Abstract
Background: We aimed to compare the clinical severity in patients who were coinfected with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and rhinovirus or monoinfected with a single one of these viruses. Methods: The study period ranged from 1 March 2020 to 28 February 2021 (one year). SARS-CoV-2 and other respiratory viruses were identified by real-time reverse-transcription-PCR as part of the routine work at Marseille University hospitals. Bacterial and fungal infections were detected by standard methods. Clinical data were retrospectively collected from medical files. This study was approved by the ethical committee of our institute. Results: A total of 6034/15,157 (40%) tested patients were positive for at least one respiratory virus. Ninety-three (4.3%) SARS-CoV-2-infected patients were coinfected with another respiratory virus, with rhinovirus being the most frequent (62/93, 67%). Patients coinfected with SARS-CoV-2 and rhinovirus were significantly more likely to report a cough than those with SARS-CoV-2 monoinfection (62% vs. 31%; p = 0.0008). In addition, they were also significantly more likely to report dyspnea than patients with rhinovirus monoinfection (45% vs. 36%; p = 0.02). They were also more likely to be transferred to an intensive care unit and to die than patients with rhinovirus monoinfection (16% vs. 5% and 7% vs. 2%, respectively) but these differences were not statistically significant. Conclusions: A close surveillance and investigation of the co-incidence and interactions of SARS-CoV-2 and other respiratory viruses is needed. The possible higher risk of increased clinical severity in SARS-CoV-2-positive patients coinfected with rhinovirus warrants further large scale studies.
1. Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) emerged in France at the end of January 2020. Since then, on 23 September 2021, the number of cases reached 6,905,071 and 115,517 associated deaths were recorded [1]. In our institute, the first case was diagnosed at the end of February 2020 and, since then (on 31 August 2021), 57,055 patients were found positive among the 525,464 patients tested. We have previously observed that the frequency of coinfections with several respiratory viruses and the type of respiratory viruses involved were a “matter of sampling time” [2]. Indeed, very important differences were reported according to the period of the study and the region of the world where it took place as the occurrence of viral coinfections requires a co-incidence of the viral epidemic periods [2,3,4,5,6]. Likewise, major differences were observed according to the epidemic mode of the respiratory viruses involved in coinfections, i.e., according to whether they showed a bell-shaped curve of incidence or circulated throughout the year with varying intensities [7]. Contrasting with our previous work that focused on the March-April 2020 period, we studied the diagnoses of infections with SARS-CoV-2 and/or other respiratory viruses over a whole year during the SARS-CoV-2 pandemic. We centered our analyses on coinfections with SARS-CoV-2 and human rhinovirus (HRV) as this latter virus is the one that was found the most frequently associated with SARS-CoV-2 (in 41% of detected coinfections) in our center [2]. This predominance has also been reproducibly observed in several other previous studies conducted in various settings and geographical areas, with HRV being reported as the most frequent or among the most frequent respiratory viruses coinfecting COVID-19 patients [2,5,6,8,9,10,11,12,13]. Nonetheless, the clinical outcome of SARS-CoV-2 infection in patients co-infected with HRV remains unknown. In addition, HRV has a less marked seasonality compared to other respiratory viruses as it circulates throughout the year, although with various levels of incidence [7]. This is of particular interest in the study of respiratory virus coinfections with respect to the current SARS-CoV-2 pandemic. Here, we compared clinical severity in patients coinfected with a SARS-CoV-2 and HRV or monoinfected with a single of these viruses.
2. Materials and Methods
The study period ranged from 1 March 2020 to 28 February 2021 (one year). Data included the results of all diagnosis tests of infections with SARS-CoV-2 and other respiratory viruses performed as part of routine work at the clinical virology laboratory of IHU Méditerranée Infection and Assistance Publique-Hôpitaux de Marseille (Marseille university hospitals), Marseille, France. The study was approved by the ethical committee of the University Hospital Institute Méditerranée Infection (N°: 2020-029).
SARS-CoV-2 infections were diagnosed on nasopharyngeal samples collected from patients tested by real-time reverse-transcription-PCR (qPCR) using various reagents and protocols, including one previously described [14], as well as the BGI real-time fluorescent RT-PCR (BGI Genomics, Shanghai Fosun Long March Medical Science Co., Ltd., Shenzhen, China), the Biofire FilmArray Respiratory panel 2 plus (Biomérieux, Marcy-l’Etoile, France), the GeneXpert Xpert Xpress SARS-CoV-2 (Cepheid, Sunnyvale, CA, USA), or the VitaPCR SARS-CoV-2 (Credo Diagnostics Biomedical, Singapore) assays. Other viral respiratory infections were diagnosed using the FTD Respiratory pathogens 21 (Fast Track Diagnosis, Luxembourg), the Biofire FilmArray Respiratory panel 2 plus, the Respiratory Multi-Well System r-gene (Argene, bioMérieux, Marcy l’Etoile, France), or the GeneXpert Xpert Flu/RSV (Cepheid, Sunnyvale, CA, USA) assays. Co-infections were assessed on the same respiratory sample. The presence of bacterial and/or fungal pathogens was documented by conventional culture methods coupled with a MALDI-TOF mass spectrometry identification [15,16,17].
