Tribute to John C. Martin at the Twentieth Anniversary of the Breakthrough of Tenofovir in the Treatment of HIV Infections
Abstract
1. Prologue
2. Introduction to the Antiviral Drug Field: Ganciclovir
2.1. Switch to the Anti-HIV Drug Field: Didanosine
2.2. 2′,3′-Didehydro-2′,3′-dideoxythymidine (Stavudine, d4T)
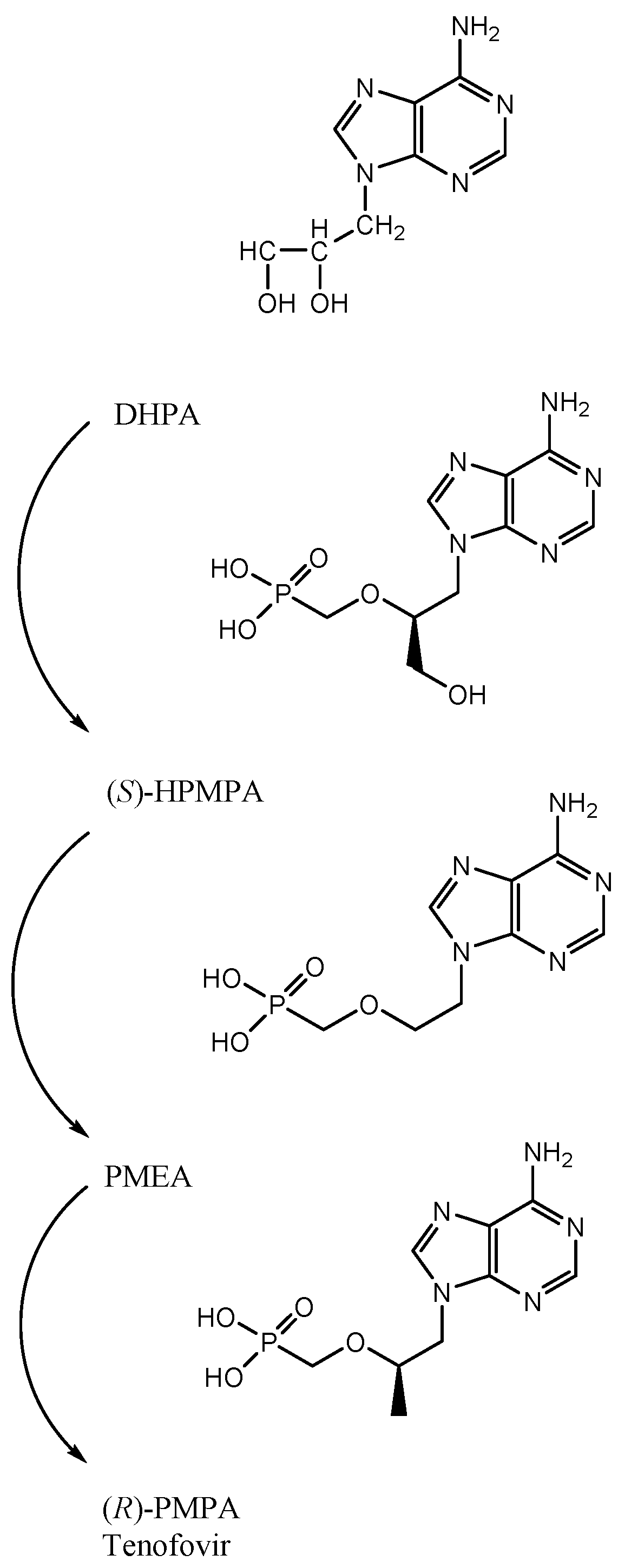
2.3. The Breakthrough of the ANPs (Acyclic Nucleoside Phosphonates)
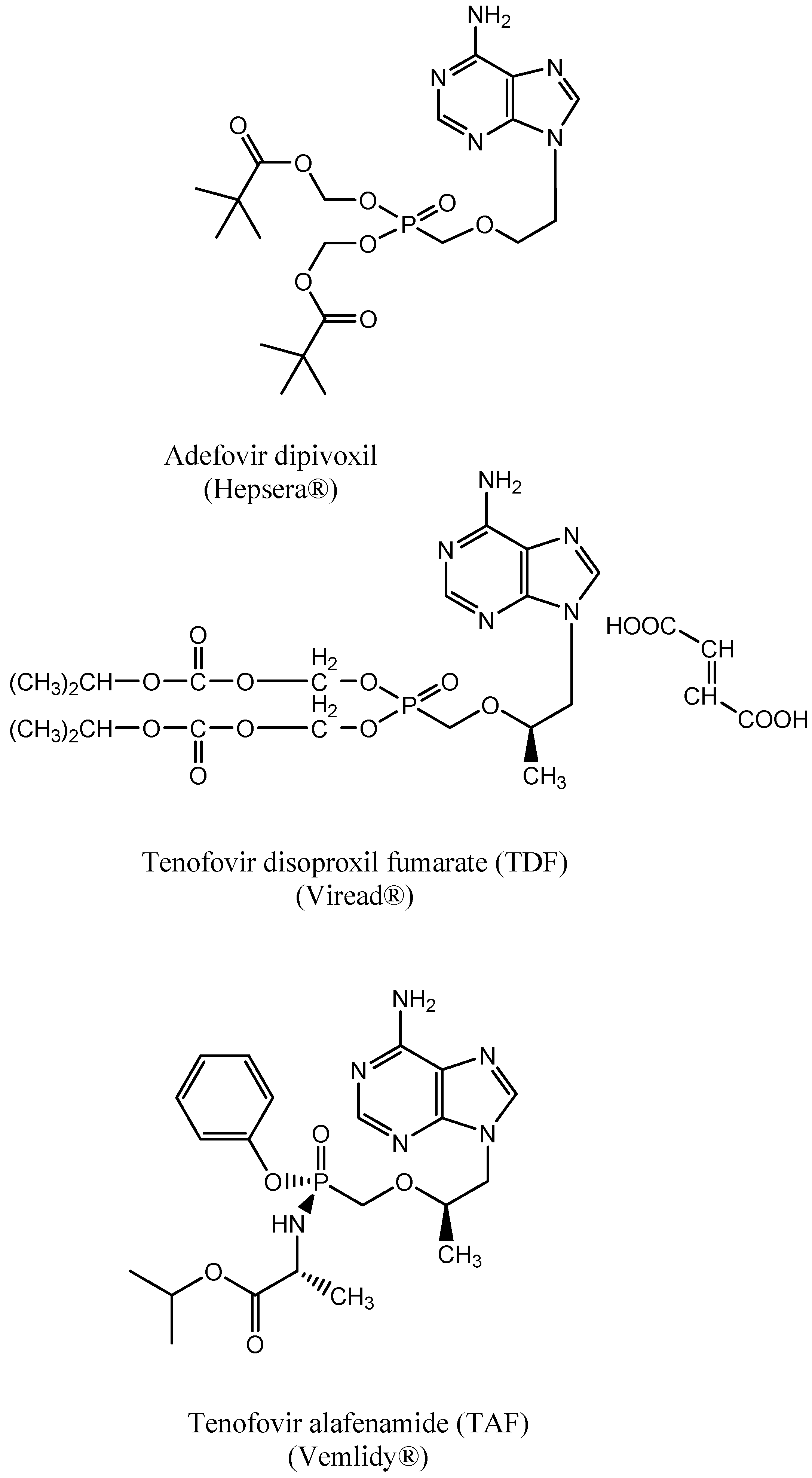
3. PMEA: 9-(2-Methoxyethyl) Adenine (Adefovir)
4. S-1-(3-Hydroxypropyl-2-methyphosphonyl) Cytosine (Cidofovir)
5. First Description of (R)-PMPA, Later Named Tenofovir
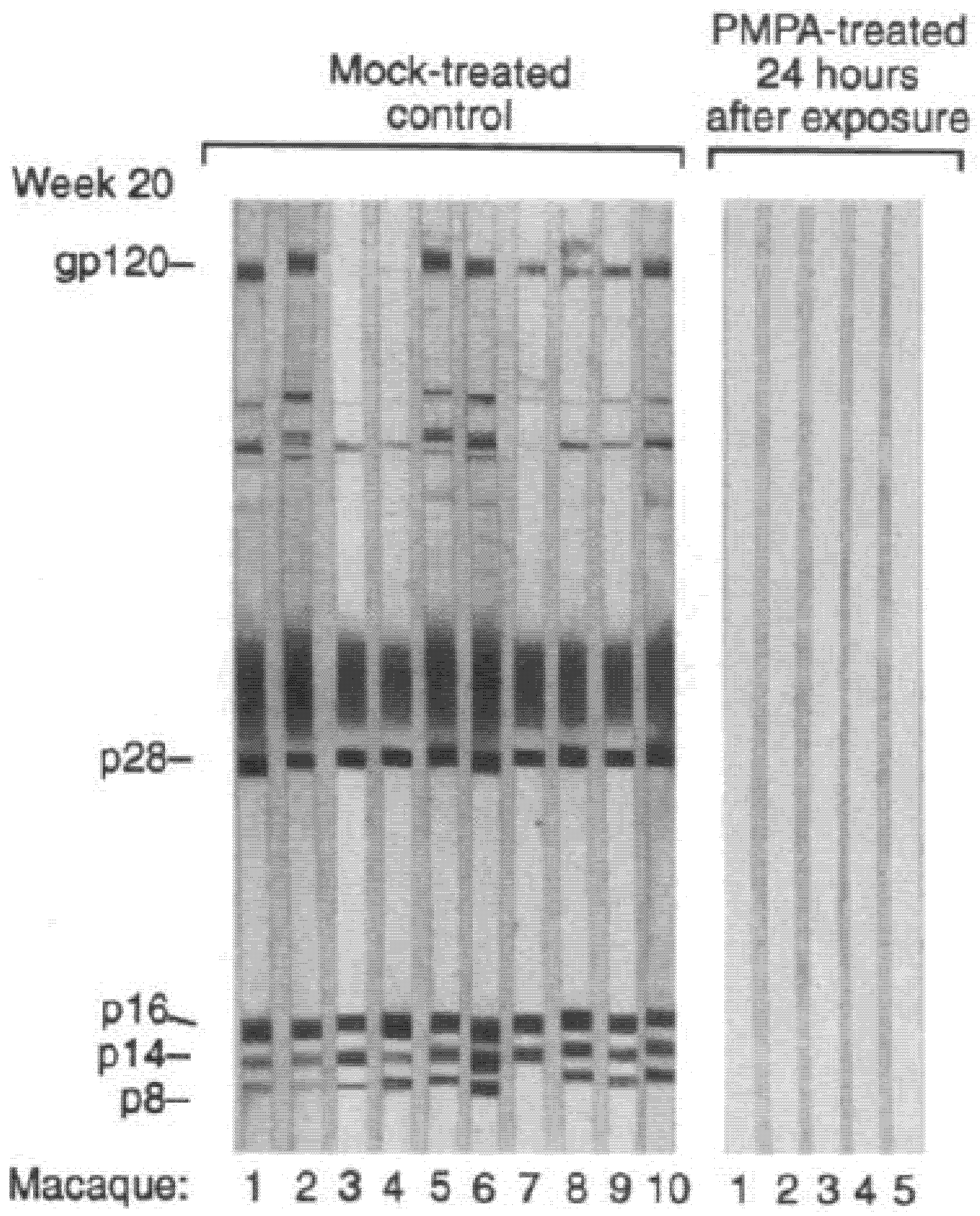
6. Complete Protection of SIV Infection by (R)-PMPA
7. Tenofovir Disoproxil
8. Tenofovir Disoproxil Fumarate (TDF)
9. TDF for the Treatment of HBV Infections
10. Truvada®: Combination of TDF with Emtricitabine
11. Combinations of TDF with Other Anti-HIV Drugs
12. Advent of Tenofovir Alafenamide (TAF)
13. TAF for the Treatment of HBV Infections
14. Combinations of TAF with Other Anti-HIV Drugs
15. TDF and TAF as Part of PrEP (Pre-Exposure Prophylaxis)
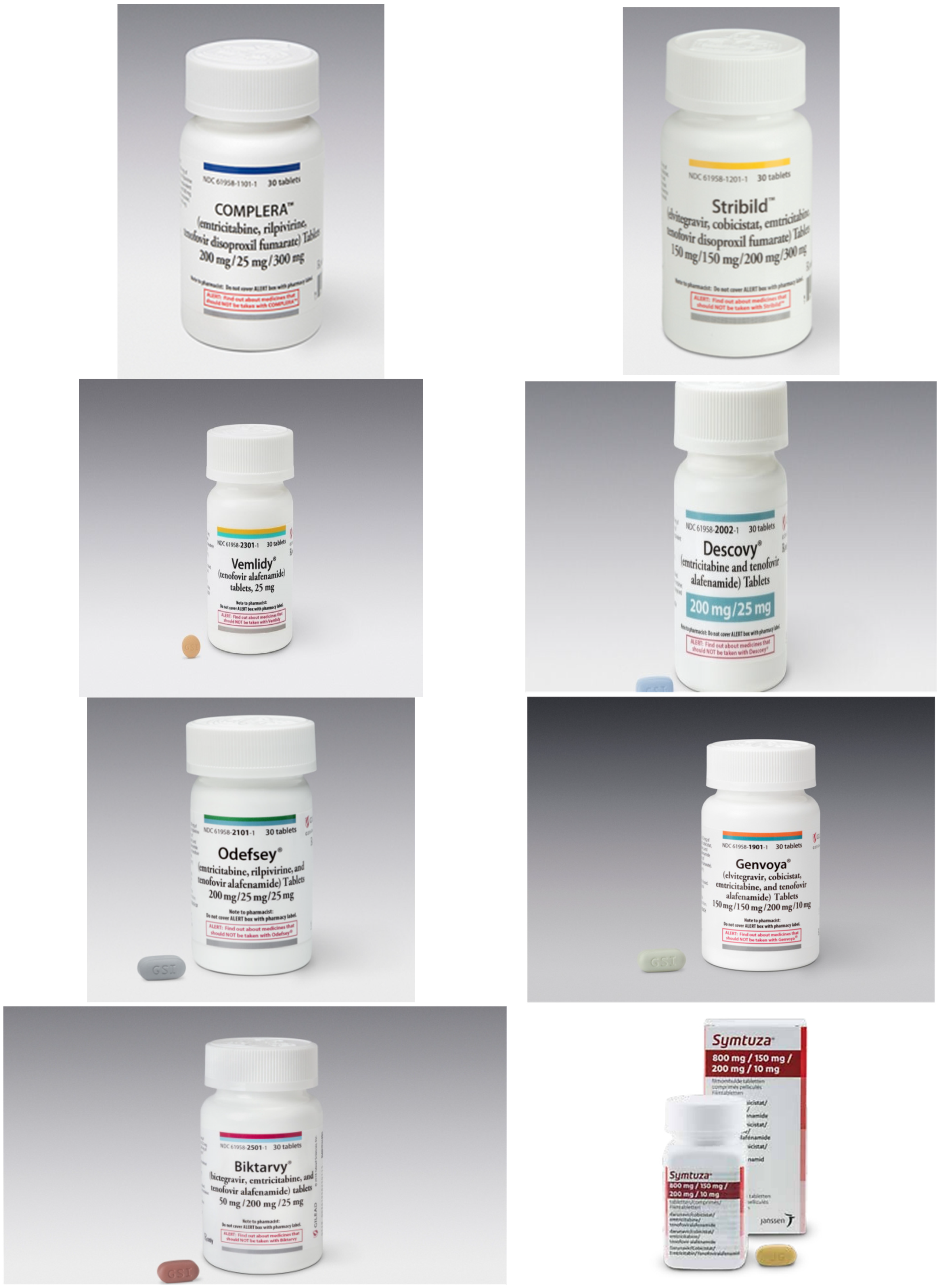
16. Total Number of Tenofovir Preparations Now on the Market
| TDF | : | Tenofovir disoproxil fumarate | : | Viread® | ||||
| TDF | + | Emtricitabine (Emtriva®) | : | Truvada® | ||||
| TDF | + | Emtricitabine | + | Efavirenz (Sustiva®, Stocrin®) | : | Atripla® | ||
| TDF | + | Emtricitabine | + | Rilpivirine (Edurant®) | : | Complera®, Eviplera® | ||
| TDF | + | Emtricitabine | Elvitegravir | + | Cobicistat | : | Stribild® | |
| TAF | : | Tenofovir alafenamide | : | Vemlidy® | ||||
| TAF | + | Emtricitabine | : | Descovy® | ||||
| TAF | + | Emtricitabine | + | Rilpivirine | : | Odefsey® | ||
| TAF | + | Emtricitabine | + | Elvitegravir | + | Cobicistat | : | Genvoya® |
| TAF | + | Emtricitabine | + | Bictegravir | : | Biktarvy® | ||
| TAF | + | Emtricitabine | + | Darunavir | + | Cobicistat | : | Symtuza® |
| TDF | + | Lamivudine | : | Cimduo™ | ||||
| TDF | + | Lamivudine | + | Efavirenz (600 mg) | : | Symfi™ | ||
| TDF | + | Lamivudine | + | Efavirenz (400 mg) | : | Symfi Lo™ | ||
| TDF | + | Lamivudine | + | Doravirine | : | Delstrigo™ | ||
17. Compounds in the Pipeline
