Characterization of Canine Influenza Virus A (H3N2) Circulating in Dogs in China from 2016 to 2018
Abstract
:1. Introduction
2. Materials and Methods
2.1. Virological Investigation
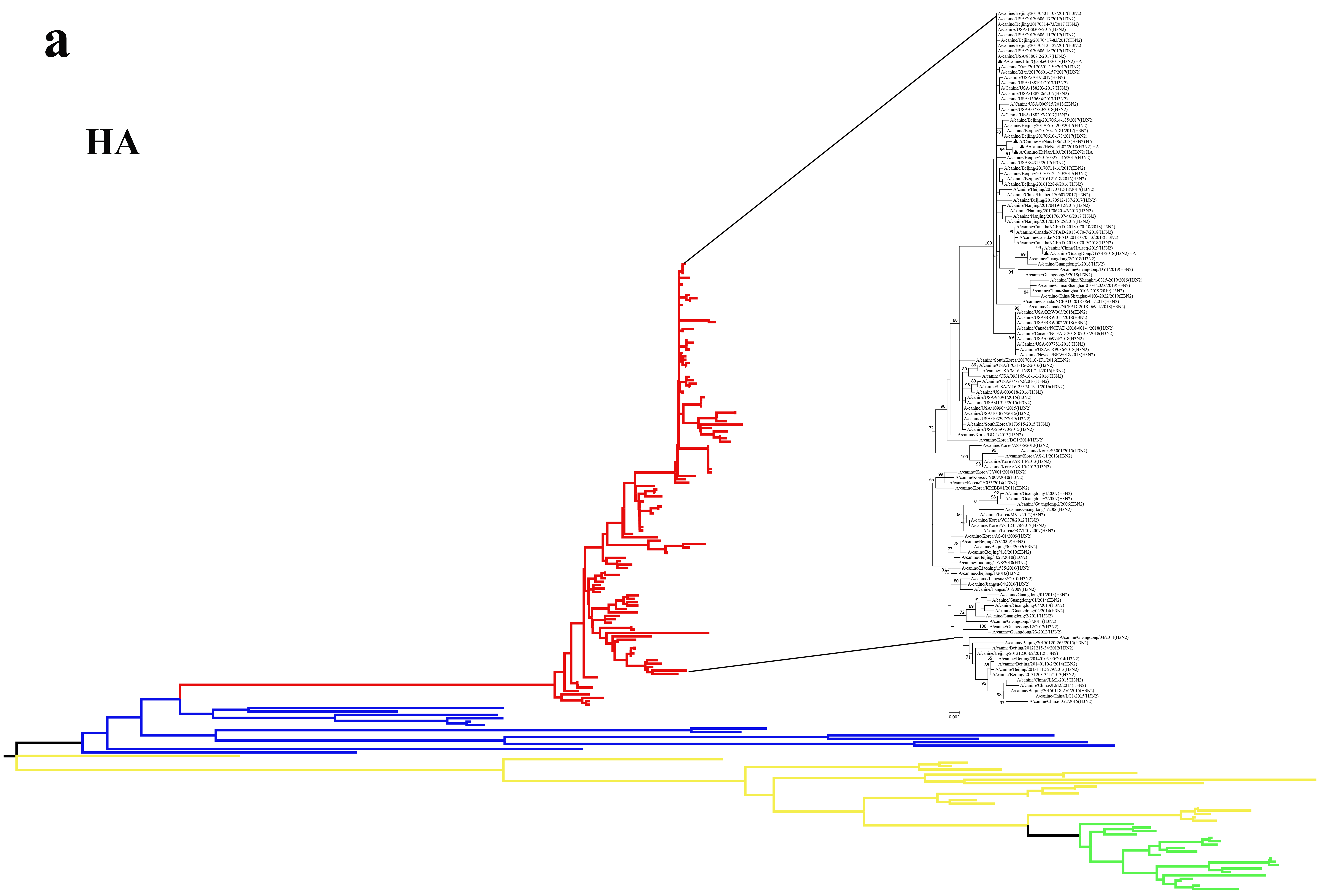
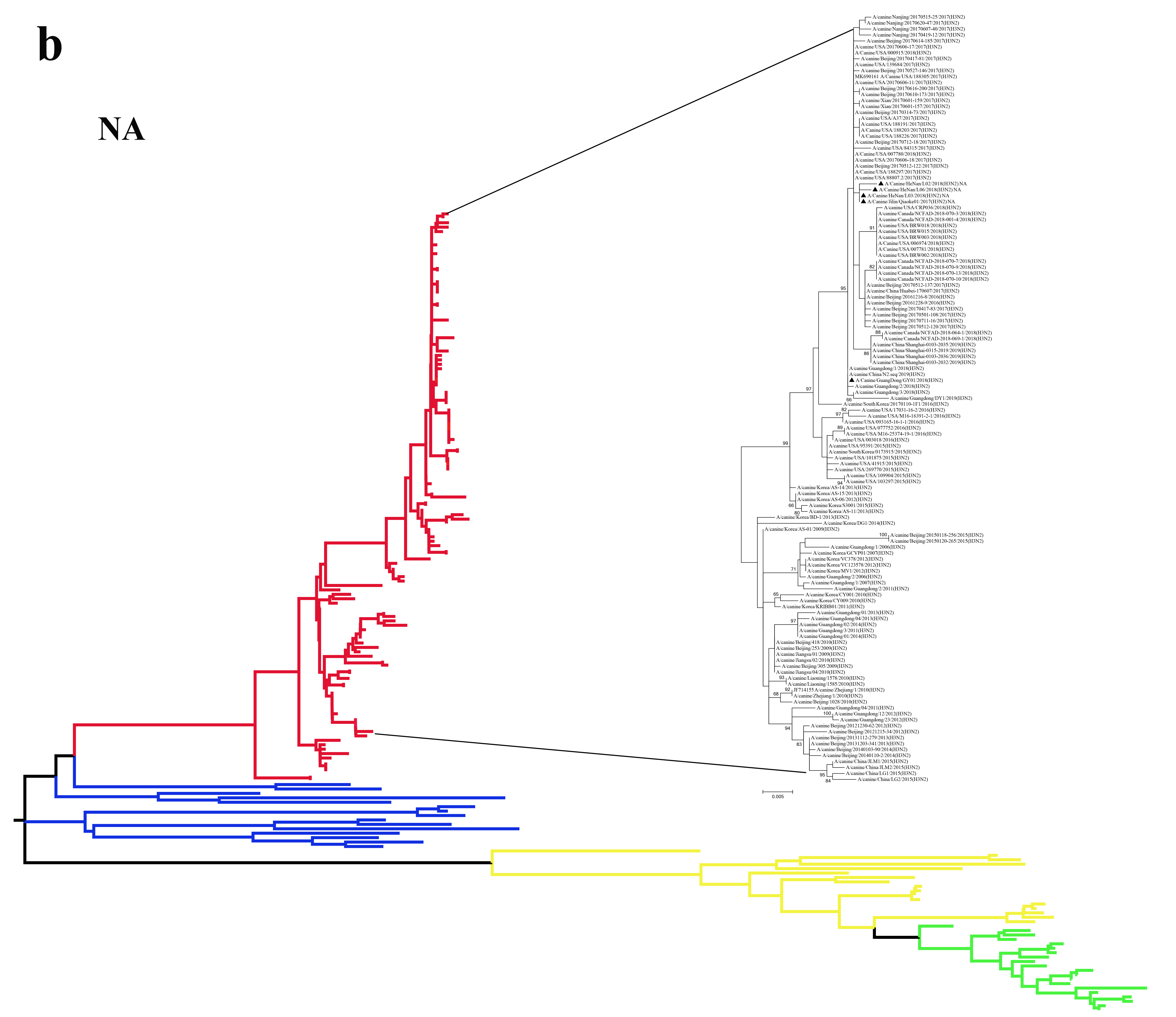
2.2. Phylogenetic Analysis
2.3. Seroepidemiological Investigation
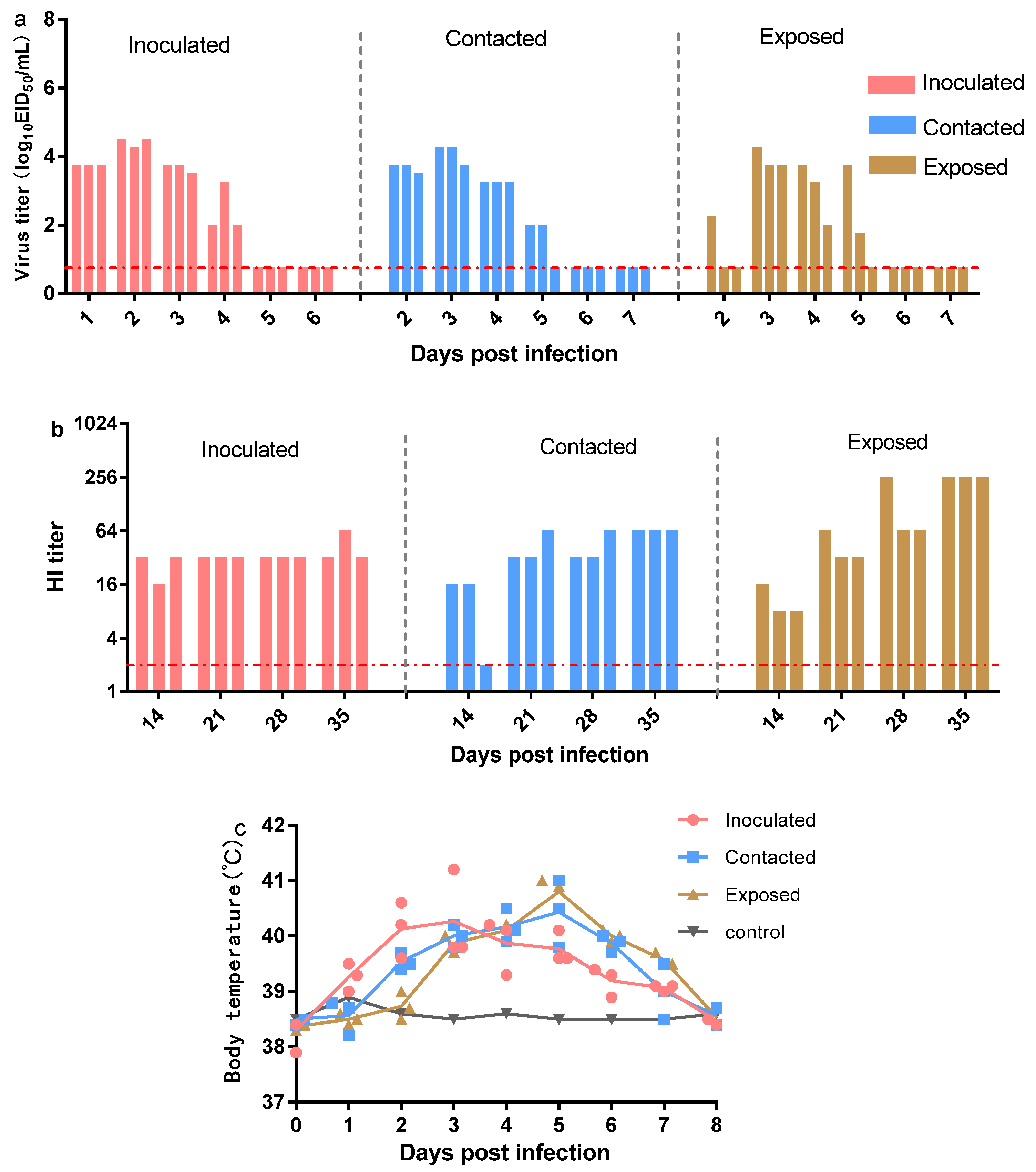
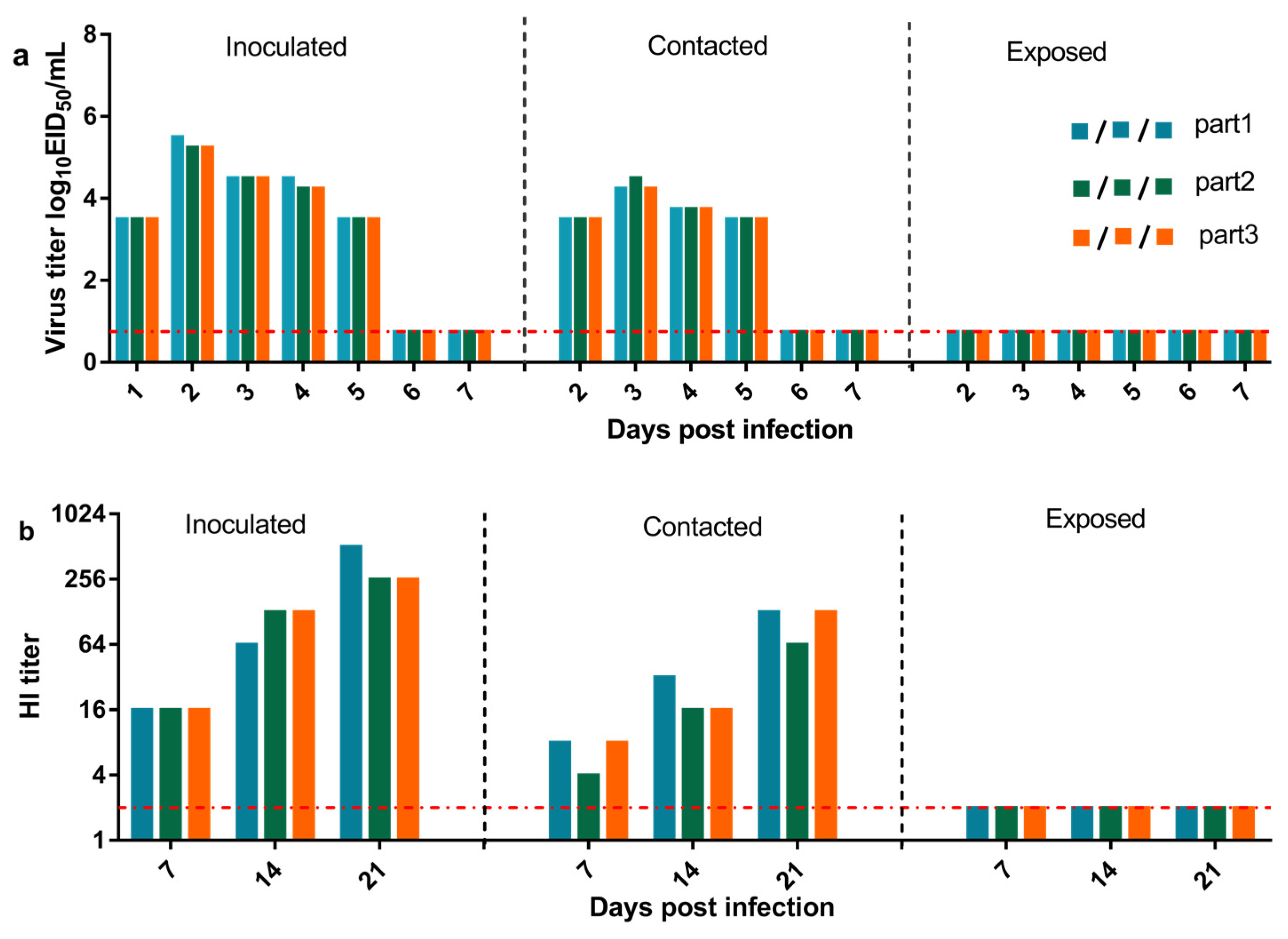
2.4. Replication and Transmission in Beagles
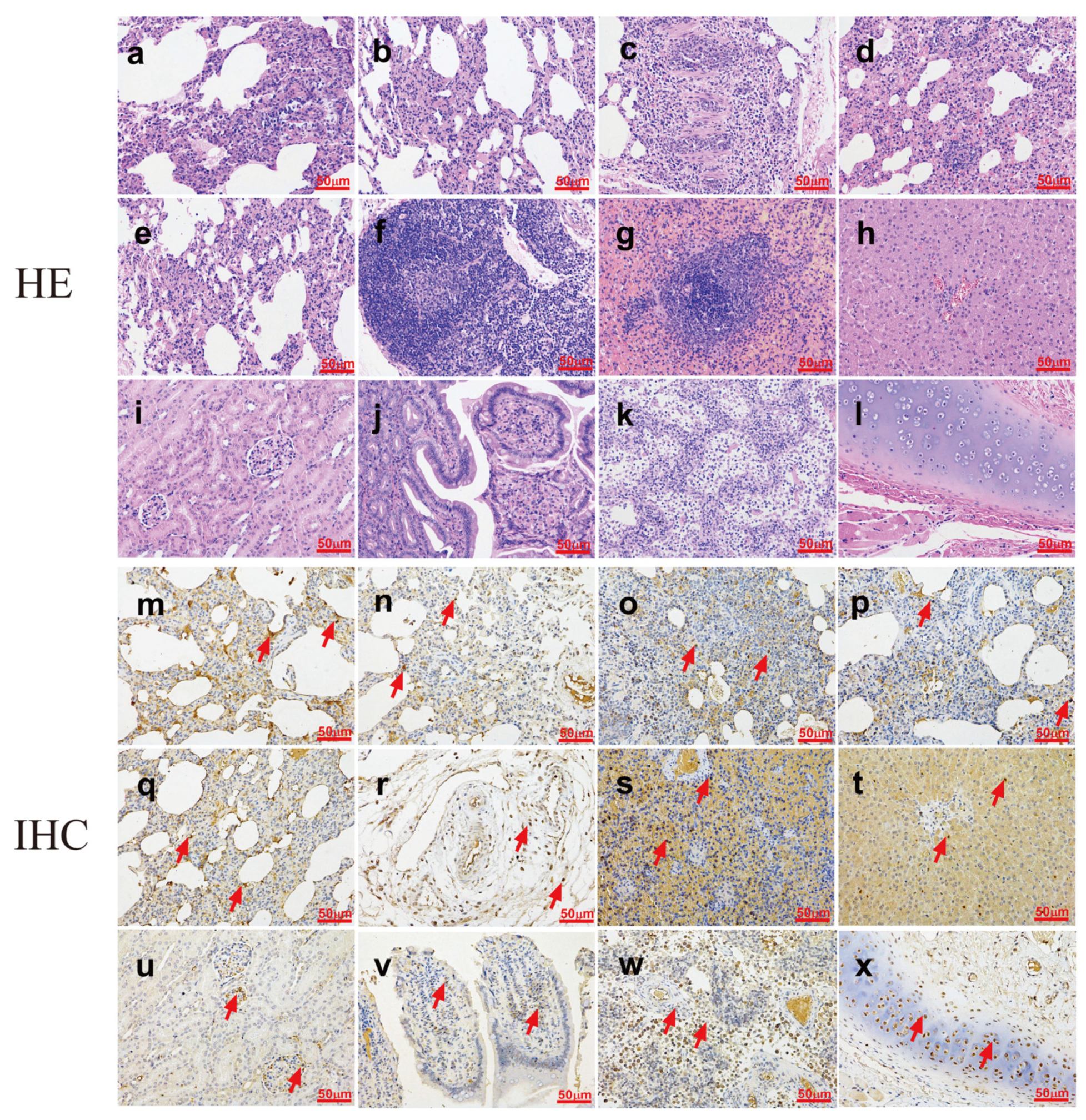
2.5. Pathogenesis in Mice
2.6. Replication and Transmission in Guinea Pig
2.7. Infection of Chickens and Ducks
3. Results
3.1. Virological Investigation
3.2. Phylogenetic Analysis
3.3. Seroepidemiological Investigation
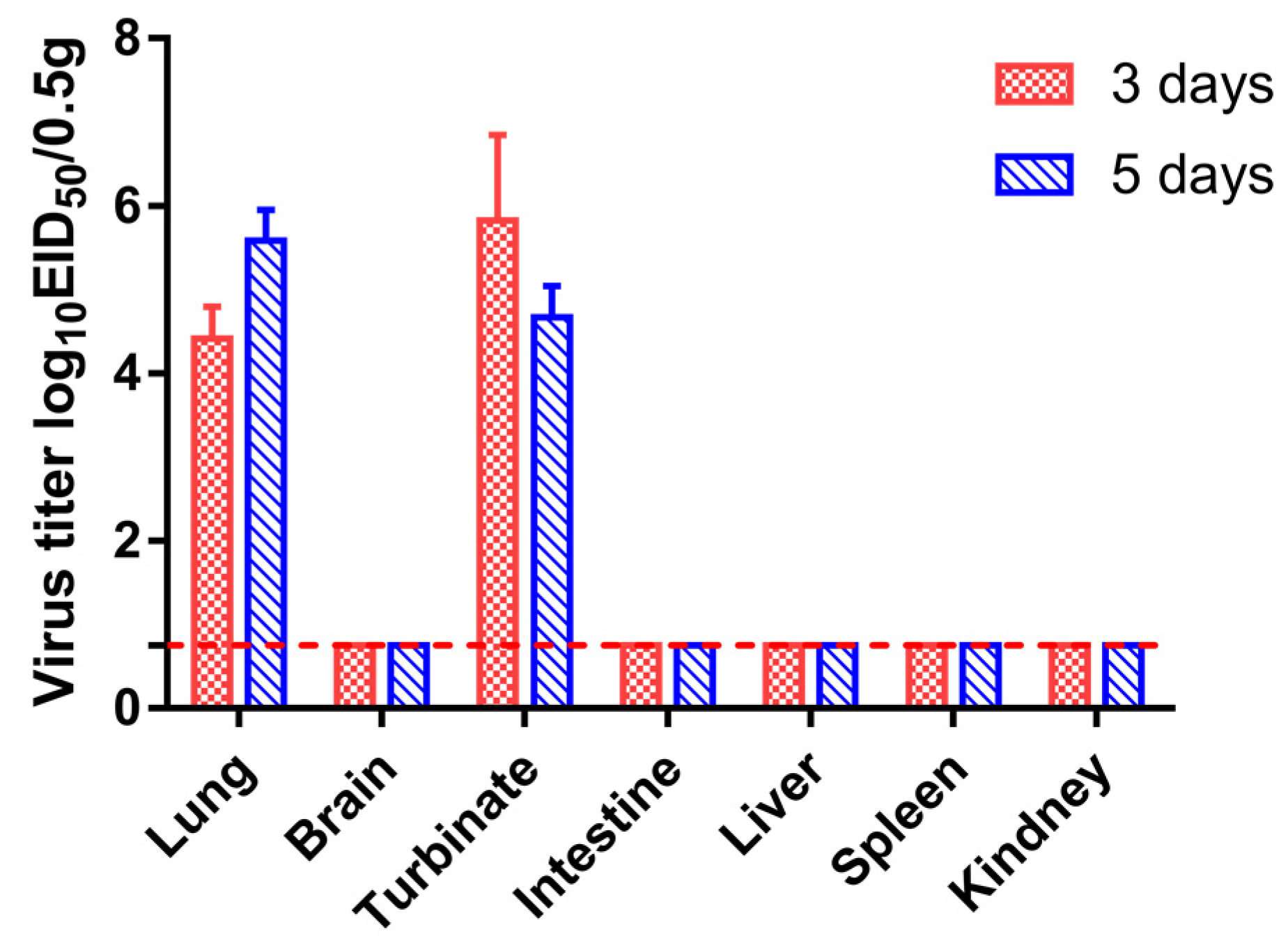
3.4. Replication and Transmission of Canine H3N2 Influenza Virus in Beagles
3.5. Pathogenesis in Mice
3.6. Replication and Transmission in Guinea Pigs
3.7. Infection of Chickens and Ducks
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Medina, R.A.; Garcia-Sastre, A. Influenza A viruses: New research developments. Nat. Rev. Genet. 2011, 9, 590–603. [Google Scholar] [CrossRef]
- Yoon, S.-W.; Webby, R.J.; Webster, R.G. Evolution and Ecology of Influenza A Viruses. Curr. Top. Microbiol. Immunol. 2014, 385, 359–375. [Google Scholar]
