Abstract
Non-typhoid Salmonella (NTS) represents one of the major causes of foodborne diseases, which are made worse by the increasing emergence of antibiotic resistance. Thus, NTS are a significant and common public health concern. The purpose of this study is to investigate whether selection for phage-resistance alters bacterial phenotype, making this approach suitable for candidate vaccine preparation. We therefore compared two strains of Salmonella enterica serovar Rissen: RR (the phage-resistant strain) and RW (the phage-sensitive strain) in order to investigate a potential cost associated with the bacterium virulence. We tested the ability of both RR and RW to infect phagocytic and non-phagocytic cell lines, the activity of virulence factors associated with the main Type-3 secretory system (T3SS), as well as the canonic inflammatory mediators. The mutant RR strain—compared to the wildtype RW strain—induced in the host a weaker innate immune response. We suggest that the mitigated inflammatory response very likely is due to structural modifications of the lipopolysaccharide (LPS). Our results indicate that phage-resistance might be exploited as a means for the development of LPS-based antibacterial vaccines.
1. Introduction
Salmonella enterica (S. enterica) is a Gram-negative bacterium, causing salmonellosis, one of the major threats to human health. Approximately 2500 Salmonella serovars have been identified [1] and classified as typhoid or non-typhoid strains, according to host specificity and clinical manifestation [2,3].
Non-typhoid Salmonella (NTS) species—specifically the S. Typhimurium or Enteritidis—are the most frequent cause of worldwide foodborne gastroenteritis, causing 155,000 deaths every year [4]. In the last decades, S. Rissen—so far a rare serotype—has been reported to play a significant role in the onset of foodborne diseases [5]. Even though self-limiting gastroenteritis is the main clinical manifestation of Salmonella infection, more severe complications—such as extra-intestinal infections or bacteremia—can occur in immunocompromised patients [5]. Antimicrobial agents are the primary strategy to counteract infectious diseases. However, the increased resistance of Salmonella to traditional antimicrobial drugs makes it difficult to prevent Salmonella infections. In this context, vaccination may represent a valid alternative.
Vaccines are designed to prevent infections and reduce the associated morbidity and mortality [6]. In detail, vaccines train the host immune system to recognize and neutralize the pathogen [7], promoting all the steps of the immune response and the production of cellular mediators responsible for the occurrence of the disease symptoms. Further, vaccines initiate a measured immune response, well tolerated by the host, which does not cause immunopathology.
So far, a vaccine protecting against NTS is not yet available [8].
Bacteriophages are viruses specifically targeting bacteria [9]. They represent the most numerous organisms in the biosphere [10], and their competitive coevolution with the host has contributed to the development, by the host, of many resistance mechanisms [11,12]. Bacteria can evade phage attacks by using different strategies. One of these consists of preventing phage adsorption modifying surface structures (usually referred to as phage receptors) [12,13,14]. Such modification has a cost for the bacterium, consisting of altering its virulence. However, a limit for the bacterium may result in an advantage for the host, becoming a potential tool for vaccine development [15,16,17].
The lipopolysaccharide (LPS) plays an important role in both phage adsorption and infection of Gram-negative bacteria [18,19]. In a previous study, we demonstrated differences in the LPS biosynthesis and morphology between the bacteriophage-sensitive (RW) and the resistant S. Rissen strains (RR) [20]. More specifically, we detected reduced expression levels of the phosphomannomutase1 and phosphomannomutase2 genes in the RR resistant strain, compared to that of RW. Thus, RR was shown to produce a LPS lacking mannose in the O-antigen portion. Furthermore, LPS is a pathogen-associated molecular pattern known to interact with the host Toll-like receptor 4 (TLR4) and activate a strong defense immune response [21]. At the same time, several studies have demonstrated that modified LPSs are poor stimulators of TLR4 and trigger a mild immune response—properties which make them useful for a good candidate vaccine [22]. In this context, we compared in vitro the host inflammatory response following infection with RR or RW strains. RW displayed a stronger inflammation compared to RR, potentially attributable to differences in the LPS structure between the two strains. Based on these findings, modified LPS of the phage-resistant S. Rissen could represent a potential candidate for vaccine development.
2. Materials and Methods
2.1. Bacterial Strains and Culture Conditions
Non-typhoid Salmonella enterica subsp. enterica serovar Rissen strain RW, Salmonella bongori, Salmonella nottingham and Salmonella typhimurium were isolated from a food matrix and characterized by Istituto Zooprofilattico Sperimentale Del Mezzogiorno (Portici, Naples, Italy). The S. Rissen strain RR was derived from the RW strain following selection for resistance to phage ϕ1, as previously described [17]. Both the Salmonella strains were grown in Nutrient Broth (Scharlab, S.L., Barcelona, Spain) at 37 °C under vigorous agitation (200 rpm).
