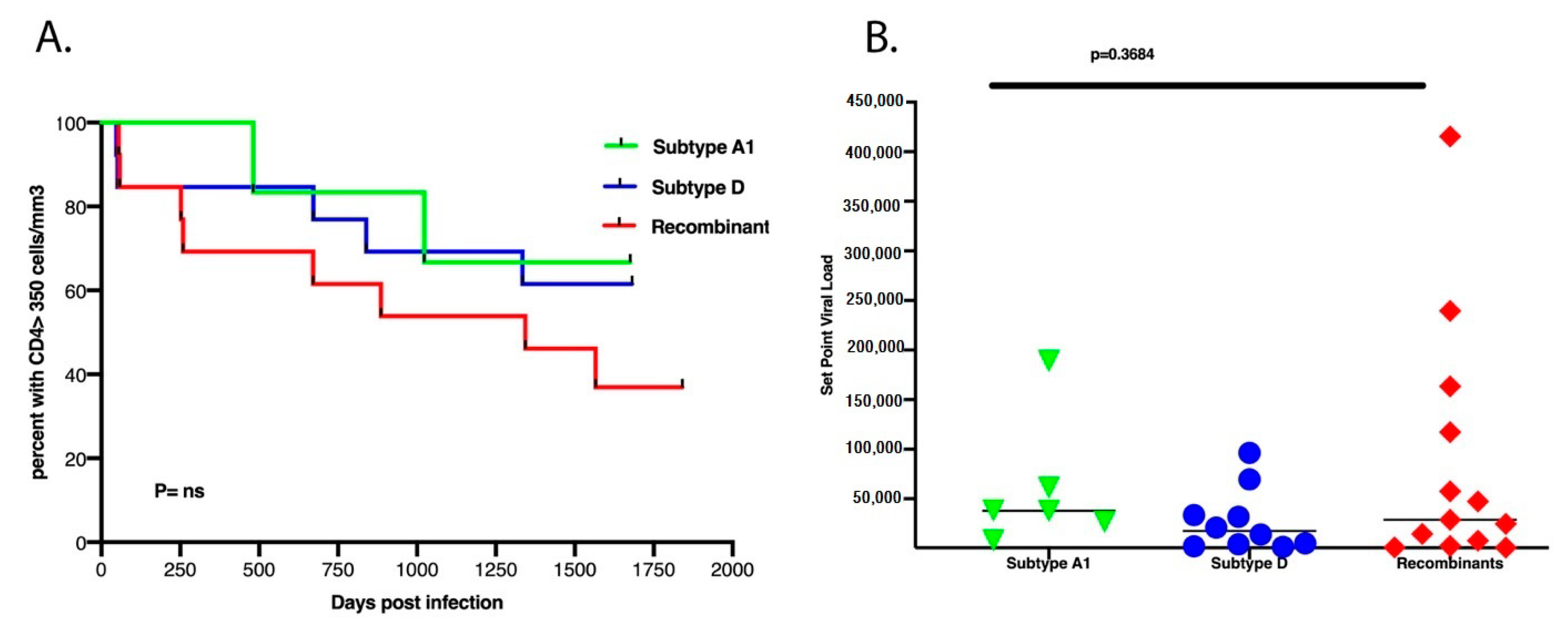
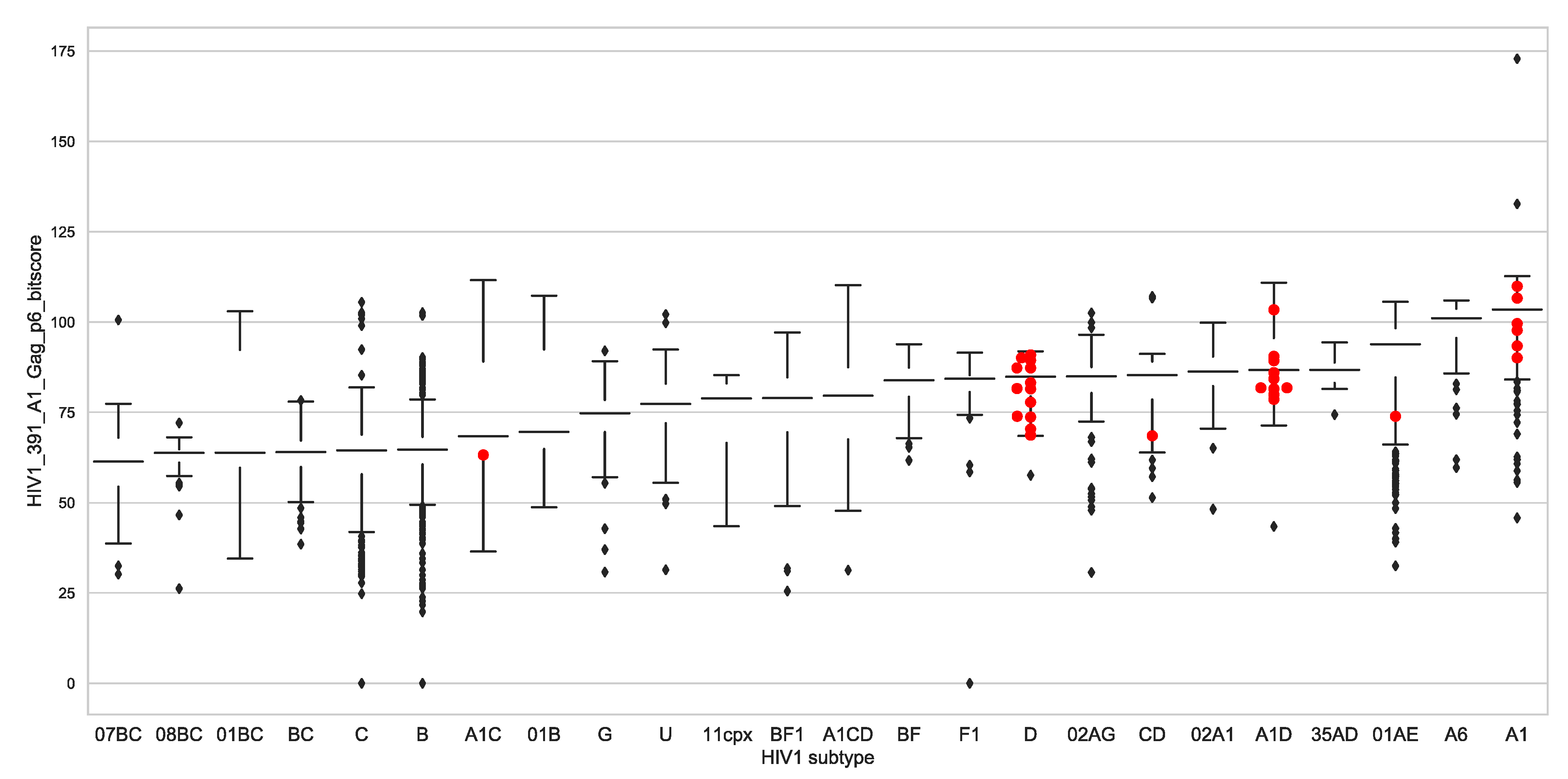
3.3. There Was No Difference in Set Point Viral Load, CD4+ T Cell Count Decline and Subtypes
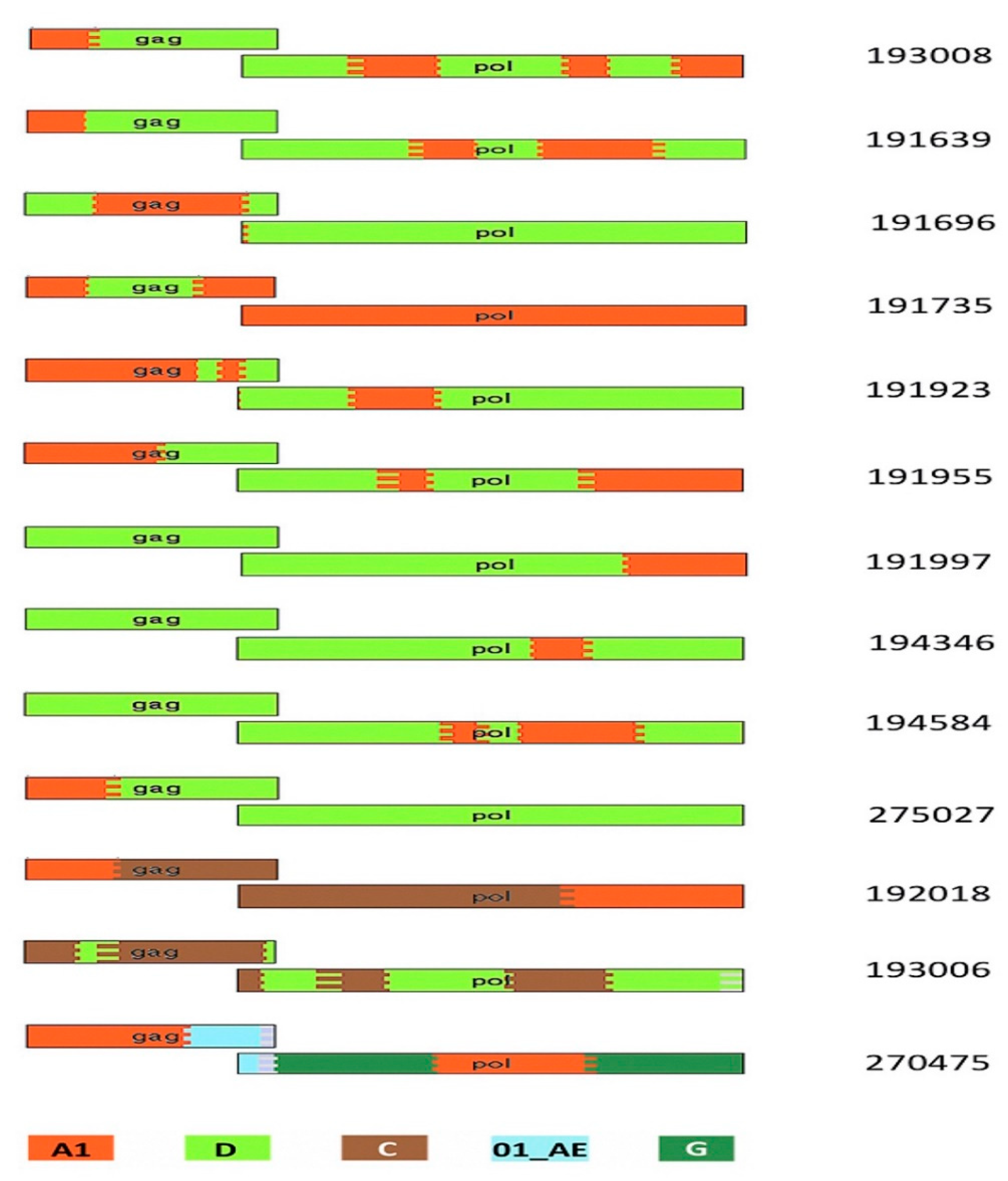
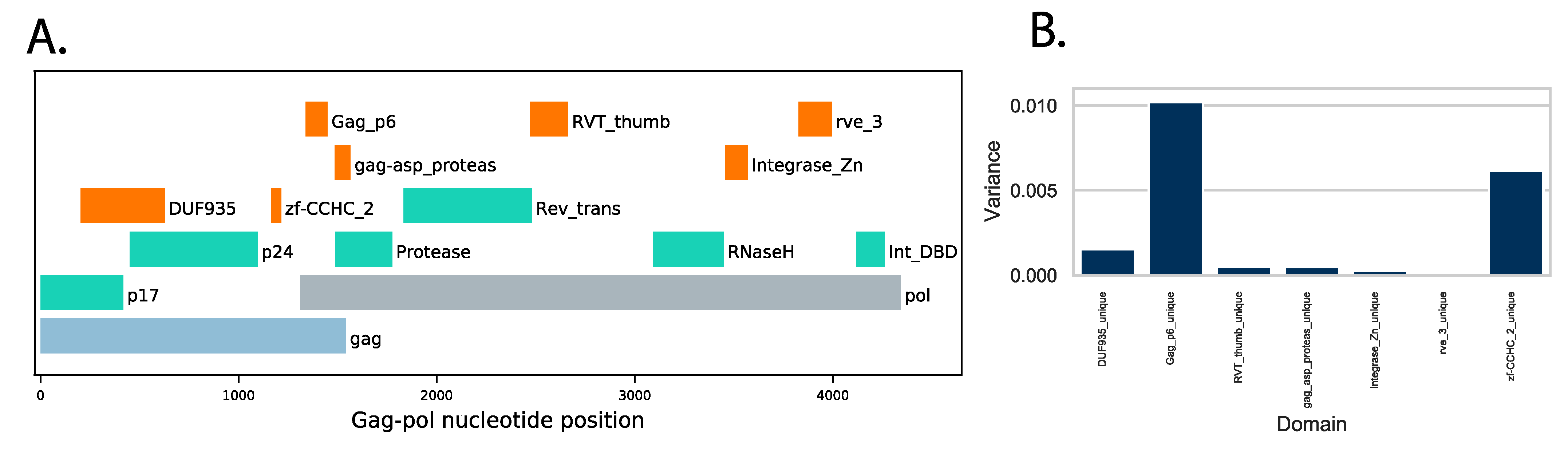
3.4. Protein Domain Diversity of Gag-Pol Regions
3.5. Variation of Gag-Pol Domains Linked to Elevated VRC
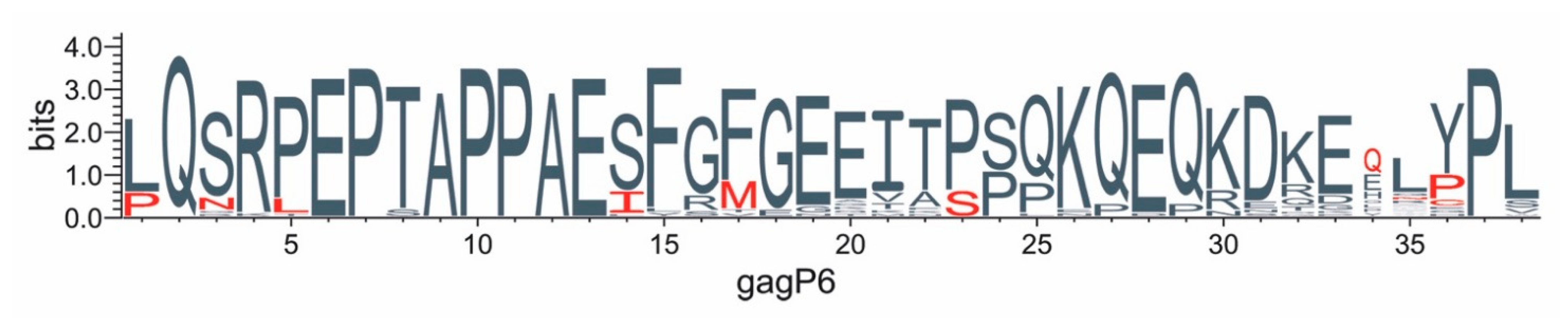
3.6. Protein Changes in Gag-P6 Region
3.7. Global Gag-P6 Domain Variation
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fiebig, E.W.; Wright, D.J.; Rawal, B.D.; Garrett, P.E.; Schumacher, R.T.; Peddada, L.; Heldebrant, C.; Smith, R.; Conrad, A.; Kleinman, S.H.; et al. Dynamics of HIV Viremia and Antibody Seroconversion in Plasma Donors: Implications for Diagnosis and Staging of Primary HIV Infection. Aids 2003, 17, 1871–1879. [Google Scholar] [CrossRef] [PubMed]
- Hansmann, A.; Schim van der Loeff, M.F.; Kaye, S.; Awasana, A.A.; Sarge-Njie, R.; O’Donovan, D.; Ariyoshi, K.; Alabi, A.; Milligan, P.; Whittle, H.C. Baseline Plasma Viral Load and CD4 Cell Percentage Predict Survival in HIV-1- and HIV-2-Infected Women in a Community-Based Cohort in The Gambia. J. Acquir. Immune Defic. Syndr. 2005, 38, 335–341. [Google Scholar] [PubMed]
- Price, M.A.; Rida, W.; Kilembe, W.; Karita, E.; Inambao, M.; Ruzagira, E.; Kamali, A.; Sanders, E.J.; Anzala, O.; Hunter, E.; et al. Control of the HIV-1 Load Varies by Viral Subtype in a Large Cohort of African Adults With Incident HIV-1 Infection. J. Infect. Dis. 2019, 220, 432–441. [Google Scholar] [CrossRef] [PubMed]
- Prentice, H.A.; Price, M.A.; Porter, T.R.; Cormier, E.; Mugavero, M.J.; Kamali, A.; Karita, E.; Lakhi, S.; Sanders, E.J.; Anzala, O.; et al. Dynamics of Viremia in Primary HIV-1 Infection in Africans: Insights from Analyses of Host and Viral Correlates. Virology 2014, 449, 254–262. [Google Scholar] [CrossRef]
- Huang, W.; Eshleman, S.H.; Toma, J.; Fransen, S.; Stawiski, E.; Paxinos, E.E.; Whitcomb, J.M.; Young, A.M.; Donnell, D.; Mmiro, F.; et al. Coreceptor Tropism in Human Immunodeficiency Virus Type 1 Subtype D: High Prevalence of CXCR4 Tropism and Heterogeneous Composition of Viral Populations. J. Virol. 2007, 81, 7885–7893. [Google Scholar] [CrossRef]
- Kaleebu, P.; Nankya, I.L.; Yirrell, D.L.; Shafer, L.A.; Kyosiimire-Lugemwa, J.; Lule, D.B.; Morgan, D.; Beddows, S.; Weber, J.; Whitworth, J.A.G. Relation between Chemokine Receptor Use, Disease Stage, and HIV-1 Subtypes A and D: Results from a Rural Ugandan Cohort. J. Acquir. Immune Defic. Syndr. 2007, 45, 28–33. [Google Scholar] [CrossRef]