- Shi, W.; Lei, F.; Zhu, C.-D.; Sievers, F.; Higgins, D.G. A Complete Analysis of HA and NA Genes of Influenza A Viruses. PLoS ONE 2010, 5, e14454. [Google Scholar] [CrossRef]
- Fouchier, R.A.M.; Munster, V.; Wallensten, A.; Bestebroer, T.M.; Herfst, S.; Smith, D.; Rimmelzwaan, G.F.; Olsen, B.; Osterhaus, A.D.M.E. Characterization of a Novel Influenza A Virus Hemagglutinin Subtype (H16) Obtained from Black-Headed Gulls. J. Virol. 2005, 79, 2814–2822. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saeed, A.A.; Hussein, M.F. Avian influenza. Saudi Med. J. 2006, 27, 585–595. [Google Scholar] [PubMed]
- Urbaniak, K.; Kowalczyk, A.; Markowska-Daniel, I. Influenza A viruses of avian origin circulating in pigs and other mammals. Acta Biochim. Pol. 2014, 61, 433–439. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ou, J.; Chen, R.; Yan, Z.; Ou, S.; Dong, N.; Lu, G.; Li, S. Codon usage bias of H3N8 equine influenza virus—An evolutionary perspective. J. Infect. 2020, 80, 671–693. [Google Scholar] [CrossRef] [PubMed]