For patients coinfected with SARS-CoV-2 and HRV, demographic and clinical data were retrospectively retrieved when they were available from medical files, including comorbidities, main symptoms, in/outpatient status, transfer to an intensive care unit (ICU), and death. To evaluate the relationship between SARS-CoV-2-HRV coinfections and the clinical profiles and outcomes of the patients, we selected patients monoinfected either with SARS-CoV-2 or with HRV as controls groups, matched with cases by, in order of priority, gender, age, comorbidities, and date of diagnosis. Infections with SARS-CoV-2 and/or another respiratory virus diagnosed during the period from 1 March to 30 April 2020 for 1711 patients from the present study were described in a previous work that only focused on diagnoses performed in March and April 2020 [2].
Statistical analyses were carried out using R 4.0.2 (https://cran.r-project.org/, accessed on 1 June 2021) and Stata version 15.1 (http://www.stata.com, accessed on 1 June 2021). Qualitative variables were presented by percentage. The univariable and multivariable analyses were conducted to evaluate the epidemiological and clinical profiles of patients coinfected with SARS-CoV-2 and HRV compared to monoinfected patients. Unadjusted associations between multiple factors (socio-demographic characteristic, comorbidities, and clinical profiles) and SARS-CoV-2 infection with or without HRV coinfection were investigated. Multivariable analysis was performed using logistic regression. Variables with a p-value < 0.2 in the univariate analysis were included in the multivariate analysis. The results were presented by an odd ratio (OR) with a 95% confidence interval (CI). A p-value < 0.05 was considered statistically significant.
3. Results
During the study, 15,157 patients were tested for SARS-CoV-2 and other respiratory viruses, with 6034 (40%) testing positive for at least one virus. The distribution of infections with respiratory viruses, including SARS-CoV-2, diagnosed from 1 March 2020 to 28 February 2021 (one year), is shown on Figure 1. Ninety-three (4.3%) SARS-CoV-2-infected patients were coinfected with another virus, with HRV being the most frequent (n = 62/93; 67%), followed by adenoviruses (14; 15%) (Table 1). Among the 3875 SARS-CoV-2-negative patients, HRV was also the virus the most frequently detected in 2337 cases (39%), followed by adenoviruses (446; 7%).
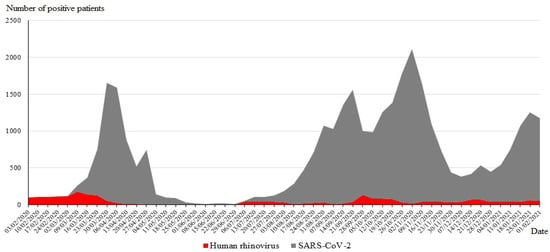
Figure 1.
Temporal distribution of the SARS-CoV-2 and HRV diagnoses from 1 March 2020 to 28 February 2021.

Table 1.
Epidemiological and virological features of SARS-CoV-2-negative and -positive patients coinfected with other respiratory viruses using the FTD Respiratory pathogens 21 (Fast Track Diagnosis, Luxembourg), the Biofire FilmArray Respiratory panel 2 plus (Biomérieux, Marcy-l’Etoile, France), the Respiratory Multi-Well System r-gene (Argene, BioMérieux), or the GeneXpert Xpert Flu/RSV (Cepheid, Sunnyvale, CA, USA) assays.
We aimed to compare the clinical severity and outcome of coinfections with SARS-CoV-2 and HRV and of monoinfections with one of these two viruses. Four of the sixty-two SARS-CoV-2-HRV-coinfected patients (7%) were excluded due to the lack of clinical data, including two males and two females with ages of 9, 12, 25, and 26 years. Using a randomly matched selection based on gender and age, 58 patients with SARS-CoV-2 monoinfection and 58 patients with a HRV monoinfection were selected as controls (Table 2); 43% of these patients were children under 15 years of age and 57% were male.

Table 2.
Descriptive analysis of SARS-CoV-2 coinfected patients and controls.