- Kiwanuka, N.; Robb, M.; Laeyendecker, O.; Kigozi, G.; Wabwire-Mangen, F.; Makumbi, F.E.; Nalugoda, F.; Kagaayi, J.; Eller, M.; Eller, L.A.; et al. HIV-1 Viral Subtype Differences in the Rate of CD4+ T-Cell Decline among HIV Seroincident Antiretroviral Naive Persons in Rakai District, Uganda. J. Acquir. Immune Defic. Syndr. 2010, 54, 180–184. [Google Scholar] [CrossRef]
- Baeten, J.M.; Chohan, B.; Lavreys, L.; Chohan, V.; McClelland, R.S.; Certain, L.; Mandaliya, K.; Jaoko, W.; Overbaugh, J. HIV-1 Subtype D Infection Is Associated with Faster Disease Progression than Subtype A in Spite of Similar Plasma HIV-1 Loads. J. Infect. Dis. 2007, 195, 1177–1180. [Google Scholar] [CrossRef]
- Kiwanuka, N.; Laeyendecker, O.; Robb, M.; Kigozi, G.; Arroyo, M.; McCutchan, F.; Eller, L.A.; Eller, M.; Makumbi, F.; Birx, D.; et al. Effect of Human Immunodeficiency Virus Type 1 (HIV-1) Subtype on Disease Progression in Persons from Rakai, Uganda, with Incident HIV-1 Infection. J. Infect. Dis. 2008, 197, 707–713. [Google Scholar] [CrossRef]
- Kaleebu, P.; French, N.; Mahe, C.; Yirrell, D.; Watera, C.; Lyagoba, F.; Nakiyingi, J.; Rutebemberwa, A.; Morgan, D.; Weber, J.; et al. Effect of Human Immunodeficiency Virus (HIV) Type 1 Envelope Subtypes A and D on Disease Progression in a Large Cohort of HIV-1–Positive Persons in Uganda. J. Infect. Dis. 2002, 185, 1244–1250. [Google Scholar] [CrossRef]
- Ssemwanga, D.; Nsubuga, R.N.; Mayanja, B.N.; Lyagoba, F.; Magambo, B.; Yirrell, D.; Van der Paal, L.; Grosskurth, H.; Kaleebu, P. Effect of HIV-1 Subtypes on Disease Progression in Rural Uganda: A Prospective Clinical Cohort Study. PLoS ONE 2013, 8, e71768. [Google Scholar] [CrossRef] [PubMed]
- Prince, J.L.; Claiborne, D.T.; Carlson, J.M.; Schaefer, M.; Yu, T.; Lahki, S.; Prentice, H.A.; Yue, L.; Vishwanathan, S.A.; Kilembe, W.; et al. Role of Transmitted Gag CTL Polymorphisms in Defining Replicative Capacity and Early HIV-1 Pathogenesis. PLoS Pathog. 2012, 8, e1003041. [Google Scholar] [CrossRef] [PubMed]
