Composition of Eukaryotic Viruses and Bacteriophages in Individuals with Acute Gastroenteritis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethics Information
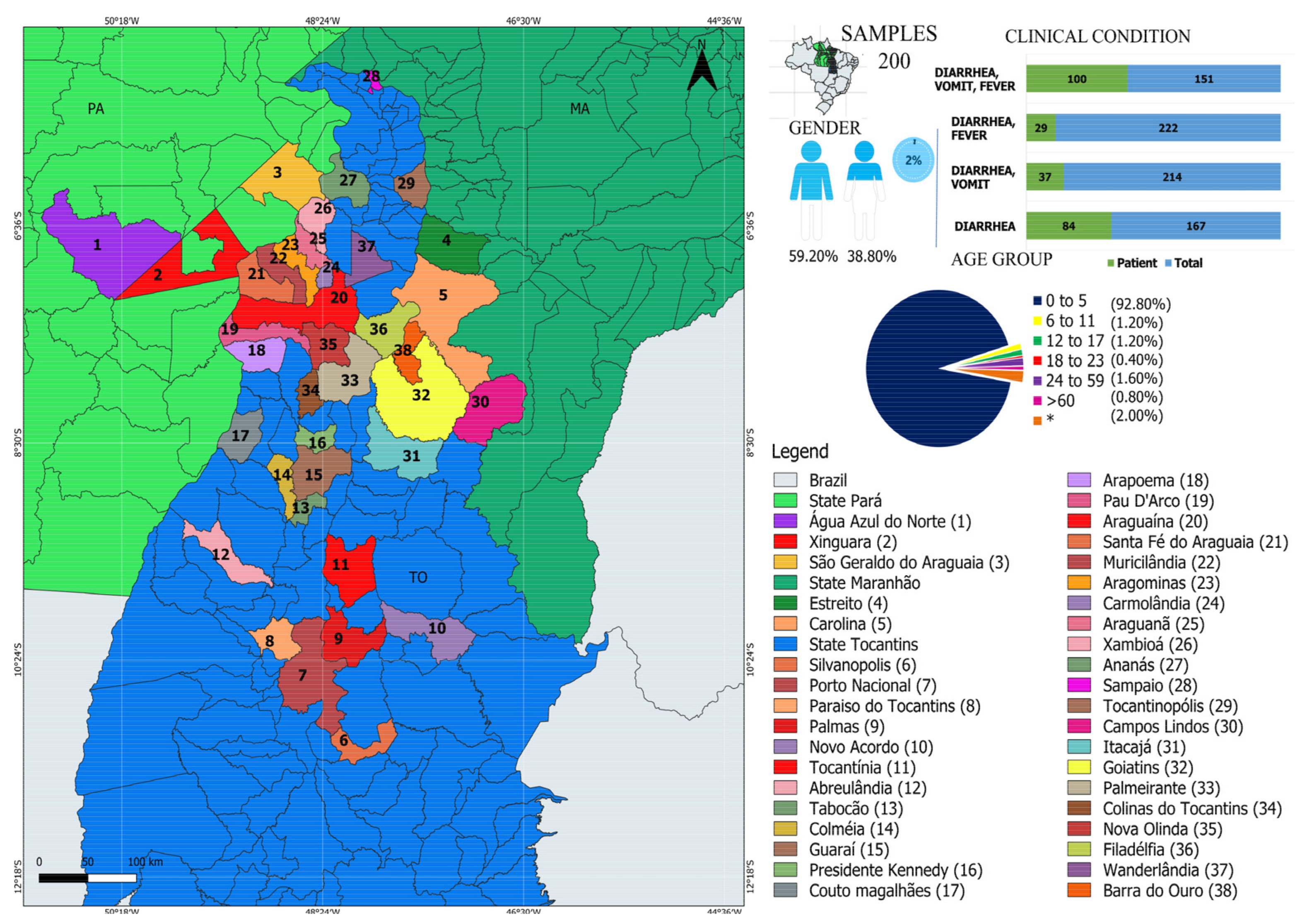
2.2. Study Population and Sample Collection
2.3. Sample Screening
2.4. Viral-like Particle Metagenomics
2.5. Bioinformatics Analysis
2.6. Statistics Analysis
3. Results
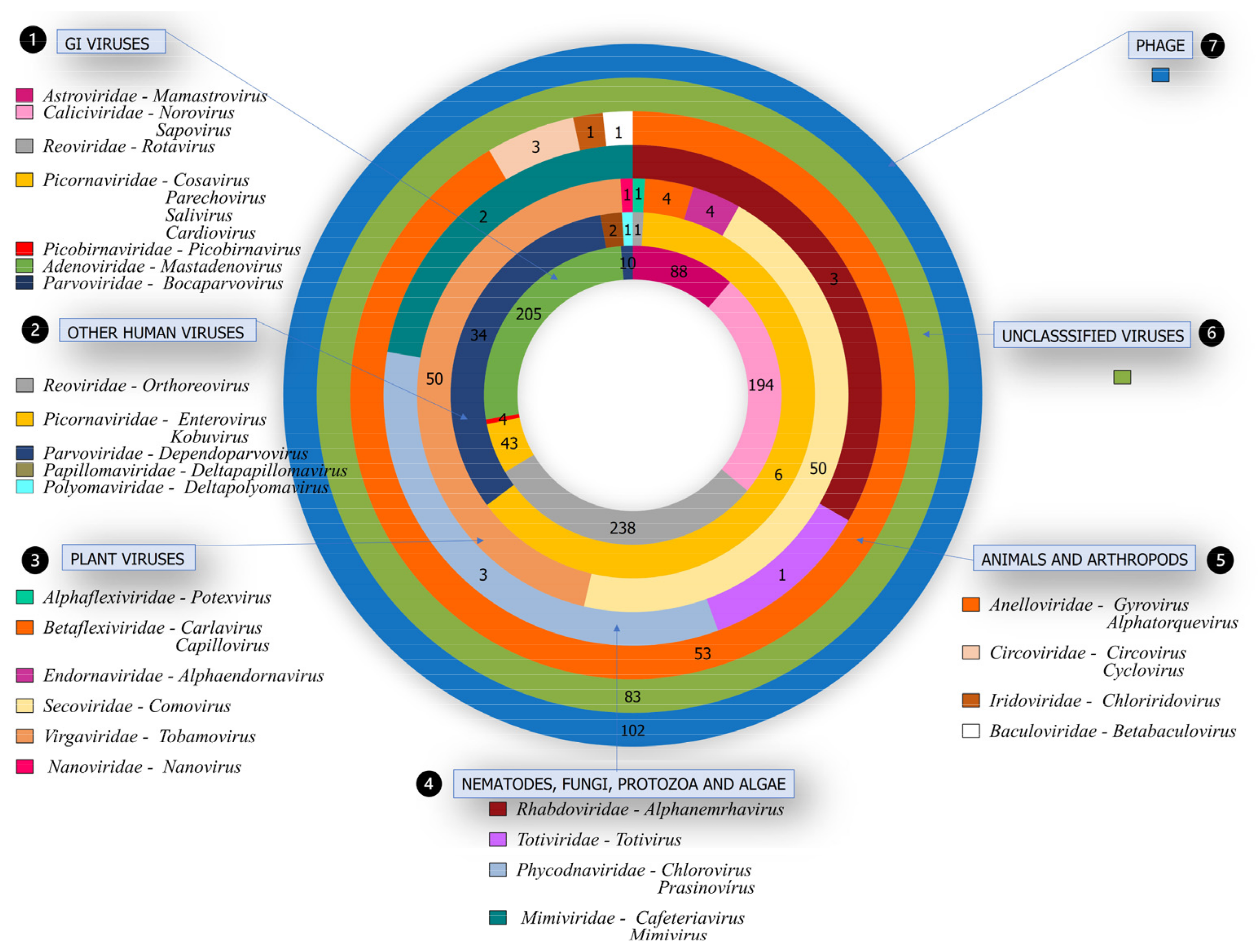
3.1. Characterization of Viruses in Individuals with GI
3.2. Viral Families Detected by NGS
3.3. Pathogenic Viruses Associated with GI
3.4. Other Viruses in Human Hosts Not Related to GI
3.5. Viruses from Different Hosts
3.6. Unclassified Viruses
3.7. Bacteriophages
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Reyes, A.; Haynes, M.; Hanson, N.; Angly, F.E.; Heath, A.C.; Rohwer, F.; Gordon, J.I. Viruses in the Faecal Microbiota of Monozygotic Twins and Their Mothers. Nature 2010, 466, 334–338. [Google Scholar] [CrossRef] [PubMed]
- Minot, S.; Sinha, R.; Chen, J.; Li, H.; Keilbaugh, S.A.; Wu, G.D.; Lewis, J.D.; Bushman, F.D. The Human Gut Virome: Inter-Individual Variation and Dynamic Response to Diet. Genome Res. 2011, 21, 1616–1625. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernandez-Cassi, X.; Martínez-Puchol, S.; Silva-Sales, M.; Cornejo, T.; Bartolome, R.; Bofill-Mas, S.; Girones, R. Unveiling Viruses Associated with Gastroenteritis Using a Metagenomics Approach. Viruses 2020, 12, 1432. [Google Scholar] [CrossRef] [PubMed]
- Troeger, C.; Forouzanfar, M.; Rao, P.C.; Khalil, I.; Brown, A.; Reiner, R.C.; Fullman, N.; Thompson, R.L.; Abajobir, A.; Ahmed, M.; et al. Estimates of Global, Regional, and National Morbidity, Mortality, and Aetiologies of Diarrhoeal Diseases: A Systematic Analysis for the Global Burden of Disease Study 2015. Lancet Infect. Dis. 2017, 17, 909–948. [Google Scholar] [CrossRef] [Green Version]
- Liu, L.; Oza, S.; Hogan, D.; Chu, Y.; Perin, J.; Zhu, J.; Lawn, J.E.; Cousens, S.; Mathers, C.; Black, R.E. Global, Regional, and National Causes of under-5 Mortality in 2000–15: An Updated Systematic Analysis with Implications for the Sustainable Development Goals. Lancet 2016, 388, 3027–3035. [Google Scholar] [CrossRef] [Green Version]
- Nora-Krukle, Z.; Vilmane, A.; Xu, M.; Rasa, S.; Ziemele, I.; Silina, E.; Söderlund-Venermo, M.; Gardovska, D.; Murovska, M. Human Bocavirus Infection Markers in Peripheral Blood and Stool Samples of Children with Acute Gastroenteritis. Viruses 2018, 10, 639. [Google Scholar] [CrossRef] [Green Version]
- Adam, M.A.; Wang, J.; Enan, K.-A.; Shen, H.; Wang, H.; El Hussein, A.R.; Musa, A.B.; Khidir, I.M.; Ma, X. Molecular Survey of Viral and Bacterial Causes of Childhood Diarrhea in Khartoum State, Sudan. Front. Microbiol. 2018, 9, 112. [Google Scholar] [CrossRef]