2.2. Cell Lines and Culture Conditions
AGS (human Caucasian gastric adenocarcinoma) and HT-29 (human Caucasian colon adenocarcinoma) cells were grown in Dulbecco’s modified Eagle’s medium, high glucose (DMEM; Microtech, Pozzuoli, Naples, Italy), and supplemented with 10% fetal bovine serum (FBS; Microtech, Pozzuoli, NA, Italy), 1% penicillin/streptomycin (Gibco, Waltham, MA, USA) and 1% L-glutamine (Gibco, Waltham, MA, USA). U937 (human myeloid leukemia) cells were grown in RPMI-1640 (Microtech, Pozzuoli, NA, Italy) and supplemented with 10% fetal bovine serum (FBS; Microtech, Pozzuoli, NA, Italy), 1% penicillin/streptomycin (Gibco, Waltham, MA, USA) and 1% L-glutamine (Gibco, Waltham, MA, USA). All cell lines were maintained in 5% CO2 at 37 °C. U937 cells were induced to differentiate into macrophages by exposing them to phorbol-12-myristate-13-acetate (PMA, 100 ng/mL; Sigma Aldrich, St. Louis, MO, USA) for 48 h. Cells were then washed twice, and the culture medium was replaced with RPMI-1640 without PMA, followed by a resting period of 24 h.
2.3. Salmonella Invasion Assay
All the Salmonella strains were analyzed for their capacity to colonize the following human cell lines: AGS and HT-29 (non-phagocytic epithelial cell lines), and U937 differentiated into macrophages (phagocytic cell line). Cells were seeded at the density of 1 × 106 per well in 12-well plates and incubated overnight at 37 °C in the presence of 5% CO2 and without antibiotics. Salmonella invasion capabilities were evaluated as previously described [20]. Briefly, cell monolayers were infected with 108 CFU/mL in a 12-well plate, at MOI (multiplicity of infection) = 1:100 and incubated for 2 h at 37 °C. After incubation, cell monolayers were washed with PBS (Phosphate buffered saline) and incubated in the presence of 100 μg/mL gentamicin for 30 min. Again, cells were washed with PBS (pH 7.3) and lysed in 1 mL of fresh PBS by scraping. Viable intracellular bacteria were counted after plating serial dilutions in nutrient broth. Results were expressed as the mean ± standard error of the mean (SEM) of the number of intracellular bacteria, expressed in Log10 CFU/mL. Experiments were performed in duplicate and repeated at least three times.
2.4. Expression Levels of Virulence Genes
The presence of 7 genes related to the virulence of Salmonella spp. was detected in RW and RR strains by end-point PCR and electrophoretic run on an automated qiaxcel instrument (Qiagen, Hilden, Germany). Their expression levels were evaluated by qRT-PCR at 2, 4 and 6 hpi on the AGS cell line. The selected virulence factors are related to the presence of prophages (grvA, gogB, sspH1, sodC1, gtgE) or plasmids (spvC) [21].
2.5. Infection on AGS Cell Line
Cells were seeded at the density of 1 × 106 per well in 12-well plates and incubated overnight at 37 °C in the presence of 5% CO2, without antibiotics. The next day, cells were infected with RR or RW strains (MOI 1:100) for 2 h, 4 h and 6 h. After infection, cells were washed with PBS, and gentamicin (100 μg/mL) was added for 30 min. AGS cells were then lysed and collected using 1 mL of TRIzol LS reagent (Thermo Fisher Scientific, Waltham, MA, USA); whereas bacteria were collected and lysed using scraping and 500 µL of TRIzol reagent. All the samples were stored at −80 °C until the analysis.
2.6. RNA Extraction and RT-qPCR
Total RNA extraction was performed using TRIzol LS reagent (Thermo Fisher Scientific, Waltham, MA, USA) following the manufacturer’s instructions. The quality and quantity of RNA was estimated using NanoDrop 2000 c (Thermo Fisher Scientific, Waltham, MA, USA) and then reverse-transcribed using the high-capacity cDNA Reverse transcription kit (Thermo Fisher Scientific, Waltham, MA, USA). Gene transcript levels were measured using Power SYBR Green PCR Master Mix (Applied Biosystem, Waltham, MA, USA) on a StepOne Real-Time PCR System (Thermo Fisher Scientific, Waltham, MA, USA), according to the standard mode thermal cycling conditions, as indicated by Spatuzza et al. [22]. Relative expression levels of analyzed genes were determined using probes listed in Table S1. The 2−ΔΔCT method was used to calculate relative changes in gene expression determined from real-time quantitative PCR experiments [23,24]. Target gene expression levels were normalized using housekeeping genes (recA for Salmonella and GAPDH for AGS cell line).
2.7. Cytokine Determination by Bio-Plex Assay
Bio-Plex Pro Human Th17 Cytokine Assay (BioRad, Hercules, CA, USA) was performed to detect the level of cytokines in supernatants of AGS cells infected with the RR or RW strain. The assay detects multiple analytes simultaneously in a single sample [25,26].