- Wright, J.K.; Novitsky, V.; Brockman, M.A.; Brumme, Z.L.; Brumme, C.J.; Carlson, J.M.; Heckerman, D.; Wang, B.; Losina, E.; Leshwedi, M.; et al. Influence of Gag-Protease-Mediated Replication Capacity on Disease Progression in Individuals Recently Infected with HIV-1 Subtype C. J. Virol. 2011, 85, 3996–4006. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Claiborne, D.T.; Prince, J.L.; Scully, E.; Macharia, G.; Micci, L.; Lawson, B.; Kopycinski, J.; Deymier, M.J.; Vanderford, T.H.; Nganou-Makamdop, K.; et al. Replicative Fitness of Transmitted HIV-1 Drives Acute Immune Activation, Proviral Load in Memory CD4+ T Cells, and Disease Progression. Proc. Natl. Acad. Sci. USA 2015, 112, E1480–E1489. [Google Scholar] [CrossRef] [PubMed]
- Prado, J.G.; Prendergast, A.; Thobakgale, C.; Molina, C.; Tudor-Williams, G.; Ndung’u, T.; Walker, B.D.; Goulder, P. Replicative Capacity of Human Immunodeficiency Virus Type 1 Transmitted from Mother to Child Is Associated with Pediatric Disease Progression Rate. J. Virol. 2010, 84, 492–502. [Google Scholar] [CrossRef]
- Baalwa, J.; Wang, S.; Parrish, N.F.; Decker, J.M.; Keele, B.F.; Learn, G.H.; Yue, L.; Ruzagira, E.; Ssemwanga, D.; Kamali, A.; et al. Molecular Identification, Cloning and Characterization of Transmitted/Founder HIV-1 Subtype A, D and A/D Infectious Molecular Clones. Virology 2013, 436, 33–48. [Google Scholar] [CrossRef]
- Abraha, A.; Nankya, I.L.; Gibson, R.; Demers, K.; Tebit, D.M.; Johnston, E.; Katzenstein, D.; Siddiqui, A.; Herrera, C.; Fischetti, L.; et al. CCR5- and CXCR4-Tropic Subtype C Human Immunodeficiency Virus Type 1 Isolates Have a Lower Level of Pathogenic Fitness than Other Dominant Group M Subtypes: Implications for the Epidemic. J. Virol. 2009, 83, 5592–5605. [Google Scholar] [CrossRef]
- Ariën, K.K.; Abraha, A.; Quiñones-Mateu, M.E.; Kestens, L.; Vanham, G.; Arts, E.J. The Replicative Fitness of Primary Human Immunodeficiency Virus Type 1 (HIV-1) Group M, HIV-1 Group O, and HIV-2 Isolates. J. Virol. 2005, 79, 8979–8990. [Google Scholar] [CrossRef]
- Ball, S.C.; Abraha, A.; Collins, K.R.; Marozsan, A.J.; Baird, H.; Quiñones-Mateu, M.E.; Penn-Nicholson, A.; Murray, M.; Richard, N.; Lobritz, M.; et al. Comparing the Ex Vivo Fitness of CCR5-Tropic Human Immunodeficiency Virus Type 1 Isolates of Subtypes B and C. J. Virol. 2003, 77, 1021–1038. [Google Scholar] [CrossRef]
- Ojwach, D.B.A.; MacMillan, D.; Reddy, T.; Novitsky, V.; Brumme, Z.L.; Brockman, M.A.; Ndung’u, T.; Mann, J.K. Pol-Driven Replicative Capacity Impacts Disease Progression in HIV-1 Subtype C Infection. J. Virol. 2018, 92. [Google Scholar] [CrossRef]