18. Epilogue
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Martin, J.C.; Dvorak, C.A.; Smee, D.F.; Matthews, T.R.; Verheyden, J.P. 9-(1,3-Dihydroxy-2-propoxymethyl) guanine: A new potent and selective antiherpes agent. J. Med. Chem. 1983, 26, 759–761. [Google Scholar] [CrossRef]
- Mitsuya, H.; Broder, S. Inhibition of the in vitro infectivity and cytopathic effect of human T-lymphotrophic virus type III/lymphadenopathy-associated virus (HTLV-III/LAV) by 2′,3′-dideoxynucleosides. Proc. Natl. Acad. Sci. USA 1986, 83, 1911–1915. [Google Scholar] [CrossRef] [PubMed]
- Dziuban, E.J.; Raizes, E.; Koumans, E.H. A farewell to didanosine: Harm reduction and cost savings by eliminating use of didanosine. Int. J. STD AIDS 2015, 26, 903–906. [Google Scholar] [CrossRef] [PubMed]
- Gelpi, M.; Afzal, S.; Fuchs, A.; Lundgren, J.; Knudsen, A.D.; Drivsholm, N.; Mocroft, A.; Lebech, A.M.; Lindegaard, B.; Kühl, J.T.; et al. Prior exposure to thymidine analogs and didanosine is associated with long-lasting alterations in adipose tissue distribution and cardiovascular risk factors. AIDS 2019, 33, 675–683. [Google Scholar] [CrossRef] [PubMed]
- Logan, S.; Rodger, A.; Maynard-Smith, L.; O’Beirne, J.; Fernandez, T.; Ferro, F.; Smith, C.; Bhagani, S. Prevalence of significant liver disease in human immunodeficiency virus-infected patients exposed to Didanosine: A cross sectional study. World J. Hepatol. 2016, 8, 1623–1628. [Google Scholar] [CrossRef] [PubMed]
- Martin, J.C.; Hitchcock, M.J.; De Clercq, E.; Prusoff, W.H. Early nucleoside reverse transcriptase inhibitors for the treatment of HIV: A brief history of stavudine (D4T) and its comparison with other dideoxynucleosides. Antivir. Res. 2010, 85, 34–38. [Google Scholar] [CrossRef]
- Baba, M.; Pauwels, R.; Herdewijn, P.; De Clercq, E.; Desmyter, J.; Vandeputte, M. Both 2′,3′-dideoxythymidine and its 2′,3′-unsaturated derivative (2′,3′-dideoxythymidinene) are potent and selective inhibitors of human immunodeficiency virus replication in vitro. Biochem. Biophys. Res. Commun. 1987, 142, 128–134. [Google Scholar] [CrossRef]