- Mohammad, H.A.; Madi, N.M.; Al-Nakib, W. Analysis of Viral Diversity in Stool Samples from Infants and Children with Acute Gastroenteritis in Kuwait Using Metagenomics Approach. Virol. J. 2020, 17, 10. [Google Scholar] [CrossRef] [Green Version]
- Lanata, C.F.; Fischer-Walker, C.L.; Olascoaga, A.C.; Torres, C.X.; Aryee, M.J.; Black, R.E.; Child Health Epidemiology Reference Group of the World Health Organization and UNICEF. Global Causes of Diarrheal Disease Mortality in Children <5 Years of Age: A Systematic Review. PLoS ONE 2013, 8, e72788. [Google Scholar] [CrossRef] [Green Version]
- Justino, M.C.A.; Campos, E.A.; Mascarenhas, J.D.P.; Soares, L.S.; Sylvia de Fátima, S.G.; Furlaneto, I.P.; Pavão, M.J.C., Jr.; Maciel, T.S.; Farias, F.P.; Bezerra, O.M.; et al. Rotavirus Antigenemia as a Common Event among Children Hospitalised for Severe, Acute Gastroenteritis in Belém, Northern Brazil. BMC Pediatr. 2019, 19, 193. [Google Scholar] [CrossRef]
- Varela, M.F.; Rivadulla, E.; Lema, A.; Romalde, J.L. Human Sapovirus among Outpatients with Acute Gastroenteritis in Spain: A One-Year Study. Viruses 2019, 11, 144. [Google Scholar] [CrossRef] [Green Version]
- Kapoor, A.; Li, L.; Victoria, J.; Oderinde, B.; Mason, C.; Pandey, P.; Zaidi, S.Z.; Delwart, E. Multiple Novel Astrovirus Species in Human Stool. J. Gen. Virol. 2009, 90, 2965–2972. [Google Scholar] [CrossRef] [PubMed]
- Leal, É.; Luchs, A.; de Pádua Milagres, F.A.; Komninakis, S.V.; Gill, D.E.; Alves Brito Sayão Lobato, M.C.; Brustulin, R.; das Chagas, R.T.; de Fátima Neves dos Santos Abrão, M.; de Deus Alves Soares, C.V.; et al. Recombinant Strains of Human Parechovirus in Rural Areas in the North of Brazil. Viruses 2019, 11, 488. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Watanabe, A.S.A.; Luchs, A.; Leal, É.; de Pádua Milagres, F.A.; Komninakis, S.V.; Gill, D.E.; Alves Brito Sayão Lobato, M.C.; Brustulin, R.; das Chagas, R.T.; de Fátima Neves dos Santos Abrão, M.; et al. Complete Genome Sequences of Six Human Bocavirus Strains from Patients with Acute Gastroenteritis in the North Region of Brazil. Genome Announc. 2018, 6, e00235-18. [Google Scholar] [CrossRef] [Green Version]
- Allander, T.; Tammi, M.T.; Eriksson, M.; Bjerkner, A.; Tiveljung-Lindell, A.; Andersson, B. From the Cover: Cloning of a Human Parvovirus by Molecular Screening of Respiratory Tract Samples. Proc. Natl. Acad. Sci. USA 2005, 102, 12891–12896. [Google Scholar] [CrossRef] [Green Version]
- Da Costa, A.C.; Luchs, A.; de Pádua Milagres, F.A.; Komninakis, S.V.; Gill, D.E.; Alves Brito Sayão Lobato, M.C.; Brustulin, R.; das Chagas, R.T.; de Fátima Neves dos Santos Abrão, M.; de Deus Alves Soares, C.V.; et al. Near Full Length Genome of a Recombinant (E/D) Cosavirus Strain from a Rural Area in the Central Region of Brazil. Sci. Rep. 2018, 8, 12304. [Google Scholar] [CrossRef]