- Kiguoya, M.W.; Mann, J.K.; Chopera, D.; Gounder, K.; Lee, G.Q.; Hunt, P.W.; Martin, J.N.; Ball, T.B.; Kimani, J.; Brumme, Z.L.; et al. Subtype-Specific Differences in Gag-Protease-Driven Replication Capacity Are Consistent with Intersubtype Differences in HIV-1 Disease Progression. J. Virol. 2017, 91. [Google Scholar] [CrossRef] [PubMed]
- Neogi, U.; Engelbrecht, S.; Claassen, M.; Jacobs, G.B.; van Zyl, G.; Preiser, W.; Sonnerborg, A. Mutational Heterogeneity in P6 Gag Late Assembly (L) Domains in HIV-1 Subtype C Viruses from South Africa. AIDS Res. Hum. Retrovir. 2016, 32, 80–84. [Google Scholar] [CrossRef] [PubMed]
- van Domselaar, R.; Njenda, D.T.; Rao, R.; Sönnerborg, A.; Singh, K.; Neogi, U. HIV-1 Subtype C with PYxE Insertion Has Enhanced Binding of Gag-P6 to Host Cell Protein ALIX and Increased Replication Fitness. J. Virol. 2019, 93, e00077-19. [Google Scholar] [CrossRef] [PubMed]
- Martins, A.N.; Waheed, A.A.; Ablan, S.D.; Huang, W.; Newton, A.; Petropoulos, C.J.; Brindeiro, R.D.M.; Freed, E.O. Elucidation of the Molecular Mechanism Driving Duplication of the HIV-1 PTAP Late Domain. J. Virol. 2016, 90, 768–779. [Google Scholar] [CrossRef] [PubMed]
- Kamali, A.; Price, M.A.; Lakhi, S.; Karita, E.; Inambao, M.; Sanders, E.J.; Anzala, O.; Latka, M.H.; Bekker, L.-G.; Kaleebu, P.; et al. Creating an African HIV Clinical Research and Prevention Trials Network: HIV Prevalence, Incidence and Transmission. PLoS ONE 2015, 10, e0116100. [Google Scholar] [CrossRef]
- Amornkul, P.N.; Karita, E.; Kamali, A.; Rida, W.N.; Sanders, E.J.; Lakhi, S.; Price, M.A.; Kilembe, W.; Cormier, E.; Anzala, O.; et al. Disease Progression by Infecting HIV-1 Subtype in a Seroconverter Cohort in Sub-Saharan Africa. Aids 2013, 27, 2775–2786. [Google Scholar] [CrossRef]
- Salazar-Gonzalez, J.F.; Salazar, M.G.; Keele, B.F.; Learn, G.H.; Giorgi, E.E.; Li, H.; Decker, J.M.; Wang, S.; Baalwa, J.; Kraus, M.H.; et al. Genetic Identity, Biological Phenotype, and Evolutionary Pathways of Transmitted/Founder Viruses in Acute and Early HIV-1 Infection. J. Exp. Med. 2009, 206, 1273–1289. [Google Scholar] [CrossRef]
- de Oliveira, T.; Deforche, K.; Cassol, S.; Salminen, M.; Paraskevis, D.; Seebregts, C.; Snoeck, J.; van Rensburg, E.J.; Wensing, A.M.J.; van de Vijver, D.A.; et al. An Automated Genotyping System for Analysis of HIV-1 and Other Microbial Sequences. Bioinformatics 2005, 21, 3797–3800. [Google Scholar] [CrossRef]