- Hamamoto, Y.; Nakashima, H.; Matsui, T.; Matsuda, A.; Ueda, T.; Yamamoto, N. Inhibitory effect of 2′,3′-didehydro-2′,3′-dideoxynucleosides on infectivity, cytopathic effects, and replication of human immunodeficiency virus. Antimicrob. Agents Chemother. 1987, 31, 907–910. [Google Scholar] [CrossRef]
- Tai-Shun, L.; Schinazi, R.F.; Prusoff, W.H. Potent and selective in vitro activity of 3′-deoxythymidin-2′-ene (3′-deoxy-2′,3′-didehydrothymidine) against human immunodeficiency virus. Biochem. Pharmacol. 1987, 36, 2713–2718. [Google Scholar] [CrossRef]
- Mateo, M.G.; Gutierrez, M.D.M.; Vidal, F.; Domingo, P. Stavudine extended release (once-daily, Bristol-Myers Squibb) for the treatment of HIV/AIDS. Expert Opin. Pharmacother. 2013, 14, 1055–1064. [Google Scholar] [CrossRef]
- Spaulding, A.; Rutherford, G.W.; Siegfried, N. Stavudine or zidovudine in three-drug combination therapy for initial treatment of HIV infection in antiretroviral-naïve individuals. Cochrane Database Syst. Rev. 2010, Cd008651. [Google Scholar] [CrossRef] [PubMed]
- Stephan, C.; Hill, A.; Sawyer, W.; van Delft, Y.; Moecklinghoff, C. Impact of baseline HIV-1 RNA levels on initial highly active antiretroviral therapy outcome: A meta-analysis of 12,370 patients in 21 clinical trials. HIV Med. 2013, 14, 284–292. [Google Scholar] [CrossRef] [PubMed]
- De Clercq, E.; Holy, A.; Rosenberg, I.; Sakuma, T.; Balzarini, J.; Maudgal, P.C. A novel selective broad-spectrum anti-DNA virus agent. Nature 1986, 323, 467. [Google Scholar] [CrossRef] [PubMed]
- De Clercq, E.; Sakuma, T.; Baba, M.; Pauwels, R.; Balzarini, J.; Rosenberg, I.; Holy, A. Antiviral activity of phosphonylmethoxyalkyl derivatives of purine and pyrimidines. Antivir. Res. 1987, 8, 261–272. [Google Scholar] [CrossRef]
- Balzarini, J.; Naesens, L.; Herdewijn, P.; Rosenberg, I.; Holy, A.; Pauwels, R.; Baba, M.; Johns, D.G.; De Clercq, E. Marked in vivo antiretrovirus activity of 9-(2-phosphonylmethoxyethyl)adenine, a selective anti-human immunodeficiency virus agent. Proc. Natl. Acad. Sci. USA 1989, 86, 332–336. [Google Scholar] [CrossRef] [PubMed]
- Pauwels, R.; Balzarini, J.; Schols, D.; Baba, M.; Desmyter, J.; Rosenberg, I.; Holy, A.; De Clercq, E. Phosphonylmethoxyethyl purine derivatives, a new class of anti-human immunodeficiency virus agents. Antimicrob. Agents Chemother. 1988, 32, 1025–1030. [Google Scholar] [CrossRef] [PubMed]