- Runstadler, J.A.; Puryear, W. A Brief Introduction to Influenza A Virus in Marine Mammals. Methods Mol. Biol. 2020, 2123, 429–450. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Ma, K.; Liu, Y. Influenza A virus infection in dogs: Epizootiology, evolution and prevention—A review. Acta Vet. Hung. 2016, 64, 125–139. [Google Scholar] [CrossRef]
- Su, S.; Wang, L.; Fu, X.; He, S.; Hong, M.; Zhou, P.; Lai, A.; Gray, G.; Li, S. Equine Influenza A(H3N8) Virus Infection in Cats. Emerg. Infect. Dis. 2014, 20, 2096–2099. [Google Scholar] [CrossRef] [PubMed]
- Roberton, S.; Bell, D.; Smith, G.J.; Nicholls, J.M.; Chan, K.; Nguyen, D.; Tran, P.; Streicher, U.; Poon, L.; Chen, H.; et al. Avian influenza H5N1 in viverrids: Implications for wildlife health and conservation. Proc. R. Soc. B Boil. Sci. 2006, 273, 1729–1732. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karlsson, E.; Engel, G.A.; Feeroz, M.; San, S.; Rompis, A.; Lee, B.P.Y.-H.; Shaw, E.; Oh, G.; Schillaci, M.A.; Grant, R.; et al. Influenza Virus Infection in Nonhuman Primates. Emerg. Infect. Dis. 2012, 18, 1672–1675. [Google Scholar] [CrossRef]
- Levy, H.; Fiddaman, S.R.; Djurhuus, A.; Black, C.E.; Kraberger, S.; Smith, A.L.; Hart, T.; Varsani, A. Identification of Circovirus Genome in a Chinstrap Penguin (Pygoscelis antarcticus) and Adélie Penguin (Pygoscelis adeliae) on the Antarctic Peninsula. Viruses 2020, 12, 858. [Google Scholar] [CrossRef]