Concurrent infections with bacteria or microscopic fungi were found in ten patients. Three patients coinfected with SARS-CoV-2 and HRV were also coinfected with Candida albicans in one case, Stenotrophomonas maltophilia in one case, and with multiple bacterial and fungal pathogens (Haemophilus influenzae, Pseudomonas aeruginosa, Enterobacter gergoviae, and Candida lusitaniae) in one case. Three and four other patients in the SARS-CoV-2 and HRV monoinfection groups, respectively, were also coinfected with bacteria (Supplementary Table S1).
Table 3 shows the univariate and multivariate analyses comparing the clinical profiles and outcomes of the three groups of patients. In the univariate analysis, patients coinfected with SARS-CoV-2 and HRV were significantly more likely to report a cough than those with SARS-CoV-2 monoinfection (62% versus 31%; p = 0.0008). They were also significantly more likely to report dyspnea than patients with HRV monoinfection (45% versus 36%; p = 0.02). These associations remained significant in the multivariate analysis. In addition, patients who were coinfected with SARS-CoV-2 and HRV had a non-statistically significant increased risk of being transferred to an intensive care unit (ICU) and to die than those infected only with HRV (16% versus 5% and 6.9% versus 1.7%, respectively).

Table 3.
Coinfection with human rhinovirus and severity of COVID-19 diseases.
Finally, ten patients with SARS-CoV-2 and/or HRV infections were also diagnosed with bacteria or Candida spp. The detection of these microorganisms can indicate the most likely colonizations rather than bronchopulmonary infections. Of these ten patients, seven were hospitalized (two coinfected with SARS-CoV-2 and rhinovirus, three SARS-CoV-2-monoinfected, and two rhinovirus-monoinfected), three were transferred to an ICU (two SARS-CoV-2 and rhinovirus-coinfected and one SARS-CoV-2-monoinfected), and two died (one SARS-CoV-2 and rhinovirus-coinfected and one SARS-CoV-2-monoinfected) (Supplementary Table S1).
4. Discussion
Over a whole year from March 2020 through February 2021, rhinovirus was, by far, the most frequently diagnosed respiratory virus in our institute, either in association with SARS-CoV-2 or alone. To our knowledge, to date, no study systematically assessed the severity of infections with SARS-CoV-2 and rhinovirus, but prolonged SARS-CoV-2 persistence beyond the acute infection phase was significantly associated with HRV/enterovirus co-infection [11]. It could increase the capacity of viral transmission [11]. Previous reports were case reports [18,19]. Orozoco-Hermandez et al. reported increased severity of initially mild COVID-19 symptomatology in a 41-year-old patient with coinfection with rhinovirus or enterovirus who developed multilobar pneumonia requiring admission to ICU [18]. A case of SARS-CoV-2 and rhinovirus–enterovirus coinfection was also reported in a pregnant woman [19]. Our results notably suggest that patients coinfected with SARS-CoV-2 and rhinovirus were more likely to suffer dyspnea than those infected with rhinovirus only. A trend toward increased risks for both transfer to an ICU and death was also seen in patients coinfected with SARS-CoV-2 and rhinovirus compared to those monoinfected with rhinovirus. The putative interactions and clinical impact of HRV and SARS-CoV-2 co-infections were, thus, very scarcely addressed. Here, co-infected patients presented significantly more frequently cough, compared to those with mono-SARS-CoV-2 infection. Although the interaction between these viruses is still unclear, the co-detection of HRV has also been associated with mild COVID-19 [10,12]. One potential reason for this phenomenon is that viral coinfection is more common in young persons, aged between 15 and 64 years [10]. Besides, a recent in vitro study reported an indirect negative interaction between HRV and SARS-CoV-2 and hypothesized that HRV can trigger an interferon response that makes most cells nonpermissive to SARS-CoV-2 infection, blocking its replication [20].
The present study has some limitations. Not all patients diagnosed as infected with SARS-CoV-2 or HRV during the study period were systematically tested for the other respiratory virus. In addition, we were not able to classify these cases as concomitant infections or superinfections, as the study was not designed for this aim and same respiratory samples were tested for both viruses that have different incubation duration and may differ regarding their persistence duration. In addition, several biomarkers known to be associated with respiratory disease severity, including lactate dehydrogenase level, D-dimers value, thrombocyte count, and troponin level [21], were not considered either. Moreover, we did not provide information on the duration of symptoms which could differ between patients who were co- or monoinfected with SARS-CoV-2 and rhinovirus. Additionally, we did not follow up the patient after discharge. Finally, the duration and intensity of viral shedding were not evaluated. Since the future of the current SARS-CoV-2 pandemic is unknown, a close surveillance and investigation of the co-incidence and interactions of SARS-CoV-2 and other respiratory viruses are needed. Further large-scale studies are needed to investigate the role of co-infection between SARS-CoV-2 and HRV in severity of COVID-19.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/v13122528/s1, Table S1: Concurrent infections with bacteria or microscopic fungi among studied patients.