- Heijtink, R.A.; De Wilde, G.A.; Kruining, J.; Berk, L.; Balzarini, J.; De Clercq, E.; Holy, A.; Schalm, S.W. Inhibitory effect of 9-(2-phosphonylmethoxyethyl)-adenine (PMEA) on human and duck hepatitis B virus infection. Antivir. Res. 1993, 21, 141–153. [Google Scholar] [CrossRef]
- Yokota, T.; Konno, K.; Chonan, E.; Mochizuki, S.; Kojima, K.; Shigeta, S.; De Clercq, E. Comparative activities of several nucleoside analogs against duck hepatitis B virus in vitro. Antimicrob. Agents Chemother. 1990, 34, 1326–1330. [Google Scholar] [CrossRef] [PubMed]
- Yokota, T.; Konno, K.; Shigeta, S.; Holy, A.; Balzarini, J.; De Clercq, E. Inhibitory effects of acyclic nucleoside phosphonate analogues on hepatitis B virus DNA synthesis in HB611 cells. Antivir. Chem. Chemother. 1994, 5, 57–63. [Google Scholar] [CrossRef]
- Yokota, T.; Mochizuki, S.; Konno, K.; Mori, S.; Shigeta, S.; De Clercq, E. Inhibitory effects of selected antiviral compounds on human hepatitis B virus DNA synthesis. Antimicrob. Agents Chemother. 1991, 35, 394–397. [Google Scholar] [CrossRef]
- Marcellin, P.; Chang, T.T.; Lim, S.G.; Tong, M.J.; Sievert, W.; Shiffman, M.L.; Jeffers, L.; Goodman, Z.; Wulfsohn, M.S.; Xiong, S.; et al. Adefovir dipivoxil for the treatment of hepatitis B e antigen-positive chronic hepatitis B. N. Engl. J. Med. 2003, 348, 808–816. [Google Scholar] [CrossRef] [PubMed]
- Hadziyannis, S.J.; Tassopoulos, N.C.; Heathcote, E.J.; Chang, T.T.; Kitis, G.; Rizzetto, M.; Marcellin, P.; Lim, S.G.; Goodman, Z.; Wulfsohn, M.S.; et al. Adefovir dipivoxil for the treatment of hepatitis B e antigen-negative chronic hepatitis B. N. Engl. J. Med. 2003, 348, 800–807. [Google Scholar] [CrossRef]
- Hadziyannis, S.J.; Tassopoulos, N.C.; Heathcote, E.J.; Chang, T.T.; Kitis, G.; Rizzetto, M.; Marcellin, P.; Lim, S.G.; Goodman, Z.; Ma, J.; et al. Long-term therapy with adefovir dipivoxil for HBeAg-negative chronic hepatitis B. N. Engl. J. Med. 2005, 352, 2673–2681. [Google Scholar] [CrossRef] [PubMed]
- Snoeck, R.; Sakuma, T.; De Clercq, E.; Rosenberg, I.; Holy, A. (S)-1-(3-hydroxy-2-phosphonylmethoxypropyl)cytosine, a potent and selective inhibitor of human cytomegalovirus replication. Antimicrob. Agents Chemother. 1988, 32, 1839–1844. [Google Scholar] [CrossRef] [PubMed]