- Uyeki, T.M.; Peiris, M. Novel Avian Influenza A Virus Infections of Humans. Infect. Dis. Clin. N. Am. 2019, 33, 907–932. [Google Scholar] [CrossRef]
- Liu, J.; Xiao, H.; Wu, Y.; Liu, D.; Qi, X.; Shi, Y.; Gao, G.F. H7N9: A low pathogenic avian influenza A virus infecting humans. Curr. Opin. Virol. 2014, 5, 91–97. [Google Scholar] [CrossRef]
- Borkenhagen, L.K.; Salman, M.D.; Ma, M.-J.; Gray, G.C. Animal influenza virus infections in humans: A commentary. Int. J. Infect. Dis. 2019, 88, 113–119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Imai, M.; Kawaoka, Y. The role of receptor binding specificity in interspecies transmission of influenza viruses. Curr. Opin. Virol. 2012, 2, 160–167. [Google Scholar] [CrossRef] [Green Version]
- Wasik, B.R.; Barnard, K.N.; Ossiboff, R.J.; Khedri, Z.; Feng, K.H.; Yu, H.; Chen, X.; Perez, D.R.; Varki, A.; Parrish, C.R. Distribution of O-Acetylated Sialic Acids among Target Host Tissues for Influenza Virus. mSphere 2017, 2, e00379-16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, S.; Shi, Z.; Jiao, P.; Zhang, G.; Zhong, Z.; Tian, W.; Long, L.-P.; Cai, Z.; Zhu, X.; Liao, M.; et al. Avian-origin H3N2 canine influenza A viruses in Southern China. Infect. Genet. Evol. 2010, 10, 1286–1288. [Google Scholar] [CrossRef] [Green Version]