- Da Costa, A.; Luchs, A.; de Pádua Milagres, F.A.; Komninakis, S.; Gill, D.; Alves Brito Sayão Lobato, M.C.; Brustulin, R.; das Chagas, R.; de Fátima Neves dos Santos Abrão, M.; de Deus Alves Soares, C.V.; et al. Recombination Located over 2A-2B Junction Ribosome Frameshifting Region of Saffold Cardiovirus. Viruses 2018, 10, 520. [Google Scholar] [CrossRef] [Green Version]
- Chansaenroj, J.; Tuanthap, S.; Thanusuwannasak, T.; Duang-in, A.; Klinfueng, S.; Thaneskongtong, N.; Vutithanachot, V.; Vongpunsawad, S.; Poovorawan, Y. Human Enteroviruses Associated with and without Diarrhea in Thailand between 2010 and 2016. PLoS ONE 2017, 12, e0182078. [Google Scholar] [CrossRef] [Green Version]
- Khetsuriani, N.; Lamonte-Fowlkes, A.; Oberst, S.; Pallansch, M.A. Centers for Disease Control and Prevention Enterovirus Surveillance—United States, 1970–2005. MMWR Surveill. Summ. 2006, 55, 1–20. [Google Scholar]
- Khetsuriani, N.; Kutateladze, T.; Zangaladze, E.; Shutkova, T.; Peñaranda, S.; Nix, W.A.; Pallansch, M.A.; Oberste, M.S. High Degree of Genetic Diversity of Non-Polio Enteroviruses Identified in Georgia by Environmental and Clinical Surveillance, 2002–2005. J. Med. Microbiol. 2010, 59, 1340–1347. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barker, S.F.; Zomer, E.; O’Toole, J.; Sinclair, M.; Gibney, K.; Liew, D.; Leder, K. Cost of Gastroenteritis in Australia: A Healthcare Perspective. PLoS ONE 2018, 13, e0195759. [Google Scholar] [CrossRef] [Green Version]
- Castaño-Rodríguez, N.; Underwood, A.P.; Merif, J.; Riordan, S.M.; Rawlinson, W.D.; Mitchell, H.M.; Kaakoush, N.O. Gut Microbiome Analysis Identifies Potential Etiological Factors in Acute Gastroenteritis. Infect. Immun. 2018, 86, e00060-18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Da Costa, A.C.; Leal, E.; Gill, D.; de Pádua Milagres, F.A.; Komninakis, S.V.; Brustulin, R.; da Aparecida Rodrigues Teles, M.; Alves Brito Sayão Lobato, M.C.; das Chagas, R.T.; de Fátima Neves dos Santos Abrão, M.; et al. Discovery of Cucumis Melo Endornavirus by Deep Sequencing of Human Stool Samples in Brazil. Virus Genes 2019, 55, 332–338. [Google Scholar] [CrossRef] [PubMed]
- Kiselev, D.; Matsvay, A.; Abramov, I.; Dedkov, V.; Shipulin, G.; Khafizov, K. Current Trends in Diagnostics of Viral Infections of Unknown Etiology. Viruses 2020, 12, 211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fulci, V.; Stronati, L.; Cucchiara, S.; Laudadio, I.; Carissimi, C. Emerging Roles of Gut Virome in Pediatric Diseases. Int. J. Mol. Sci. 2021, 22, 4127. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, S.; Huq, S.; Yatsunenko, T.; Haque, R.; Mahfuz, M.; Alam, M.A.; Benezra, A.; DeStefano, J.; Meier, M.F.; Muegge, B.D.; et al. Persistent Gut Microbiota Immaturity in Malnourished Bangladeshi Children. Nature 2014, 510, 417–421. [Google Scholar] [CrossRef]