- Lalezari, J.P.; Drew, W.L.; Glutzer, E.; James, C.; Miner, D.; Flaherty, J.; Fisher, P.E.; Cundy, K.; Hannigan, J.; Martin, J.C.; et al. (S)-1-[3-hydroxy-2-(phosphonylmethoxy)propyl]cytosine (cidofovir): Results of a phase I/II study of a novel antiviral nucleotide analogue. J. Infect. Dis. 1995, 171, 788–796. [Google Scholar] [CrossRef]
- Lalezari, J.P.; Holland, G.N.; Kramer, F.; McKinley, G.F.; Kemper, C.A.; Ives, D.V.; Nelson, R.; Hardy, W.D.; Kuppermann, B.D.; Northfelt, D.W.; et al. Randomized, controlled study of the safety and efficacy of intravenous cidofovir for the treatment of relapsing cytomegalovirus retinitis in patients with AIDS. J. Acquir. Immune Defic. Syndr. Hum. Retrovirol. 1998, 17, 339–344. [Google Scholar] [CrossRef] [PubMed]
- Lalezari, J.P.; Kuppermann, B.D. Clinical experience with cidofovir in the treatment of cytomegalovirus retinitis. J. Acquir. Immune Defic. Syndr. Hum. Retrovirol. 1997, 14 (Suppl. 1), S27–S31. [Google Scholar] [CrossRef]
- Lalezari, J.P.; Stagg, R.J.; Kuppermann, B.D.; Holland, G.N.; Kramer, F.; Ives, D.V.; Youle, M.; Robinson, M.R.; Drew, W.L.; Jaffe, H.S. Intravenous cidofovir for peripheral cytomegalovirus retinitis in patients with AIDS: A randomized, controlled trial. Ann. Intern. Med. 1997, 126, 257–263. [Google Scholar] [CrossRef]
- De Clercq, E. Clinical potential of the acyclic nucleoside phosphonates cidofovir, adefovir, and tenofovir in treatment of DNA virus and retrovirus infections. Clin. Microbiol. Rev. 2003, 16, 569–596. [Google Scholar] [CrossRef]
- Stittelaar, K.J.; Neyts, J.; Naesens, L.; van Amerongen, G.; van Lavieren, R.F.; Holì, A.; De Clercq, E.; Niesters, H.G.M.; Fries, E.; Maas, C.; et al. Antiviral treatment is more effective than smallpox vaccination upon lethal monkeypox virus infection. Nature 2006, 439, 745–748. [Google Scholar] [CrossRef]
- Balzarini, J.; Holy, A.; Jindrich, J.; Dvorakova, H.; Hao, Z.; Snoeck, R.; Herdewijn, P.; Johns, D.G.; De Clercq, E. 9-[(2RS)-3-Fluoro-2-phosphonylmethoxypropyl] derivatives of purines: A class of highly selective antiretroviral agents in vitro and in vivo. Proc. Natl. Acad. Sci. USA 1991, 88, 4961–4965. [Google Scholar] [CrossRef]