- Lee, Y.-N.; Lee, D.-H.; Lee, H.-J.J.; Park, J.-K.; Yuk, S.-S.; Sung, H.-J.; Park, H.-M.; Lee, J.-B.; Park, S.-Y.; Choi, I.-S.; et al. Evidence of H3N2 canine influenza virus infection before 2007. Vet. Rec. 2012, 171, 477. [Google Scholar] [CrossRef]
- Su, S.; Cao, N.; Chen, J.; Zhao, F.; Li, H.; Zhao, M.; Wang, Y.; Huang, Z.; Yuan, L.; Wang, H.; et al. Complete Genome Sequence of an Avian-Origin H3N2 Canine Influenza A Virus Isolated in Farmed Dogs in Southern China. J. Virol. 2012, 86, 10238. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Teng, Q.; Zhang, X.; Xu, D.; Zhou, J.; Dai, X.; Chen, Z.; Li, Z. Characterization of an H3N2 canine influenza virus isolated from Tibetan mastiffs in China. Vet. Microbiol. 2013, 162, 345–352. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Hughes, J.; Murcia, P.R. Origins and Evolutionary Dynamics of H3N2 Canine Influenza Virus. J. Virol. 2015, 89, 5406–5418. [Google Scholar] [CrossRef] [Green Version]
- Watson, C.E.; Bell, C.; Toohey-Kurth, K. H3N2 Canine Influenza Virus Infection in a Dog. Vet. Pathol. 2016, 54, 527–530. [Google Scholar] [CrossRef]