- Siqueira, J.D.; Dominguez-Bello, M.G.; Contreras, M.; Lander, O.; Caballero-Arias, H.; Xutao, D.; Noya-Alarcon, O.; Delwart, E. Complex Virome in Feces from Amerindian Children in Isolated Amazonian Villages. Nat. Commun. 2018, 9, 4270. [Google Scholar] [CrossRef] [PubMed]
- Kleiner, M.; Hooper, L.V.; Duerkop, B.A. Evaluation of Methods to Purify Virus-like Particles for Metagenomic Sequencing of Intestinal Viromes. BMC Genom. 2015, 16, 7. [Google Scholar] [CrossRef] [Green Version]
- Jurasz, H.; Pawłowski, T.; Perlejewski, K. Contamination Issue in Viral Metagenomics: Problems, Solutions, and Clinical Perspectives. Front. Microbiol. 2021, 12, 745076. [Google Scholar] [CrossRef]
- Van Borm, S.; Fu, Q.; Winand, R.; Vanneste, K.; Hakhverdyan, M.; Höper, D.; Vandenbussche, F. Evaluation of a Commercial Exogenous Internal Process Control for Diagnostic RNA Virus Metagenomics from Different Animal Clinical Samples. J. Virol. Methods 2020, 283, 113916. [Google Scholar] [CrossRef]
- Klenner, J.; Kohl, C.; Dabrowski, P.W.; Nitsche, A. Comparing Viral Metagenomic Extraction Methods. Curr. Issues Mol. Biol. 2017, 59–70. [Google Scholar] [CrossRef]
- Charlys da Costa, A.; Thézé, J.; Komninakis, S.C.V.; Sanz-Duro, R.L.; Felinto, M.R.L.; Moura, L.C.C.; Barroso, I.M.d.O.; Santos, L.E.C.; Nunes, M.A.d.L.; Moura, A.A.; et al. Spread of Chikungunya Virus East/Central/South African Genotype in Northeast Brazil. Emerg. Infect. Dis. 2017, 23, 1742–1744. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deng, X.; Naccache, S.N.; Ng, T.; Federman, S.; Li, L.; Chiu, C.Y.; Delwart, E.L. An Ensemble Strategy That Significantly Improves de Novo Assembly of Microbial Genomes from Metagenomic Next-Generation Sequencing Data. Nucleic Acids Res. 2015, 43, e46. [Google Scholar] [CrossRef] [Green Version]
- Cilli, A.; Luchs, A.; Leal, E.; Gill, D.; de Padua Milagres, F.A.; Komninakis, S.V.; Brustulin, R.; da Aparecida Rodrigues Teles, M.; Alves Brito Sayão Lobato, M.C.; das Chagas, R.T.; et al. Human Sapovirus GI.2 and GI.3 from Children with Acute Gastroenteritis in Northern Brazil. Mem. Inst. Oswaldo Cruz 2019, 114, e180574. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silva-Sales, M.; Martínez-Puchol, S.; Gonzales-Gustavson, E.; Hundesa, A.; Gironès, R. High Prevalence of Rotavirus A in Raw Sewage Samples from Northeast Spain. Viruses 2020, 12, 318. [Google Scholar] [CrossRef] [Green Version]
- Tahmasebi, R.; Luchs, A.; Tardy, K.; Hefford, P.M.; Tinker, R.J.; Eilami, O.; de Padua Milagres, F.A.; Brustulin, R.; da Aparecida Rodrigues Teles, M.; dos Santos Morais, V.; et al. Viral Gastroenteritis in Tocantins, Brazil: Characterizing the Diversity of Human Adenovirus F through next-Generation Sequencing and Bioinformatics. J. Gen. Virol. 2020, 101, 1280–1288. [Google Scholar] [CrossRef]