2.8. Western Blotting Analysis
AGS cells were infected with RW or RR strains for 1 h and 2 h. Total proteins were extracted with RIPA lysis buffer (50 mM Tris-HCl, 150 mM NaCl, 0.5% Triton X-100, 0.5% deoxycholic acid, 10 mg/mL leupeptin, 2 mM phenylmethylsulfonyl fluoride and 10 mg/mL aprotinin containing protease and phosphatase inhibitors (Sigma Aldrich, St. Louis, MO, USA)). Samples were quantified using Protein Analysis Dye Reagent Concentrate (BioRad, Hercules, CA, USA). Equal quantities of protein were separated by SDS-PAGE gel and transferred to PVDF membranes using a Trans-Blot Turbo (BioRad). The membranes were blocked with 5% fat-free milk in Tris saline buffer containing 0.1% Tween-20 (TBST) at room temperature for 1 h, incubated with primary antibodies (1:1000) at 4 °C overnight, and incubated with horseradish peroxidase (HRP)-conjugated secondary antibodies (1:2000) (BioRad) at room temperature for 1 h. The signals were detected using the BioRad ChemiDoc MP image sensor after the membranes were soaked in enhanced ECL reagents (ECLTM Prime Western Reagents for Blotting Detection, Amersham, GE Healthcare, Buckinghamshire, UK). Protein bands were detected by chemiluminescence HRP substrate (Millipore, Burlington, MA, USA) and analyzed by Image J software (National Institutes of Health, version 2.1.0/1.53c). Total extracts were normalized using an anti-β-actin antibody. The following antibodies were used for the Western blot analysis: Mouse monoclonal anti-human β-actin antibody and anti-AKT mouse monoclonal antibody were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA); anti-pNFKB rabbit monoclonal antibody, anti- NFKB rabbit monoclonal, anti-IKBα rabbit monoclonal antibody, anti-pSTAT3 rabbit monoclonal antibody, anti-STAT3 rabbit monoclonal antibody and anti-pAKT rabbit monoclonal antibody were from Cell Signaling Technology (Danvers, MA, USA). The following were used as secondary antibodies: Goat Anti-Rabbit and Goat Anti-Mouse HRP (BioRad, Hercules, CA, USA). The company and concentrations of all antibodies used are presented in Table S2.
2.9. Statistical Analysis
Statistical analysis was performed using GraphPad Prism 8.0 software (San Diego, CA, USA). All data were compared using two-way ANOVA multiple comparisons. Experimental data are presented as mean ± SD of three independent experiments, performed in triplicate. Statistical analysis was considered statistically significant when p < 0.05.
3. Results
3.1. RW and RR Strains Display the Same Antigenic and Antibiotic Resistance Profiles
The slide agglutination test displayed both strains having the same antigenic determinants of the LPS O-chain (O6, O7) and of flagella (Hf, Hg). The two lines also displayed the same antibiotic profile: both were resistant to cefoxitin and sensitive to the same 20 antibiotics (Table S3). Recent studies have shown that, in bacteria, acquisition of phage resistance is often associated with loss of antibiotic resistance [27]. The RR strain instead remained resistant to cefoxitin (Table S3).
3.2. The RW and RR Strains Both Exhibit the Same Capacity to Colonize Host Cells
Colonization is a major property of Salmonella [28]. Therefore, we tested the two strains (RR and RW) for their capacity to colonize the host. The AGS (epithelial gastric adenocarcinoma) and U937 (macrophage) cell lines were incubated for 1 and 2 h with the RW or RR strain. The U937 and AGS cell lines both displayed the capacity to internalize RW and RR bacterial strains to the same extent (Figure 1A). Instead, the HT-29 cell line was not colonized by RR or RW, both at 1 and 2 h. In addition, no serovar-specific differences in HT-29 cells’ colonization were observed. We repeated the experiment using additional Salmonella serovars (S. typhimurium, bongori, enteritidis and nottingham). All Salmonella strains exhibited no capacity to colonize the HT-29 cell line (Figure 1A,B). According to the literature, studies have shown that, in bacteria, acquisition of phage resistance is associated with defects in the host cell colonization [13]. The RR strain instead behaved exactly as the wildtype (Figure 1A).
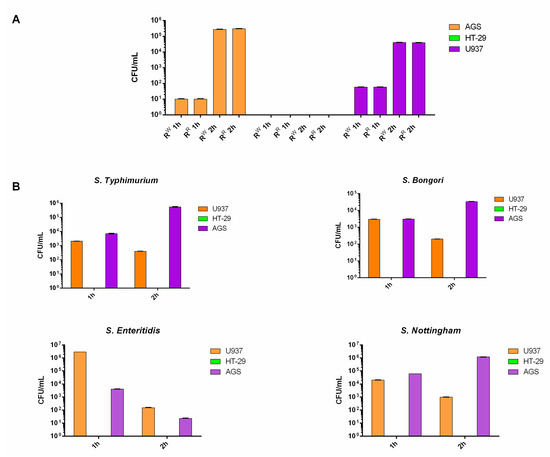
Figure 1.
RR and RW host cell colonization. (A) AGS, U937 and HT-29 cell lines were infected with the bacteriophage-resistant RR or RW strains for 1 and 2 h. (B) AGS, U937 and HT-29 cell lines were infected with Salmonella typhimurium, bongori, enteritidis or nottingham for 1 and 2 h. Results are reported as Log10 CFU/mL and represent the mean ± SD of three experiments, each performed in triplicate.
3.3. RW and RR Strains Exhibit Different Virulence Profiles
The virulence of RR and RW strains was tested, incubating the epithelial AGS cell line with both strains for 2, 4 and 6 h. The expression levels of the virulence genes (invA, sspH1, sodC1, gtgE, grvA, spvC, gogB) were measured by RT-qPCR. The invA gene—controlling colonization of epithelial cells [29]—was equally expressed in both strains (Figure 2). This result exactly concurs with the one reported in Figure 1A, displaying no difference in the colonization of the epithelial AGS cell line by RR or RW. Instead, using the same AGS cell line, significant differences between RW and RR were detected regarding sodC1, gogB, spvC, sspH1, grvA, gtgE (Figure 2). SodC1 protects the bacterium from oxidative burst [30], while gogB protects the host tissue integrity [31]. Both of these genes were expressed at a 100× higher level in RR compared to RW. SpvC and sspH1 inhibit NF-kB [32]. GrvA and gtgE help the bacteria survive in the host [33]. Both of these genes (spvC-sspH1 and grvA-gtgE) were found significantly expressed in RR only at 6 hpi compared to RW, while grvA was found significantly more expressed in RR already after 4 h of incubation. Taken together, these results indicate: (1) that RR and RW have clear different virulence profiles, and (2) that phage-resistance contributes to bacterial persistence in host cells.