Author Contributions
Conceived and designed the experiments: P.C. and P.G. Contributed materials/analysis tools: E.L.G., V.T.H., C.B., L.N., C.Z., A.B., V.B., G.D., S.R., J.-C.L., M.M., M.D., P.-E.F. and P.C. Analyzed the data: E.L.G., V.T.H., C.B., P.C. and P.G. Wrote the paper: E.L.G., V.T.H., P.G. and P.C. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the French Government under the “Investments for the Future” program managed by the National Agency for Research (ANR), Méditerranée-Infection 10-IAHU-03 and was also supported by Région Provence Alpes Côte d’Azur and European funding FEDER PRIMMI (Fonds Européen de Développement Régional-Plateformes de Recherche et d’Innovation Mutualisées Méditerranée Infection), FEDER PA 0000320 PRIMMI.
Institutional Review Board Statement
All data were generated as part of the routine work at Assistance Publique-Hôpitaux de Marseille (Marseille university hospitals). This study was approved by the ethical committee of the University Hospital Institute Méditerranée Infection (N°: 2020-029; June 2020).
Informed Consent Statement
This study results from routine standard clinical management. Access to the patients’ biological and registry data issued from the hospital information system was approved by the data protection committee of Assistance Publique-Hôpitaux de Marseille (APHM) and was recorded in the European General Data Protection Regulation registry under number RGPD/APHM 2019-73.
Data Availability Statement
The data that support the findings of this study will be available from [PC] upon reasonable request.
Conflicts of Interest
The authors have no conflict of interest to declare. Funding sources had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript.
References
- European Centre for Disease Prevention and Control. COVID-19 Situation Update Worldwide, as of Week 36, Updated 16 September 2021. Available online: https://www.ecdc.europa.eu/en/geographical-distribution-2019-ncov-cases (accessed on 23 September 2021).
- Boschi, C.; Hoang, V.T.; Giraud-Gatineau, A.; Ninove, L.; Lagier, J.-C.; La Scola, B.; Gautret, P.; Raoult, D.; Colson, P. Coinfections with SARS-CoV-2 and other respiratory viruses in Southeastern France: A matter of sampling time. J. Med. Virol. 2021, 93, 1878–1881. [Google Scholar] [CrossRef] [PubMed]
- Nowak, M.D.; Sordillo, E.M.; Gitman, M.R.; Mondolfi, A.E.P. Coinfection in SARS-CoV-2 infected patients: Where are influenza virus and rhinovirus/enterovirus? J. Med. Virol. 2020, 92, 1699–1700. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Quinn, J.; Pinsky, B.; Shah, N.H.; Brown, I. Rates of Co-infection Between SARS-CoV-2 and Other Respiratory Pathogens. JAMA 2020, 323, 2085–2086. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aghbash, P.S.; Eslami, N.; Shirvaliloo, M.; Baghi, H.B. Viral coinfections in COVID-19. J. Med. Virol. 2021, 93, 5310–5322. [Google Scholar] [CrossRef] [PubMed]
- Musuuza, J.S.; Watson, L.; Parmasad, V.; Putman-Buehler, N.; Christensen, L.; Safdar, N. Prevalence and outcomes of co-infection and superinfection with SARS-CoV-2 and other pathogens: A systematic review and meta-analysis. PLoS ONE 2021, 16, e0251170. [Google Scholar] [CrossRef] [PubMed]
- Moriyama, M.; Hugentobler, W.J.; Iwasaki, A. Seasonality of Respiratory Viral Infections. Annu. Rev. Virol. 2020, 7, 83–101. [Google Scholar] [CrossRef] [PubMed]
- Vandini, S.; Biagi, C.; Fischer, M.; Lanari, M. Impact of Rhinovirus Infections in Children. Viruses 2019, 11, 521. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hazra, A.; Collison, M.; Pisano, J.; Kumar, M.; Oehler, C.; Ridgway, J.P. Coinfections with SARS-CoV-2 and other respiratory pathogens. Infect. Control. Hosp. Epidemiol. 2020, 41, 1228–1229. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Ge, Y.; Wu, T.; Zhao, K.; Chen, Y.; Wu, B.; Zhu, F.; Zhu, B.; Cui, L. Co-infection with respiratory pathogens among COVID-2019 cases. Virus Res. 2020, 285, 198005. [Google Scholar] [CrossRef] [PubMed]