- Tinker, R.J.; da Costa, A.C.; Tahmasebi, R.; de Padua Milagres, F.A.; dos Santos Morais, V.; Pandey, R.P.; José-Abrego, A.; Brustulin, R.; da Aparecida Rodrigues Teles, M.; Cunha, M.S.; et al. Norovirus Strains in Patients with Acute Gastroenteritis in Rural and Low-Income Urban Areas in Northern Brazil. Arch. Virol. 2021, 166, 905–913. [Google Scholar] [CrossRef]
- Ribeiro, G.; Luchs, A.; de Padua Milagres, F.A.; Komninakis, S.; Gill, D.; Alves Brito Sayão Lobato, M.C.; Brustulin, R.; das Chagas, R.; de Fátima Neves dos Santos Abrão, M.; de Deus Alves Soares, C.V.; et al. Detection and Characterization of Enterovirus B73 from a Child in Brazil. Viruses 2018, 11, 16. [Google Scholar] [CrossRef] [Green Version]
- Luchs, A.; Leal, E.; Tardy, K.; de Padua Milagres, F.A.; Komninakis, S.V.; Brustulin, R.; da Aparecida Rodrigues Teles, M.; Alves Brito Sayão Lobato, M.C.; das Chagas, R.T.; de Fátima Neves dos Santos Abrão, M.; et al. The Rare Enterovirus C99 and Echovirus 29 Strains in Brazil: Potential Risks Associated to Silent Circulation. Mem. Inst. Oswaldo Cruz 2019, 114, e190160. [Google Scholar] [CrossRef] [Green Version]
- Do Socorro Fôro Ramos, E.; Rosa, U.A.; de Oliveira Ribeiro, G.; Villanova, F.; de Pádua Milagres, F.A.; Brustulin, R.; dos Santos Morais, V.; Bertanhe, M.; Marcatti, R.; Araújo, E.L.L.; et al. High Heterogeneity of Echoviruses in Brazilian Children with Acute Gastroenteritis. Viruses 2021, 13, 595. [Google Scholar] [CrossRef] [PubMed]
- Rosa, U.A.; de Oliveira Ribeiro, G.; Villanova, F.; Luchs, A.; de Pádua Milagres, F.A.; Komninakis, S.V.; Tahmasebi, R.; Alves Brito Sayão Lobato, M.C.; Brustulin, R.; das Chagas, R.T.; et al. First Identification of Mammalian Orthoreovirus Type 3 by Gut Virome Analysis in Diarrheic Child in Brazil. Sci. Rep. 2019, 9, 18599. [Google Scholar] [CrossRef]
- Tahmasebi, R.; da Costa, A.C.; Tardy, K.; Tinker, R.J.; de Padua Milagres, F.A.; Brustulin, R.; da Aparecida Rodrigues Teles, M.; Togisaki das Chagas, R.; de Deus Alves Soares, C.V.; Watanabe, A.S.A.; et al. Genomic Analyses of Potential Novel Recombinant Human Adenovirus C in Brazil. Viruses 2020, 12, 508. [Google Scholar] [CrossRef]
- Luchs, A.; Leal, E.; Komninakis, S.V.; de Pádua Milagres, F.A.; Brustulin, R.; da Aparecida Rodrigues Teles, M.; Gill, D.E.; Deng, X.; Delwart, E.; Sabino, E.C.; et al. Wuhan Large Pig Roundworm Virus Identified in Human Feces in Brazil. Virus Genes 2018, 54, 470–473. [Google Scholar] [CrossRef]
- Do Socorro Foro Ramos, E.; Alves Rosa, U.; de Oliveira Ribeiro, G.; Villanova, F.; de Pádua Milagres, F.A.; Brustulin, R.; dos Santos Morais, V.; Lima Araújo, E.L.; Pandey, R.P.; Raj, V.S.; et al. Multiple Clades of Husavirus in South America Revealed by next Generation Sequencing. PLoS ONE 2021, 16, e0248486. [Google Scholar] [CrossRef]