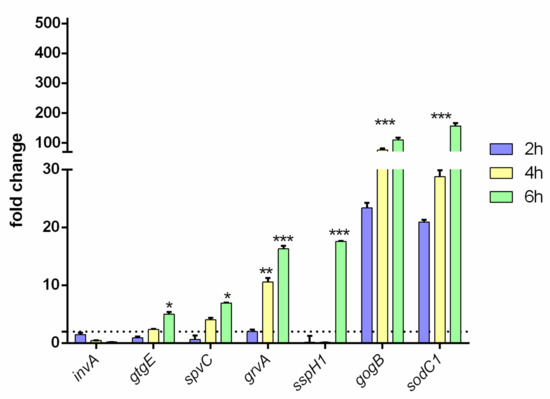
Figure 2.
Virulence profile of the RR phage-resistant strain. invA, gtgE, spvC, grvA, sspH1, gogB and sodC1 gene expression levels were measured in the epithelial AGS cell line infected with RR or RW for 2, 4 and 6 h. Results are reported as mean ± SD of three independent experiments, each performed in triplicate and labeled with asterisks (* p < 0.05; ** p < 0.01; *** p < 0.001). Relative gene expression was normalized to RW.
3.4. RW and RR Strains Induce a Different Inflammatory Host Response
Bioplex analysis indicated that RW elicits a stronger pro-inflammatory response (higher levels of IL-6, IL-8, G-CSF, MCP-1, MIP-1β and TNF-α) than RR. At the same time, both strains produce low and very close levels of IL-10 (Figure 3A). Western blot analysis confirmed these results. NF-kB, Akt and STAT3 were significantly more activated in RW than in RR infected cells (Figure 3B). Interestingly, RR infected cells displayed a reduced expression level of STAT3 and a similar expression level of NF-kB, compared to control cells. NF-kB, Akt and STAT3 pathways are known to play a critical role in the inflammatory response triggered by infections [34,35]. These data show that the phage-resistant strain RR induces a significantly lower pro-inflammatory response than RW.
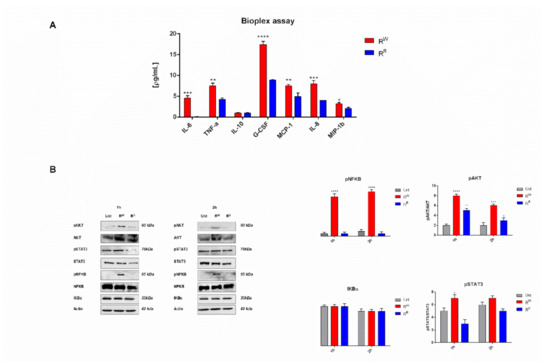
Figure 3.
Phage-resistance curbs AGS-induced inflammatory response. (A) Cytokines IL-6, TNF-α, IL-10, G-CSF, MCP-1, MIP-1β and IL-8 were measured by Bio-plex assay in AGS cells culture medium after incubation for two hours with RR or RW strains. Results are expressed as pg of cytokines secreted in mL of cell medium. Values were normalized to the basal activity (CTR) and represent mean ± SD of at least three independent experiments, each performed in triplicate (* p < 0.05; ** p < 0.01; *** p < 0.001; **** p < 0.0001). (B) Western blot and densitometric analysis of the ratio pNF-kB/NF-kB; pAkt/Akt; pSTAT3/STAT3. Actin was used for normalization. Graphs report the result of three independent experiments and represent mean ± SD (* p < 0.05; ** p < 0.01; *** p < 0.001; **** p < 0.0001).
3.5. RW and RR Strains Display a Different LpxR and TLR4 Gene Expression Level
The lpxR gene is involved in de-acylation of lipid A portion of LPS [36]. A time course RT-PCR experiment displayed that lpxR is upregulated in RR and downregulated in RW (Figure 4A). The same experiment displayed also that upregulation of lpxR increases together with incubation time (Figure 4A). A high expression of lpxR gene in RR could potentially reflect a higher level of de-acylation of the lipid A of the mutant RR strain. Instead, the low level of lpxR gene expression of the RW strain suggests that this strain has the classic hexa-acylated lipid A structure. A further confirmation of this conclusion is provided by an independent experiment carried out on additional Salmonella strains (S. bongori or enteritidis). Again, lpxR was found upregulated in RR compared to S. bongori and enteritidis, which instead expresses a level of lpxR comparable to RW. The expression of TLR4 is negatively modulated by the presence of deacylated lipid A portion of LPS [37]. In this study, the evidence that TLR4 gene is downregulated in cells incubated with RR (Figure 4B) represents one more independent proof that RR has acquired resistance to phage ɸ1 by modification of the LPS.