- Balzarini, J.; Holy, A.; Jindrich, J.; Naesens, L.; Snoeck, R.; Schols, D.; De Clercq, E. Differential antiherpesvirus and antiretrovirus effects of the (S) and (R) enantiomers of acyclic nucleoside phosphonates: Potent and selective in vitro and in vivo antiretrovirus activities of (R)-9-(2-phosphonomethoxypropyl)-2,6-diaminopurine. Antimicrob. Agents Chemother. 1993, 37, 332–338. [Google Scholar] [CrossRef]
- Lou, L.L.; Martin, J.C. Selected thoughts on hydrophobicity in drug design. Molecules 2021, 26, 875. [Google Scholar] [CrossRef]
- Tsai, C.C.; Follis, K.E.; Sabo, A.; Beck, T.W.; Grant, R.F.; Bischofberger, N.; Benveniste, R.E.; Black, R. Prevention of SIV infection in macaques by (R)-9-(2-phosphonylmethoxypropyl)adenine. Science 1995, 270, 1197–1199. [Google Scholar] [CrossRef] [PubMed]
- Mitsuya, H.; Weinhold, K.J.; Furman, P.A.; St Clair, M.H.; Lehrman, S.N.; Gallo, R.C.; Bolognesi, D.; Barry, D.W.; Broder, S. 3′-Azido-3′-deoxythymidine (BW A509U): An antiviral agent that inhibits the infectivity and cytopathic effect of human T-lymphotropic virus type III/lymphadenopathy-associated virus in vitro. Proc. Natl. Acad. Sci. USA 1985, 82, 7096–7100. [Google Scholar] [CrossRef]
- Naesens, L.; Bischofberger, N.; Augustijns, P.; Annaert, P.; Van den Mooter, G.; Arimilli, M.N.; Kim, C.U.; De Clercq, E. Antiretroviral efficacy and pharmacokinetics of oral bis(isopropyloxycarbonyloxymethyl)-9-(2-phosphonylmethoxypropyl)adenine in mice. Antimicrob. Agents Chemother. 1998, 42, 1568–1573. [Google Scholar] [CrossRef] [PubMed]
- Robbins, B.L.; Srinivas, R.V.; Kim, C.; Bischofberger, N.; Fridland, A. Anti-human immunodeficiency virus activity and cellular metabolism of a potential prodrug of the acyclic nucleoside phosphonate 9-R-(2-phosphonomethoxypropyl)adenine (PMPA), Bis(isopropyloxymethylcarbonyl)PMPA. Antimicrob. Agents Chemother. 1998, 42, 612–617. [Google Scholar] [CrossRef]
- Barditch-Crovo, P.; Deeks, S.G.; Collier, A.; Safrin, S.; Coakley, D.F.; Miller, M.; Kearney, B.P.; Coleman, R.L.; Lamy, P.D.; Kahn, J.O.; et al. Phase i/ii trial of the pharmacokinetics, safety, and antiretroviral activity of tenofovir disoproxil fumarate in human immunodeficiency virus-infected adults. Antimicrob. Agents Chemother. 2001, 45, 2733–2739. [Google Scholar] [CrossRef] [PubMed]