- Voorhees, I.E.H.; Dalziel, B.D.; Glaser, A.; Dubovi, E.J.; Murcia, P.R.; Newbury, S.; Toohey-Kurth, K.; Su, S.; Kriti, D.; Van Bakel, H.; et al. Multiple Incursions and Recurrent Epidemic Fade-Out of H3N2 Canine Influenza A Virus in the United States. J. Virol. 2018, 92. [Google Scholar] [CrossRef] [Green Version]
- Lyu, Y.; Song, S.; Zhou, L.; Bing, G.; Wang, Q.; Sun, H.; Chen, M.; Hu, J.; Wang, M.; Sun, H.; et al. Canine Influenza Virus A(H3N2) Clade with Antigenic Variation, China, 2016–2017. Emerg. Infect. Dis. 2019, 25, 161–165. [Google Scholar] [CrossRef]
- Song, D.; Moon, H.-J.; An, D.-J.; Jeoung, H.-Y.; Kim, H.; Yeom, M.-J.; Hong, M.; Nam, J.-H.; Park, S.-J.; Park, B.-K.; et al. A novel reassortant canine H3N1 influenza virus between pandemic H1N1 and canine H3N2 influenza viruses in Korea. J. Gen. Virol. 2012, 93, 551–554. [Google Scholar] [CrossRef]
- Chen, Y.; Trovão, N.S.; Wang, G.; Zhao, W.; He, P.; Zhou, H.; Mo, Y.; Wei, Z.; Ouyang, K.; Huang, W.; et al. Emergence and Evolution of Novel Reassortant Influenza A Viruses in Canines in Southern China. mBio 2018, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wasik, B.R.; Voorhees, I.E.H.; Parrish, C.R. Canine and Feline Influenza. Cold Spring Harb. Perspect. Med. 2021, 11. [Google Scholar] [CrossRef] [Green Version]