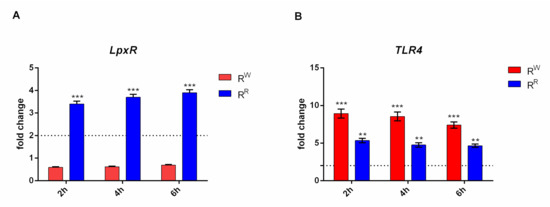
Figure 4.
RR and RW strains show a different TLR4 activation. (A) Relative gene expression of lpxR was determined by quantitative real-time PCR (RT-qPCR), performed on RNA isolated from AGS cells cultured with RR or RW for 2, 4 and 6 h. (*** p < 0.001). (B) Relative gene expression of TLR4 was determined by quantitative real-time PCR (RT-qPCR), performed on RNA isolated from AGS cells cultured with RR or RW for 2, 4 and 6 h. All samples were normalized to GAPDH as a reference housekeeping gene. Furthermore, relative gene expression was normalized to basal activity (CTR), in order to obtain relative fold expression. Graphs report the results of at least three independent experiments, represented as means ± SD (** p < 0.01; *** p < 0.001).
4. Discussion
The frequent and often inappropriate use of antibiotics in medicine and intensive farming has favored the selection of antibiotic-resistant bacteria, causing serious consequences for human health. This drawback was further emphasized by the phenomenon of phage-resistant bacteria. Any host fighting against a drug or a parasite inevitably evolves strategies to evade the antagonist and survives.
In the present study, we compare two strains of Salmonella enterica serovar Rissen, RR (the phage-resistant strain) and RW (the phage-sensitive strain), in order to know, first, whether the changes associated with the acquisition of phage-resistance affects the host cell physiology and, second, the potential mechanisms responsible for the different host-bacteria interaction.
We firstly evaluated the property of both RR and RW strains to colonize host cells. Both RR and RW were found to colonize AGS and U937 cell lines to the same extent (Figure 1A). To establish the host colonization, Salmonella uses the Type 3 Secretion System (T3SS), a complex machinery encoded by Salmonella pathogenicity islands (SPIs) [37,38], and consists of a cluster of virulence genes [39].
Therefore, to investigate the effect of acquisition of phage-resistance on bacteria virulence, we infected the epithelial AGS cell line separately with one of these two strains and analyzed some of the most representative SPIs-virulence genes. As expected, we observed similar expression levels of invA, indicating the same capacity of RR and RW to colonize AGS cells (Figure 2). Instead, marked differences were detected with gtgE, sodC1 and grvA, which were all upregulated in RR compared to RW (Figure 2), suggesting that upregulation might favor the survival of RR in AGS compared to RW [30,40]. Interestingly, we also noted increased expression levels of gogB, spvC and sspH1 genes in the RR strain (Figure 2). These data provide evidence about the capacity of RR to infect the host more efficiently, compared to RW, by modulating the host’s innate immune response and surviving longer within the host.
Upon bacterial infection, innate immunity initiates a defensive response, which leads to inflammation. Bacteria have developed strategies to elude the host immune clearance and curb the inflammatory response. Our data indicate that the above statement extends to the RR strain. In accordance with the upregulation of the sspH1 and spvC genes inhibiting NF-KB [32] in the RR strain, we found reduced activation of the nuclear transcription factor-kB (NF-kB) and of its activator Akt in the cells infected with RR (Figure 3B). NF-kB is a critical modulator of inflammation; it initiates the transcription of numerous genes, including cytokines and chemokines [41]. Consistent with this finding, we also detected reduced expression levels of pro-inflammatory cytokines and chemokines in RR infected cells (Figure 3A). More specifically, we observed lower levels of: (1) IL-8, MCP-1 and MIP-1β, responsible for the recruitment of neutrophils, monocytes and lymphocytes at the site of infection [42,43]; (2) IL-6 and TNF-α, directly involved in the early stage of pathogen-induced inflammatory response; and (3) GCS-F, involved in cell growth and differentiation [44,45,46]. Cytokines, in turn, are known to induce the activation of the transcriptional factor STAT3 [47]. Finally, in RR infected cells, we detected reduced activation of the STAT3 protein (Figure 3B). We can conclude that our data indicate the RR strain, as a potential candidate vaccine, modulates the immune response curbing inflammation.
In order to organize the immune defense against the pathogen, evolution has selected ancient receptors that recognize pathogen-associated molecular patterns (PAMPs). Lipopolysaccharide (LPS), the most important Gram-negative PAMP, has also been reported to interact with phage proteins, acting as a phage receptor [48]. Bacteria—including Salmonella species—can alter genes of the LPS biosynthesis pathway, modifying the LPS structure and inhibiting phage adsorption [49]. In a previous work, we demonstrated differences between RR and RW strains in the expression levels of two genes (phosphomannomutase1 and phosphomannomutase2) involved in the LPS biosynthetic pathway. Precisely, a comparative analysis showed that RR produces an LPS lacking mannose sugar in the O-antigen portion [17]. Further, lipid A, a principal component of the LPS [50], induces the inflammatory reaction following interaction with Toll-like receptor 4 (TLR4). Based on these considerations, we investigated whether modifications of the RR phenotype could be attributed to alterations of the LPS-lipid A portion. Salmonella species can synthesize enzymes able to covalently alter the lipid A portion, such as the 3′-O-deacylase, encoded by the lpxR gene, which is upregulated in RR (Figure 4A). The 3′-O-deacylated form of the lipid A is a poor stimulator of TLR4 [51], which favors bacteria in evading the host immune response. The downregulation of the TLR4 gene in RR infected cells (Figure 4B) further supports the idea that the phage-resistant strain has acquired the resistance by modifying the LPS structure. Before testing RR in vivo, as a potential candidate vaccine, we will further confirm biochemically that RR displays an altered LPS-lipid A portion.