- Schultz, A.-K.; Zhang, M.; Bulla, I.; Leitner, T.; Korber, B.; Morgenstern, B.; Stanke, M. JpHMM: Improving the Reliability of Recombination Prediction in HIV-1. Nucleic Acids Res. 2009, 37, W647–W651. [Google Scholar] [CrossRef]
- Siepel, A.C.; Halpern, A.L.; Macken, C.; Korber, B.T. A Computer Program Designed to Screen Rapidly for HIV Type 1 Intersubtype Recombinant Sequences. AIDS Res. Hum. Retrovir. 1995, 11, 1413–1416. [Google Scholar] [CrossRef]
- Brockman, M.A.; Tanzi, G.O.; Walker, B.D.; Allen, T.M. Use of a Novel GFP Reporter Cell Line to Examine Replication Capacity of CXCR4- and CCR5-Tropic HIV-1 by Flow Cytometry. J. Virol. Methods 2006, 131, 134–142. [Google Scholar] [CrossRef] [PubMed]
- Wright, J.K.; Naidoo, V.L.; Brumme, Z.L.; Prince, J.L.; Claiborne, D.T.; Goulder, P.J.R.; Brockman, M.A.; Hunter, E.; Ndung’u, T. Impact of HLA-B*81-Associated Mutations in HIV-1 Gag on Viral Replication Capacity. J. Virol. 2012, 86, 3193–3199. [Google Scholar] [CrossRef] [PubMed]
- Eddy, S.R. Accelerated Profile HMM Searches. PLOS Comput. Biol. 2011, 7, e1002195. [Google Scholar] [CrossRef] [PubMed]
- El-Gebali, S.; Mistry, J.; Bateman, A.; Eddy, S.R.; Luciani, A.; Potter, S.C.; Qureshi, M.; Richardson, L.J.; Salazar, G.A.; Smart, A.; et al. The Pfam Protein Families Database in 2019. Nucleic Acids Res. 2019, 47, D427–D432. [Google Scholar] [CrossRef] [PubMed]
- Solis-Reyes, S.; Avino, M.; Poon, A.; Kari, L. An Open-Source k-Mer Based Machine Learning Tool for Fast and Accurate Subtyping of HIV-1 Genomes. PLoS ONE 2018, 13. [Google Scholar] [CrossRef] [PubMed]
- Yue, L.; Prentice, H.A.; Farmer, P.; Song, W.; He, D.; Lakhi, S.; Goepfert, P.; Gilmour, J.; Allen, S.; Tang, J.; et al. Cumulative Impact of Host and Viral Factors on HIV-1 Viral-Load Control during Early Infection. J. Virol. 2013, 87, 708–715. [Google Scholar] [CrossRef]
- Hollingsworth, T.D.; Laeyendecker, O.; Shirreff, G.; Donnelly, C.A.; Serwadda, D.; Wawer, M.J.; Kiwanuka, N.; Nalugoda, F.; Collinson-Streng, A.; Ssempijja, V.; et al. HIV-1 Transmitting Couples Have Similar Viral Load Set-Points in Rakai, Uganda. PLoS Pathog. 2010, 6, e1000876. [Google Scholar] [CrossRef]
- Crooks, G.E. WebLogo: A Sequence Logo Generator. Genome Res. 2004, 14, 1188–1190. [Google Scholar] [CrossRef]
- Konings, F.A.J.; Burda, S.T.; Urbanski, M.M.; Zhong, P.; Nadas, A.; Nyambi, P.N. Human Immunodeficiency Virus Type 1 (HIV-1) Circulating Recombinant Form 02_AG (CRF02_AG) Has a Higher in Vitro Replicative Capacity than Its Parental Subtypes A and G. J. Med. Virol. 2006, 78, 523–534. [Google Scholar] [CrossRef]