- Wolf, Y.I.; Kazlauskas, D.; Iranzo, J.; Lucía-Sanz, A.; Kuhn, J.H.; Krupovic, M.; Dolja, V.V.; Koonin, E.V. Origins and Evolution of the Global RNA Virome. MBio 2018, 9. [Google Scholar] [CrossRef] [Green Version]
- Koonin, E.V.; Dolja, V.V.; Krupovic, M. Origins and Evolution of Viruses of Eukaryotes: The Ultimate Modularity. Virology 2015, 479, 2–25. [Google Scholar] [CrossRef] [Green Version]
- Japhet, M.O.; Famurewa, O.; Adesina, O.A.; Opaleye, O.O.; Wang, B.; Höhne, M.; Bock, C.T.; Mas Marques, A.; Niendorf, S. Viral Gastroenteritis among Children of 0-5 Years in Nigeria: Characterization of the First Nigerian Aichivirus, Recombinant Noroviruses and Detection of a Zoonotic Astrovirus. J. Clin. Virol. 2019, 111, 4–11. [Google Scholar] [CrossRef] [PubMed]
- Charbit-Henrion, F.; Parlato, M.; Hanein, S.; Duclaux-Loras, R.; Nowak, J.; Begue, B.; Rakotobe, S.; Bruneau, J.; Fourrage, C.; Alibeu, O.; et al. Diagnostic Yield of Next-Generation Sequencing in Very Early-Onset Inflammatory Bowel Diseases: A Multicentre Study. J. Crohn Colitis 2018, 12, 1104–1112. [Google Scholar] [CrossRef]
- Fumian, T.M.; Fioretti, J.M.; Lun, J.H.; dos Santos, I.A.L.; White, P.A.; Miagostovich, M.P. Detection of Norovirus Epidemic Genotypes in Raw Sewage Using Next Generation Sequencing. Environ. Int. 2019, 123, 282–291. [Google Scholar] [CrossRef]
- Lu, L.; Jia, R.; Zhong, H.; Xu, M.; Su, L.; Cao, L.; Dong, Z.; Dong, N.; Xu, J. Molecular Characterization and Multiple Infections of Rotavirus, Norovirus, Sapovirus, Astrovirus and Adenovirus in Outpatients with Sporadic Gastroenteritis in Shanghai, China, 2010–2011. Arch. Virol. 2015, 160, 1229–1238. [Google Scholar] [CrossRef] [PubMed]
- Qiu, F.; Shen, X.; Li, G.; Zhao, L.; Chen, C.; Duan, S.; Guo, J.; Zhao, M.; Yan, T.; Qi, J.-J.; et al. Adenovirus Associated with Acute Diarrhea: A Case-Control Study. BMC Infect. Dis. 2018, 18, 450. [Google Scholar] [CrossRef]
- Lynch, J.; Kajon, A. Adenovirus: Epidemiology, Global Spread of Novel Serotypes, and Advances in Treatment and Prevention. Semin. Respir. Crit. Care Med. 2016, 37, 586–602. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Atmar, R.L. Noroviruses: State of the Art. Food Environ. Virol. 2010, 2, 117–126. [Google Scholar] [CrossRef]
- Luchs, A.; Tardy, K.; Tahmasebi, R.; Morillo, S.G.; de Pádua Milagres, F.A.; dos Santos Morais, V.; Brustulin, R.; da Aparecida Rodrigues Teles, M.; de Azevedo, L.S.; de Souza, E.V.; et al. Human Astrovirus Types 1, 4 and 5 Circulating among Children with Acute Gastroenteritis in a Rural Brazilian State, 2010–2016. Arch. Virol. 2021, 166, 3165–3172. [Google Scholar] [CrossRef]