- Miller, M.D.; Margot, N.A.; Lamy, P.D.; Fuller, M.D.; Anton, K.E.; Mulato, A.S.; Cherrington, J.M. Adefovir and tenofovir susceptibilities of HIV-1 after 24 to 48 weeks of adefovir dipivoxil therapy: Genotypic and phenotypic analyses of study GS-96-408. J. Acquir. Immune Defic. Syndr. 2001, 27, 450–458. [Google Scholar] [CrossRef]
- Naeger, L.K.; Margot, N.A.; Miller, M.D. Increased drug susceptibility of HIV-1 reverse transcriptase mutants containing M184V and zidovudine-associated mutations: Analysis of enzyme processivity, chain-terminator removal and viral replication. Antivir. Ther. 2001, 6, 115–126. [Google Scholar] [PubMed]
- Rosenwirth, B.; ten Haaft, P.; Bogers, W.M.; Nieuwenhuis, I.G.; Niphuis, H.; Kuhn, E.M.; Bischofberger, N.; Heeney, J.L.; Uberla, K. Antiretroviral therapy during primary immunodeficiency virus infection can induce persistent suppression of virus load and protection from heterologous challenge in rhesus macaques. J. Virol. 2000, 74, 1704–1711. [Google Scholar] [CrossRef][Green Version]
- Van Rompay, K.K.; Miller, M.D.; Marthas, M.L.; Margot, N.A.; Dailey, P.J.; Canfield, D.R.; Tarara, R.P.; Cherrington, J.M.; Aguirre, N.L.; Bischofberger, N.; et al. Prophylactic and therapeutic benefits of short-term 9-[2-(R)-(phosphonomethoxy)propyl]adenine (PMPA) administration to newborn macaques following oral inoculation with simian immunodeficiency virus with reduced susceptibility to PMPA. J. Virol. 2000, 74, 1767–1774. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Marcellin, P.; Heathcote, E.J.; Buti, M.; Gane, E.; de Man, R.A.; Krastev, Z.; Germanidis, G.; Lee, S.S.; Flisiak, R.; Kaita, K.; et al. Tenofovir disoproxil fumarate versus adefovir dipivoxil for chronic hepatitis B. N. Engl. J. Med. 2008, 359, 2442–2455. [Google Scholar] [CrossRef] [PubMed]
- Eisenberg, E.J.; He, G.X.; Lee, W.A. Metabolism of GS-7340, a novel phenyl monophosphoramidate intracellular prodrug of PMPA, in blood. Nucleosides Nucleotides Nucleic Acids 2001, 20, 1091–1098. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.A.; He, G.X.; Eisenberg, E.; Cihlar, T.; Swaminathan, S.; Mulato, A.; Cundy, K.C. Selective intracellular activation of a novel prodrug of the human immunodeficiency virus reverse transcriptase inhibitor tenofovir leads to preferential distribution and accumulation in lymphatic tissue. Antimicrob. Agents Chemother. 2005, 49, 1898–1906. [Google Scholar] [CrossRef] [PubMed]