- Njai, H.F.; Gali, Y.; Vanham, G.; Clybergh, C.; Jennes, W.; Vidal, N.; Butel, C.; Mpoudi-Ngolle, E.; Peeters, M.; Ariën, K.K. The Predominance of Human Immunodeficiency Virus Type 1 (HIV-1) Circulating Recombinant Form 02 (CRF02_AG) in West Central Africa May Be Related to Its Replicative Fitness. Retrovirology 2006, 3, 40. [Google Scholar] [CrossRef]
- Goepfert, P.A.; Lumm, W.; Farmer, P.; Matthews, P.; Prendergast, A.; Carlson, J.M.; Derdeyn, C.A.; Tang, J.; Kaslow, R.A.; Bansal, A.; et al. Transmission of HIV-1 Gag Immune Escape Mutations Is Associated with Reduced Viral Load in Linked Recipients. J. Exp. Med. 2008, 205, 1009–1017. [Google Scholar] [CrossRef] [PubMed]
- Brockman, M.A.; Schneidewind, A.; Lahaie, M.; Schmidt, A.; Miura, T.; Desouza, I.; Ryvkin, F.; Derdeyn, C.A.; Allen, S.; Hunter, E.; et al. Escape and Compensation from Early HLA-B57-Mediated Cytotoxic T-Lymphocyte Pressure on Human Immunodeficiency Virus Type 1 Gag Alter Capsid Interactions with Cyclophilin A. J. Virol. 2007, 81, 12608–12618. [Google Scholar] [CrossRef] [PubMed]
- Müller, B.; Patschinsky, T.; Kräusslich, H.-G. The Late-Domain-Containing Protein P6 Is the Predominant Phosphoprotein of Human Immunodeficiency Virus Type 1 Particles. J. Virol. 2002, 76, 1015–1024. [Google Scholar] [CrossRef] [PubMed]
- Aralaguppe, S.G.; Winner, D.; Singh, K.; Sarafianos, S.G.; Quiñones-Mateu, M.E.; Sönnerborg, A.; Neogi, U. Increased Replication Capacity Following Evolution of PYxE Insertion in Gag-P6 Is Associated with Enhanced Virulence in HIV-1 Subtype C from East Africa. J. Med. Virol. 2017, 89, 106–111. [Google Scholar] [CrossRef] [PubMed]
- Neogi, U.; Rao, S.D.; Bontell, I.; Verheyen, J.; Rao, V.R.; Gore, S.C.; Soni, N.; Shet, A.; Schülter, E.; Ekstrand, M.L.; et al. Novel Tetra-Peptide Insertion in Gag-P6 ALIX-Binding Motif in HIV-1 Subtype C Associated with Protease Inhibitor Failure in Indian Patients. Aids 2014, 28, 2319–2322. [Google Scholar] [CrossRef]
- Gorelick, R.J.; Nigida, S.M.; Bess, J.W.; Arthur, L.O.; Henderson, L.E.; Rein, A. Noninfectious Human Immunodeficiency Virus Type 1 Mutants Deficient in Genomic RNA. J. Virol. 1990, 64, 3207–3211. [Google Scholar] [CrossRef]
- Gorelick, R.J.; Chabot, D.J.; Rein, A.; Henderson, L.E.; Arthur, L.O. The Two Zinc Fingers in the Human Immunodeficiency Virus Type 1 Nucleocapsid Protein Are Not Functionally Equivalent. J. Virol. 1993, 67, 4027–4036. [Google Scholar] [CrossRef]