- Aktaş, O.; Aydin, H.; Timurkan, M.O. A Molecular Study on the Prevalence and Coinfections of Rotavirus, Norovirus, Astrovirus and Adenovirus in Children with Gastroenteritis. Minerva Pediatr. 2019, 71, 431–437. [Google Scholar] [CrossRef] [PubMed]
- Miller, R.R.; Montoya, V.; Gardy, J.L.; Patrick, D.M.; Tang, P. Metagenomics for Pathogen Detection in Public Health. Genome Med. 2013, 5, 81. [Google Scholar] [CrossRef] [Green Version]
- Yip, C.C.Y.; Lo, K.-L.; Que, T.-L.; Lee, R.A.; Chan, K.-H.; Yuen, K.-Y.; Woo, P.C.Y.; Lau, S.K.P. Epidemiology of Human Parechovirus, Aichi Virus and Salivirus in Fecal Samples from Hospitalized Children with Gastroenteritis in Hong Kong. Virol. J. 2014, 11, 182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Okitsu, S.; Khamrin, P.; Thongprachum, A.; Nishimura, S.; Kalesaran, A.F.C.; Takanashi, S.; Shimizu, H.; Hayakawa, S.; Mizuguchi, M.; Ushijima, H. Detection and Molecular Characterization of Human Cosavirus in a Pediatric Patient with Acute Gastroenteritis, Japan. Infect. Genet. Evol. 2014, 28, 125–129. [Google Scholar] [CrossRef]
- Khamrin, P.; Thongprachum, A.; Shimizu, H.; Okitsu, S.; Mizuguchi, M.; Hayakawa, S.; Maneekarn, N.; Ushijima, H. Detection of Human Bocavirus 1 and 2 from Children with Acute Gastroenteritis in Japan. J. Med. Virol. 2012, 84, 901–905. [Google Scholar] [CrossRef]
- Ugai, S.; Iwaya, A.; Taneichi, H.; Hirokawa, C.; Aizawa, Y.; Hatakeyama, S.; Saitoh, A. Clinical Characteristics of Saffold Virus Infection in Children. Pediatr. Infect. Dis. J. 2019, 38, 781–785. [Google Scholar] [CrossRef]
- Ghosh, S.; Kobayashi, N.; Nagashima, S.; Naik, T.N.Y. 2009 Molecular Characterization of Full-Length Genomic Segment 2 of a Bovine Picobirnavirus (PBV) Strain: Evidence for High Genetic Diversity with Genogroup I PBVs. J. Gen. Virol. 2009, 90, 2519–2524. [Google Scholar] [CrossRef]
- De Cassia Compagnoli Carmona, R.; Caetano Machado, B.; Aparecida de Sousa, C.; Vieira, H.R.; Moraes Alves, M.R.; Farias de Souza, K.A.; Souza Gregório, D.; Costa Vilanova, B.; do Carmo Sampaio Tavares Timenetsky, M. Distribution of Species Enterovirus B in Patients with Central Nervous System Infections in São Paulo State, Brazil. J. Med. Virol. 2020, 92, 3849–3856. [Google Scholar] [CrossRef]
- Ramalho, E.; Sousa, I.; Burlandy, F.; Costa, E.; Dias, A.; Serrano, R.; Oliveira, M.; Lopes, R.; Debur, M.; Burger, M.; et al. Identification and Phylogenetic Characterization of Human Enteroviruses Isolated from Cases of Aseptic Meningitis in Brazil, 2013–2017. Viruses 2019, 11, 690. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, C.; Zhou, S.; Xue, W.; Shen, L.; Huang, W.; Zhang, Y.; Li, X.; Wang, J.; Zhang, H.; Ma, X. Comprehensive Virome Analysis Reveals the Complexity and Diversity of the Viral Spectrum in Pediatric Patients Diagnosed with Severe and Mild Hand-Foot-and-Mouth Disease. Virology 2018, 518, 116–125. [Google Scholar] [CrossRef]