5. Conclusions
In conclusion, this study reports a bacteriophage-resistant Salmonella rissen strain, which increases its pathogenicity, most likely due to the potential modification of the LPS-lipid A portion. Literature reports several studies describing vaccines based on modified lipid A portion [52]. Here, we propose a valid alternative to the LPS-synthetic vaccines, consisting of exploiting the capacity of phage-resistant bacteria to modify naturally the LPS-toxic portion.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/v13122468/s1, Table S1: Primers used for RT-qPCR, Table S2: Antibody used for Western blot and Table S3: Antibody used for Western blot.
Author Contributions
Conceptualization, R.C.; methodology, M.P. and C.P.; validation, R.C.; formal analysis, A.M.I.M., A.R. and P.C.; data curation, M.P.; writing—original draft preparation, P.C. and M.P.; writing—review and editing, D.I.; supervision, R.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study era available within the article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Chattaway, M.A.; Langridge, G.C.; Wain, J. Salmonella nomenclature in the genomic era: A time for change. Sci. Rep. 2021, 11, 1–8. [Google Scholar] [CrossRef]
- Gal-Mor, O.; Boyle, E.C.; Grassl, G.A. Same species, different diseases: How and why typhoidal and non-typhoidal Salmonella enterica serovars differ. Front. Microbiol. 2014, 5, 391. [Google Scholar] [CrossRef] [PubMed]
- Wemyss, M.; Pearson, J.S. Host Cell Death Responses to Non-typhoidal Salmonella Infection. Front. Immunol. 2019, 10, 1758. [Google Scholar] [CrossRef]
- Crump, J.A.; Sjölund-Karlsson, M.; Gordon, M.; Parry, C.M. Epidemiology, clinical presentation, laboratory diagnosis, antimicrobial resistance, and antimicrobial management of invasive salmonella infections. Clin. Microbiol. Rev. 2015, 28, 901–937. [Google Scholar] [CrossRef] [PubMed]
- Campos, J.; Mourão, J.; Peixe, L.; Antunes, P. Non-typhoidal salmonella in the pig production chain: A comprehensive analysis of its impact on human health. Pathogens 2019, 8, 19. [Google Scholar] [CrossRef] [PubMed]
- Micoli, F.; Bagnoli, F.; Rappuoli, R.; Serruto, D. The role of vaccines in combatting antimicrobial resistance. Nat. Rev. Genet. 2021, 19, 287–302. [Google Scholar] [CrossRef] [PubMed]
- Jansen, K.U.; Knirsch, C.; Anderson, A.S. The role of vaccines in preventing bacterial antimicrobial resistance. Nat. Med. 2018, 24, 10–19. [Google Scholar] [CrossRef]
- Worsena, C.R.; Miller, A.S.; King, M.A. Salmonella Infections. Pediatr. Rev. 2019, 40, 543–545. [Google Scholar] [CrossRef] [PubMed]
- Clokie, M.R.J.; Millard, A.D.; Letarov, A.V.; Heaphy, S. Phages in nature. Bacteriophage 2011, 1, 31–45. [Google Scholar] [CrossRef]
- Hatfull, G.F. Dark Matter of the Biosphere: The amazing world of bacteriophage diversity. J. Virol. 2015, 89, 8107–8110. [Google Scholar] [CrossRef] [PubMed]
- Koskella, B.; Brockhurst, M.A. Bacteria–phage coevolution as a driver of ecological and evolutionary processes in microbial communities. FEMS Microbiol. Rev. 2014, 38, 916–931. [Google Scholar] [CrossRef]
- Rostøl, J.T.; Marraffini, L. (Ph)ighting Phages: How bacteria resist their parasites. Cell Host Microbe 2019, 25, 184–194. [Google Scholar] [CrossRef]
- Oechslin, F. Resistance development to bacteriophages occurring during bacteriophage therapy. Viruses 2018, 10, 351. [Google Scholar] [CrossRef] [PubMed]
- Labrie, S.J.; Samson, J.E.; Moineau, S. Bacteriophage resistance mechanisms. Nat. Rev. Microbiol. 2010, 8, 317–327. [Google Scholar] [CrossRef]
- Filippov, A.A.; Sergueev, K.V.; He, Y.; Huang, X.-Z.; Gnade, B.T.; Mueller, A.J.; Fernandez-Prada, C.M.; Nikolich, M.P. Bacteriophage-Resistant Mutants in Yersinia pestis: Identification of Phage Receptors and Attenuation for Mice. PLoS ONE 2011, 6, e25486. [Google Scholar] [CrossRef]
- Capparelli, R.; Nocerino, N.; Lanzetta, R.; Silipo, A.; Amoresano, A.; Giangrande, C.; Becker, K.; Blaiotta, G.; Evidente, A.; Cimmino, A.; et al. Bacteriophage-resistant staphylococcus aureus mutant confers broad immunity against staphylococcal infection in mice. PLoS ONE 2010, 5, e11720. [Google Scholar] [CrossRef]