- De Clercq, E. Role of tenofovir alafenamide (TAF) in the treatment and prophylaxis of HIV and HBV infections. Biochem. Pharmacol. 2018, 153, 2–11. [Google Scholar] [CrossRef]
- De Clercq, E. Tenofovir at the crossroad of the therapy and prophylaxis of HIV and HBV infections. J. Cell. Immunol. 2020, 2, 23–30. [Google Scholar]
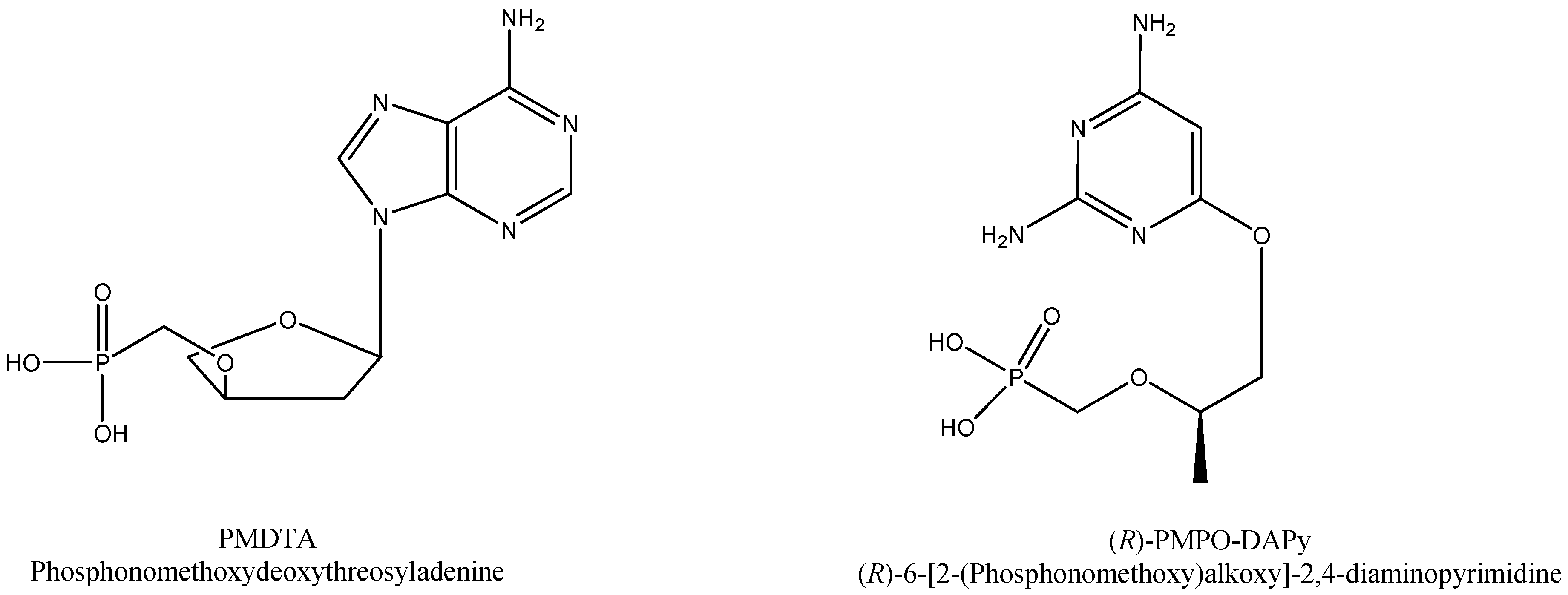
- Wu, T.; Froeyen, M.; Kempeneers, V.; Pannecouque, C.; Wang, J.; Busson, R.; De Clercq, E.; Herdewijn, P. Deoxythreosyl phosphonate nucleosides as selective anti-HIV agents. J. Am. Chem. Soc. 2005, 127, 5056–5065. [Google Scholar] [CrossRef]
- De Clercq, E.; Andrei, G.; Balzarini, J.; Leyssen, P.; Naesens, L.; Neyts, J.; Pannecouque, C.; Snoeck, R.; Ying, C.; Hocková, D.; et al. Antiviral potential of a new generation of acyclic nucleoside phosphonates, the 6-[2-(phosphonomethoxy)alkoxy]-2,4-diaminopyrimidines. Nucleosides Nucleotides Nucleic Acids 2005, 24, 331–341. [Google Scholar] [CrossRef] [PubMed]
- De Clercq, E. Acyclic nucleoside phosphonates: An unfinished story. Collect. Czechoslov. Chem. Commun. 2011, 76, 829–842. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Clercq, E. Tribute to John C. Martin at the Twentieth Anniversary of the Breakthrough of Tenofovir in the Treatment of HIV Infections. Viruses 2021, 13, 2410. https://doi.org/10.3390/v13122410
De Clercq E. Tribute to John C. Martin at the Twentieth Anniversary of the Breakthrough of Tenofovir in the Treatment of HIV Infections. Viruses. 2021; 13(12):2410. https://doi.org/10.3390/v13122410
Chicago/Turabian StyleDe Clercq, Erik. 2021. "Tribute to John C. Martin at the Twentieth Anniversary of the Breakthrough of Tenofovir in the Treatment of HIV Infections" Viruses 13, no. 12: 2410. https://doi.org/10.3390/v13122410
APA StyleDe Clercq, E. (2021). Tribute to John C. Martin at the Twentieth Anniversary of the Breakthrough of Tenofovir in the Treatment of HIV Infections. Viruses, 13(12), 2410. https://doi.org/10.3390/v13122410