- Mogotsi, M.T.; Mwangi, P.N.; Bester, P.A.; Mphahlele, M.J.; Seheri, M.L.; O’Neill, H.G.; Nyaga, M.M. Metagenomic Analysis of the Enteric RNA Virome of Infants from the Oukasie Clinic, North West Province, South Africa, Reveals Diverse Eukaryotic Viruses. Viruses 2020, 12, 1260. [Google Scholar] [CrossRef]
- Zhang, T.; Breitbart, M.; Lee, W.H.; Run, J.-Q.; Wei, C.L.; Soh, S.W.L.; Hibberd, M.L.; Liu, E.T.; Rohwer, F.; Ruan, Y. RNA Viral Community in Human Feces: Prevalence of Plant Pathogenic Viruses. PLoS Biol. 2005, 4, e3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liang, G.; Bushman, F.D. The Human Virome: Assembly, Composition and Host Interactions. Nat. Rev. Microbiol. 2021, 19, 514–527. [Google Scholar] [CrossRef]
- Norman, J.M.; Handley, S.A.; Baldridge, M.T.; Droit, L.; Liu, C.Y.; Keller, B.C.; Kambal, A.; Monaco, C.L.; Zhao, G.; Fleshner, P.; et al. Disease-Specific Alterations in the Enteric Virome in Inflammatory Bowel Disease. Cell 2015, 160, 447–460. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kanangat, S.; Skaljic, I. Microbiome Analysis, the Immune Response and Transplantation in the Era of next Generation Sequencing. Hum. Immunol. 2021, 82, 883–901. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
do Socorro Fôro Ramos, E.; de Oliveira Ribeiro, G.; Villanova, F.; de Padua Milagres, F.A.; Brustulin, R.; Araújo, E.L.L.; Pandey, R.P.; Raj, V.S.; Deng, X.; Delwart, E.; et al. Composition of Eukaryotic Viruses and Bacteriophages in Individuals with Acute Gastroenteritis. Viruses 2021, 13, 2365. https://doi.org/10.3390/v13122365
do Socorro Fôro Ramos E, de Oliveira Ribeiro G, Villanova F, de Padua Milagres FA, Brustulin R, Araújo ELL, Pandey RP, Raj VS, Deng X, Delwart E, et al. Composition of Eukaryotic Viruses and Bacteriophages in Individuals with Acute Gastroenteritis. Viruses. 2021; 13(12):2365. https://doi.org/10.3390/v13122365
Chicago/Turabian Styledo Socorro Fôro Ramos, Endrya, Geovani de Oliveira Ribeiro, Fabiola Villanova, Flávio Augusto de Padua Milagres, Rafael Brustulin, Emerson Luiz Lima Araújo, Ramendra Pati Pandey, V. Samuel Raj, Xutao Deng, Eric Delwart, and et al. 2021. "Composition of Eukaryotic Viruses and Bacteriophages in Individuals with Acute Gastroenteritis" Viruses 13, no. 12: 2365. https://doi.org/10.3390/v13122365
APA Styledo Socorro Fôro Ramos, E., de Oliveira Ribeiro, G., Villanova, F., de Padua Milagres, F. A., Brustulin, R., Araújo, E. L. L., Pandey, R. P., Raj, V. S., Deng, X., Delwart, E., Luchs, A., da Costa, A. C., & Leal, É. (2021). Composition of Eukaryotic Viruses and Bacteriophages in Individuals with Acute Gastroenteritis. Viruses, 13(12), 2365. https://doi.org/10.3390/v13122365