- Yang, H.; Chen, Y.; Qiao, C.; He, X.; Zhou, H.; Sun, Y.; Yin, H.; Meng, S.; Liu, L.; Zhang, Q.; et al. Prevalence, genetics, and transmissibility in ferrets of Eurasian avian-like H1N1 swine influenza viruses. Proc. Natl. Acad. Sci. USA 2015, 113, 392–397. [Google Scholar] [CrossRef] [Green Version]

| Sample Source | Number of Samples | Positive Number | Positive Rate (%) | HI Titer | ||
|---|---|---|---|---|---|---|
| 8–32 | 64–256 | 512–1024 | ||||
| Pet dogs (2017) | 913 | 61 | 6.68 | 33 | 25 | 3 |
| Stray dogs (2017) | 130 | 63 | 48.46 | 51 | 12 | 0 |
| Pet dogs (2018) | 2284 | 363 | 18.89 | 117 | 200 | 46 |
| Dogs with respiratory symptoms (2018) | 208 | 41 | 19.71 | 17 | 19 | 5 |
| Chinese rural dogs (2018) | 44 | 0 | 0 | - | - | - |
| Total | 3579 | 528 | 14.75 | 218 | 256 | 54 |
| Group | Day | Seroconversion (HI Antibody Titer Range) | |||
|---|---|---|---|---|---|
| Inoculated | Contacted | Exposed | Control | ||
| Chicken | 7 | 0/7 | 0/3 | 0/3 | 0/3 |
| 14 | 3/7 (64, 64, 32) | 0/3 | 0/3 | 0/3 | |
| 21 | 3/7 (128, 128, 128) | 0/3 | 0/3 | 0/3 | |
| Duck | 7 | 0/3 | 0/3 | 0/3 | 0/3 |
| 14 | 0/3 | 0/3 | 0/3 | 0/3 | |