- Gorelick, R.J.; Gagliardi, T.D.; Bosche, W.J.; Wiltrout, T.A.; Coren, L.V.; Chabot, D.J.; Lifson, J.D.; Henderson, L.E.; Arthur, L.O. Strict Conservation of the Retroviral Nucleocapsid Protein Zinc Finger Is Strongly Influenced by Its Role in Viral Infection Processes: Characterization of HIV-1 Particles Containing Mutant Nucleocapsid Zinc-Coordinating Sequences. Virology 1999, 256, 92–104. [Google Scholar] [CrossRef]
- Grigorov, B.; Décimo, D.; Smagulova, F.; Péchoux, C.; Mougel, M.; Muriaux, D.; Darlix, J.-L. Intracellular HIV-1 Gag Localization Is Impaired by Mutations in the Nucleocapsid Zinc Fingers. Retrovirology 2007, 4, 54. [Google Scholar] [CrossRef]
- Demirov, D.G.; Freed, E.O. Retrovirus Budding. Virus Res. 2004, 106, 87–102. [Google Scholar] [CrossRef]
- Strack, B.; Calistri, A.; Craig, S.; Popova, E.; Göttlinger, H.G. AIP1/ALIX Is a Binding Partner for HIV-1 P6 and EIAV P9 Functioning in Virus Budding. Cell 2003, 114, 689–699. [Google Scholar] [CrossRef]
- von Schwedler, U.K.; Stuchell, M.; Müller, B.; Ward, D.M.; Chung, H.-Y.; Morita, E.; Wang, H.E.; Davis, T.; He, G.-P.; Cimbora, D.M.; et al. The Protein Network of HIV Budding. Cell 2003, 114, 701–713. [Google Scholar] [CrossRef]

| Participant ID | Subtype a | VRC Score | Participant Gender | Participant Age | Days Post-EDI | Set Point Viral Load | Visit CD4 Count (Cells/µL) |
|---|---|---|---|---|---|---|---|
| 191084 | A1 | 0.21 | Female | 29 | 27 | 61,309 | 777 |
| 191637 | A1 | 0.18 | Male | 31 | 85 | 26,595 | 634 |
| 191734 | A1 | 0.35 | Male | 44 | 67 | 38,081 | 462 |
| 191918 | A1 | 0.11 | Male | 22 | 56 | 8005 | 878 |
| 194180 | A1 | 0.3 | Male | 46 | 59 | 37,767 | 438 |
| 270909 | A1 | 0.22 | Male | 41 | 50 | 189,054 | 536 |
| Mean * | -- | -- | -- | 35.5 | 57.33 | 60,135 | 621 |
| 191996 | D | 0.53 | Female | 37 | 55 | 1599 | 806 |
| 192002 | D | 0.37 | Female | 27 | 50 | 2122 | 569 |
| 194020 | D | 0.55 | Male | 33 | 36 | ND | 783 |
| 194037 | D | 0.55 | Male | 34 | 51 | 33,550 | 531 |
| 194374 | D | 0.43 | Male | 33 | 35 | 31,954 | 401 |
| 194535 | D | 0.48 | Male | 39 | 47 | ND | 281 |
| 194603 | D | 0.58 | Female | 33 | 52 | ND | 887 |
| 194604 | D | 0.53 | Female | 35 | 44 | 69,512 | 677 |
| 270015 | D | 0.15 | Male | 58 | 11 | 14,064 | 355 |
| 270535 | D | 0.07 | Male | 31 | 73 | 5197 | 796 |
| 275026 | D | 0.46 | Female | 21 | 51 | 96,368 | 277 |