- Brotons, P.; Jordan, I.; Bassat, Q.; Henares, D.; de Sevilla, M.F.; Ajanovic, S.; Redin, A.; Fumado, V.; Baro, B.; Claverol, J.; et al. The Positive Rhinovirus/Enterovirus Detection and SARS-CoV-2 Persistence beyond the Acute Infection Phase: An Intra-Household Surveillance Study. Viruses 2021, 13, 1598. [Google Scholar] [CrossRef] [PubMed]
- Wee, L.E.; Ko, K.K.K.; Ho, W.Q.; Kwek, G.T.C.; Tan, T.T.; Wijaya, L. Community-acquired viral respiratory infections amongst hospitalized inpatients during a COVID-19 outbreak in Singapore: Co-infection and clinical outcomes. J. Clin. Virol. 2020, 128, 104436. [Google Scholar] [CrossRef] [PubMed]
- Peci, A.; Tran, V.; Guthrie, J.; Li, Y.; Nelson, P.; Schwartz, K.; Eshaghi, A.; Buchan, S.; Gubbay, J. Prevalence of Co-Infections with Respiratory Viruses in Individuals Investigated for SARS-CoV-2 in Ontario, Canada. Viruses 2021, 13, 130. [Google Scholar] [CrossRef] [PubMed]
- Amrane, S.; Tissot-Dupont, H.; Doudier, B.; Eldin, C.; Hocquart, M.; Mailhe, M.; Dudouet, P.; Ormières, E.; Ailhaud, L.; Parola, P.; et al. Rapid viral diagnosis and ambulatory management of suspected COVID-19 cases presenting at the infectious diseases referral hospital in Marseille, France, -January 31st to March 1st, 2020: A respiratory virus snapshot. Travel Med. Infect. Dis. 2020, 36, 101632. [Google Scholar] [CrossRef] [PubMed]
- Dubourg, G.; Baron, S.; Cadoret, F.; Couderc, C.; Fournier, P.-E.; Lagier, J.-C.; Raoult, D. From Culturomics to Clinical Microbiology and Forward. Emerg. Infect. Dis. 2018, 24, 1683–1690. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cassagne, C.; Normand, A.-C.; Bonzon, L.; L’Ollivier, C.; Gautier, M.; Jeddi, F.; Ranque, S.; Piarroux, R. Routine identification and mixed species detection in 6,192 clinical yeast isolates. Med. Mycol. 2015, 54, 256–265. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gautier, M.; Ranque, S.; Normand, A.-C.; Becker, P.; Packeu, A.; Cassagne, C.; L’Ollivier, C.; Hendrickx, M.; Piarroux, R. Matrix-assisted laser desorption ionization time-of-flight mass spectrometry: Revolutionizing clinical laboratory diagnosis of mould infections. Clin. Microbiol. Infect. 2014, 20, 1366–1371. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Orozco-Hernández, J.P.; Montoya-Martínez, J.J.; Pacheco-Gallego, M.C.; Céspedes-Roncancio, M.; Porras-Hurtado, G.L. SARS-CoV-2 and rhinovirus/enterovirus co-infection in a critically ill young adult patient in Colombia. Biomedica 2020, 40 (Suppl. S2), 34–43. (In Spanish) [Google Scholar] [CrossRef] [PubMed]
- Heiselman, C.J.; Iovino, N.; Herrera, K.M. A case report of co-infection with rhinovirus and SARS-CoV-2 in pregnancy. Case Rep. Périnat. Med. 2020, 9, 20200028. [Google Scholar] [CrossRef]
- Dee, K.; Goldfarb, D.M.; Haney, J.; Amat, J.A.R.; Herder, V.; Stewart, M.; Szemiel, A.M.; Baguelin, M.; Murcia, P.R. Human Rhinovirus Infection Blocks Severe Acute Respiratory Syndrome Coronavirus 2 Replication Within the Respiratory Epithelium: Implications for COVID-19 Epidemiology. J. Infect. Dis. 2021, 224, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Henry, B.M.; Benoit, S.W.; Oliveira, M.H.; Hsieh, W.C.; Benoit, J.; Ballout, R.A.; Plebani, M.; Lippi, G. Laboratory abnormalities in children with mild and severe coronavirus disease 2019 (COVID-19): A pooled analysis and review. Clin. Biochem. 2020, 81, 1–8. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).