- Capparelli, R.; Nocerino, N.; Iannaccone, M.; Ercolini, D.; Parlato, M.; Chiara, M.; Iannelli, D. Bacteriophage Therapy of Salmonella enterica:A Fresh Appraisal of Bacteriophage Therapy. J. Infect. Dis. 2010, 201, 52–61. [Google Scholar] [CrossRef]
- Rakhuba, D.V.; Kolomiets, E.I.; Dey, E.S.; Novik, G.I. Bacteriophage receptors, mechanisms of phage adsorption and penetration into host cell. Pol. J. Microbiol. 2010, 59, 145–155. [Google Scholar] [CrossRef]
- Kulikov, E.E.; Golomidova, A.K.; Prokhorov, N.; Ivanov, P.A.; Letarov, A.V. High-throughput LPS profiling as a tool for revealing of bacteriophage infection strategies. Sci. Rep. 2019, 9, 1–10. [Google Scholar] [CrossRef]
- Papaianni, M.; Contaldi, F.; Fulgione, A.; Woo, S.L.; Casillo, A.; Corsaro, M.M.; Parrilli, E.; Marcolungo, L.; Rossato, M.; Delledonne, M.; et al. Role of phage ϕ1 in two strains of Salmonella rissen, sensitive and resistant to phage ϕ1. BMC Microbiol. 2018, 18, 208. [Google Scholar] [CrossRef]
- Ematsuura, M. Structural modifications of bacterial lipopolysaccharide that facilitate gram-negative bacteria evasion of host innate immunity. Front. Immunol. 2013, 4, 109. [Google Scholar] [CrossRef]
- Zariri, A.; Van Der Ley, P. Biosynthetically engineered lipopolysaccharide as vaccine adjuvant. Expert Rev. Vaccines 2015, 14, 861–876. [Google Scholar] [CrossRef]
- Roche, S.M.; Gracieux, P.; Milohanic, E.; Albert, I.; Virlogeux-Payant, I.; Témoin, S.; Grépinet, O.; Kerouanton, A.; Jacquet, C.; Cossart, P.; et al. Investigation of specific substitutions in virulence genes characterizing phenotypic groups of low-virulence field strains of listeria monocytogenes. Appl. Environ. Microbiol. 2005, 71, 6039–6048. [Google Scholar] [CrossRef] [PubMed]
- Borriello, G.; Lucibelli, M.G.; Pesciaroli, M.; Carullo, M.R.; Graziani, C.; Ammendola, S.; Battistoni, A.; Ercolini, D.; Pasquali, P.; Galiero, G. Diversity of Salmonella spp. serovars isolated from the intestines of water buffalo calves with gastroenteritis. BMC Vet. Res. 2012, 8, 201. [Google Scholar] [CrossRef]
- Spatuzza, C.; Schiavone, M.; Di Salle, E.; Janda, E.; Sardiello, M.; Fiume, G.; Fierro, O.; Simonetta, M.; Argiriou, N.; Faraonio, R.; et al. Physical and functional characterization of the genetic locus of IBtk, an inhibitor of Bruton’s tyrosine kinase: Evidence for three protein isoforms of IBtk. Nucleic Acids Res. 2008, 36, 4402–4416. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Front. Plant Sci. 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Hu, N.; Qian, L.; Hu, Y.; Shou, J.-Z.; Wang, C.; Giffen, C.; Wang, Q.-H.; Wang, Y.; Goldstein, A.M.; Emmert-Buck, M.; et al. Quantitative real-time RT-PCR validation of differential mRNA expression of SPARC, FADD, Fascin, COL7A1, CK4, TGM3, ECM1, PPL and EVPLin esophageal squamous cell carcinoma. BMC Cancer 2006, 6, 33. [Google Scholar] [CrossRef]
- Iannelli, D.; D’Apice, L.; Cottone, C.; Viscardi, M.; Scala, F.; Zoina, A.; Del Sorbo, G.; Spigno, P.; Capparelli, R. Simultaneous detection of cucumber mosaic virus, tomato mosaic virus and potato virus Y by flow cytometry. J. Virol. Methods 1997, 69, 137–145. [Google Scholar] [CrossRef]
- Lotze, M. Measuring Immunity: Basic Biology and Clinical Assessment; Elsevier Academic Press: San Diego, CA, USA; London, UK, 2005; ISBN 978-1-42-375541-8. [Google Scholar]
- Burmeister, A.R.; Fortier, A.; Roush, C.; Lessing, A.J.; Bender, R.G.; Barahman, R.; Grant, R.; Chan, B.K.; Turner, P.E. Pleiotropy complicates a trade-off between phage resistance and antibiotic resistance. Proc. Natl. Acad. Sci. USA 2020, 117, 11207–11216. [Google Scholar] [CrossRef]
- Zhou, D.; Galán, J. Salmonella entry into host cells: The work in concert of type III secreted effector proteins. Microbes Infect. 2001, 3, 1293–1298. [Google Scholar] [CrossRef]
- Galán, J.E.; Ginocchio, C.; Costeas, P. Molecular and functional characterization of the Salmonella invasion gene invA: Homology of InvA to members of a new protein family. J. Bacteriol. 1992, 174, 4338–4349. [Google Scholar] [CrossRef]