| 275031 | D | 0.44 | Male | 31 | 25 | 4260 | 795 |
| 194065 | D | 0.58 | Male | 41 | 42 | 20,890 | 470 |
| Mean ** | -- | -- | -- | 34.85 | 44 | 27,952 | 587 |
| 193008 | A1D | 1.34 | Male | 27 | 23 | 57,464 | 798 |
| 191639 | A1D | 0.47 | Male | 50 | 50 | 117,145 | 1149 |
| 191696 | A1D | 0.33 | Male | 29 | 50 | 239,477 | 398 |
| 191735 | A1D | 0.38 | Male | 22 | 56 | 2482 | 754 |
| 191923 | A1D | 1.11 | Female | 31 | 55 | 28,929 | 242 |
| 191955 | A1D | 0.76 | Female | 39 | 23 | 696 | 997 |
| 191997 | A1D | 0.97 | Male | 31 | 57 | 1002 | 764 |
| 194346 | A1D | 0.75 | Male | 29 | 31 | 415,426 | 346 |
| 194584 | A1D | 0.43 | Female | 33 | 25 | 7780 | 580 |
| 275027 | A1D | 0.73 | Female | 22 | 61 | 163,395 | 651 |
| 192018 | A1C | 0.96 | Male | 22 | 29 | 24,769 | 352 |
| 193006 | CD | 1.16 | Male | 24 | 52 | 47,355 | 478 |
| 270475 | 01AE | 1.13 | Female | 28 | 43 | 14,528 | 531 |
| Mean *** | -- | -- | -- | 29.77 | 42.69 | 86,188 | 618 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kapaata, A.; Balinda, S.N.; Xu, R.; Salazar, M.G.; Herard, K.; Brooks, K.; Laban, K.; Hare, J.; Dilernia, D.; Kamali, A.; et al. HIV-1 Gag-Pol Sequences from Ugandan Early Infections Reveal Sequence Variants Associated with Elevated Replication Capacity. Viruses 2021, 13, 171. https://doi.org/10.3390/v13020171
Kapaata A, Balinda SN, Xu R, Salazar MG, Herard K, Brooks K, Laban K, Hare J, Dilernia D, Kamali A, et al. HIV-1 Gag-Pol Sequences from Ugandan Early Infections Reveal Sequence Variants Associated with Elevated Replication Capacity. Viruses. 2021; 13(2):171. https://doi.org/10.3390/v13020171
Chicago/Turabian StyleKapaata, Anne, Sheila N. Balinda, Rui Xu, Maria G. Salazar, Kimberly Herard, Kelsie Brooks, Kato Laban, Jonathan Hare, Dario Dilernia, Anatoli Kamali, and et al. 2021. "HIV-1 Gag-Pol Sequences from Ugandan Early Infections Reveal Sequence Variants Associated with Elevated Replication Capacity" Viruses 13, no. 2: 171. https://doi.org/10.3390/v13020171
APA StyleKapaata, A., Balinda, S. N., Xu, R., Salazar, M. G., Herard, K., Brooks, K., Laban, K., Hare, J., Dilernia, D., Kamali, A., Ruzagira, E., Mukasa, F., Gilmour, J., Salazar-Gonzalez, J. F., Yue, L., Cotten, M., Hunter, E., & Kaleebu, P. (2021). HIV-1 Gag-Pol Sequences from Ugandan Early Infections Reveal Sequence Variants Associated with Elevated Replication Capacity. Viruses, 13(2), 171. https://doi.org/10.3390/v13020171