| 21 | 0/3 | 0/3 | 0/3 | 0/3 1 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Zhang, X.; Liu, Y.; Feng, Y.; Wang, T.; Ge, Y.; Kong, Y.; Sun, H.; Xiang, H.; Zhou, B.; et al. Characterization of Canine Influenza Virus A (H3N2) Circulating in Dogs in China from 2016 to 2018. Viruses 2021, 13, 2279. https://doi.org/10.3390/v13112279
Li Y, Zhang X, Liu Y, Feng Y, Wang T, Ge Y, Kong Y, Sun H, Xiang H, Zhou B, et al. Characterization of Canine Influenza Virus A (H3N2) Circulating in Dogs in China from 2016 to 2018. Viruses. 2021; 13(11):2279. https://doi.org/10.3390/v13112279
Chicago/Turabian StyleLi, Yuanguo, Xinghai Zhang, Yuxiu Liu, Ye Feng, Tiecheng Wang, Ye Ge, Yunyi Kong, Hongyu Sun, Haiyang Xiang, Bo Zhou, and et al. 2021. "Characterization of Canine Influenza Virus A (H3N2) Circulating in Dogs in China from 2016 to 2018" Viruses 13, no. 11: 2279. https://doi.org/10.3390/v13112279
APA StyleLi, Y., Zhang, X., Liu, Y., Feng, Y., Wang, T., Ge, Y., Kong, Y., Sun, H., Xiang, H., Zhou, B., Fang, S., Xia, Q., Hu, X., Sun, W., Wang, X., Meng, K., Lv, C., Li, E., Xia, X., ... Jin, N. (2021). Characterization of Canine Influenza Virus A (H3N2) Circulating in Dogs in China from 2016 to 2018. Viruses, 13(11), 2279. https://doi.org/10.3390/v13112279