- Ho, T.D.; Slauch, J.M. Characterization of grvA, an Antivirulence Gene on the Gifsy-2 Phage in Salmonella enterica Serovar Typhimurium. J. Bacteriol. 2001, 183, 611–620. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Qazi, I.H.; Wang, L.; Zhou, G.; Han, H. Salmonella Virulence and Immune Escape. Microorganisms 2020, 8, 407. [Google Scholar] [CrossRef] [PubMed]
- Ho, T.D.; Figueroa-Bossi, N.; Wang, M.; Uzzau, S.; Bossi, L.; Slauch, J.M. Identification of GtgE, a Novel Virulence Factor Encoded on the Gifsy-2 Bacteriophage of Salmonella enterica Serovar Typhimurium. J. Bacteriol. 2002, 184, 5234–5239. [Google Scholar] [CrossRef]
- Ranade, K.; Poteete, A.R. Superinfection exclusion (sieB) genes of bacteriophages P22 and lambda. J. Bacteriol. 1993, 175, 4712–4718. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lu, R.; Zhang, Y.-G.; Sun, J. STAT3 activation in infection and infection-associated cancer. Mol. Cell. Endocrinol. 2017, 451, 80–87. [Google Scholar] [CrossRef]
- Reynolds, C.M.; Ribeiro, A.A.; McGrath, S.C.; Cotter, R.J.; Raetz, C.R.H.; Trent, M.S. An Outer Membrane Enzyme Encoded by Salmonella typhimurium lpxR That Removes the 3′-Acyloxyacyl Moiety of Lipid A. J. Biol. Chem. 2006, 281, 21974–21987. [Google Scholar] [CrossRef] [PubMed]
- Lou, L.; Zhang, P.; Piao, R.; Wang, Y. Salmonella Pathogenicity Island 1 (SPI-1) and Its Complex Regulatory Network. Front. Cell. Infect. Microbiol. 2019, 9, 270. [Google Scholar] [CrossRef] [PubMed]
- Park, D.; Lara-Tejero, M.; Waxham, M.N.; Li, W.; Hu, B.; Galán, J.E.; Liu, J. Visualization of the type III secretion mediated Salmonella–host cell interface using cryo-electron tomography. eLife 2018, 7. [Google Scholar] [CrossRef]
- Que, F.; Wu, S.; Huang, R. Salmonella Pathogenicity Island 1(SPI-1) at Work. Curr. Microbiol. 2013, 66, 582–587. [Google Scholar] [CrossRef]
- Rushing, M.D.; Slauch, J.M. Either periplasmic tethering or protease resistance is sufficient to allow a SodC to protect Salmonella enterica serovar Typhimurium from phagocytic superoxide. Mol. Microbiol. 2011, 82, 952–963. [Google Scholar] [CrossRef] [PubMed]
- Santoro, M.; Rossi, A.; Amici, C. New embo member’s review: NF-kappaB and virus infection: Who controls whom. EMBO J. 2003, 22, 2552–2560. [Google Scholar] [CrossRef]
- Mukaida, N.; Harada, A.; Matsushima, K. Interleukin-8 (IL-8) and monocyte chemotactic and activating factor (MCAF/MCP-1), chemokines essentially involved in inflammatory and immune reactions. Cytokine Growth Factor Rev. 1998, 9, 9–23. [Google Scholar] [CrossRef]
- Maurer, M.; von Stebut, E. Macrophage inflammatory protein-1. Int. J. Biochem. Cell Biol. 2004, 36, 1882–1886. [Google Scholar] [CrossRef]
- Lissoni, P.; Messina, G.; Pelizzoni, F.; Rovelli, F.; Brivio, F.; Monzon, A.; Crivelli, N.; Lissoni, A.; Tassoni, S.; Sassola, A.; et al. The Fascination of Cytokine Immuno-logical Science. J. Infect. Rev. Artic. 2020, 3, 18–28. [Google Scholar]
- Arango Duque, G.; Descoteaux, A. Macrophage Cytokines: Involvement in Immunity and Infectious Diseases. Front. Immunol. 2014, 5, 491. [Google Scholar] [CrossRef] [PubMed]
- Eckmann, L.; Kagnoff, M.F. Cytokines in host defense against Salmonella. Microbes Infect. 2001, 3, 1191–1200. [Google Scholar] [CrossRef]
- Steward-Tharp, S.M.; Laurence, A.; Kanno, Y.; Kotlyar, A.; Villarino, A.V.; Sciume, G.; Kuchen, S.; Resch, W.; Wohlfert, E.A.; Jiang, K.; et al. A mouse model of HIES reveals pro- and anti-inflammatory functions of STAT3. Blood 2014, 123, 2978–2987. [Google Scholar] [CrossRef]
- León, M.; Bastías, R. Virulence reduction in bacteriophage resistant bacteria. Front. Microbiol. 2015, 6, 343. [Google Scholar] [CrossRef] [PubMed]
- Jarrell, K.F.; Kropinski, A. Isolation and characterization of a bacteriophage specific for the lipopolysaccharide of rough derivatives of Pseudomonas aeruginosa strain PAO. J. Virol. 1981, 38, 529–538. [Google Scholar] [CrossRef]
- Nijland, R.; Hofland, T.; Van Strijp, J.A.G. Recognition of LPS by TLR4: Potential for Anti-Inflammatory Therapies. Mar. Drugs 2014, 12, 4260–4273. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).