Identification of Cacao Mild Mosaic Virus (CaMMV) and Cacao Yellow Vein-Banding Virus (CYVBV) in Cocoa (Theobroma cacao) Germplasm
Abstract
:1. Introduction
2. Materials and Methods
2.1. PCR Screening of Cocoa Germplasm
2.1.1. Plant Material
2.1.2. DNA Extraction
2.1.3. Primer Design
2.1.4. PCR Conditions
2.2. Development of LAMP Assay for Detection of CYVBV
2.2.1. Primer Design
2.2.2. LAMP Assay
2.3. Identification of CYVBV and CaMMV Viruses in Genomic Sequencing Datasets of Cocoa
2.4. Sequence Assembly, Alignment and Evolutionary Analysis
3. Results
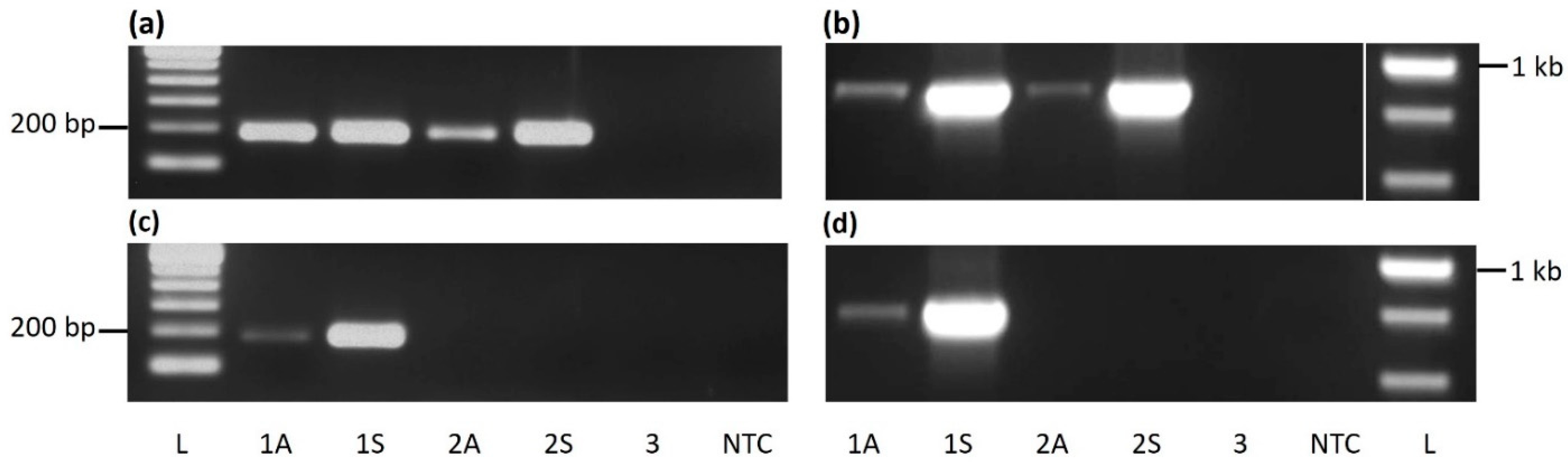
3.1. PCR Detection of CaMMV and CYVBV
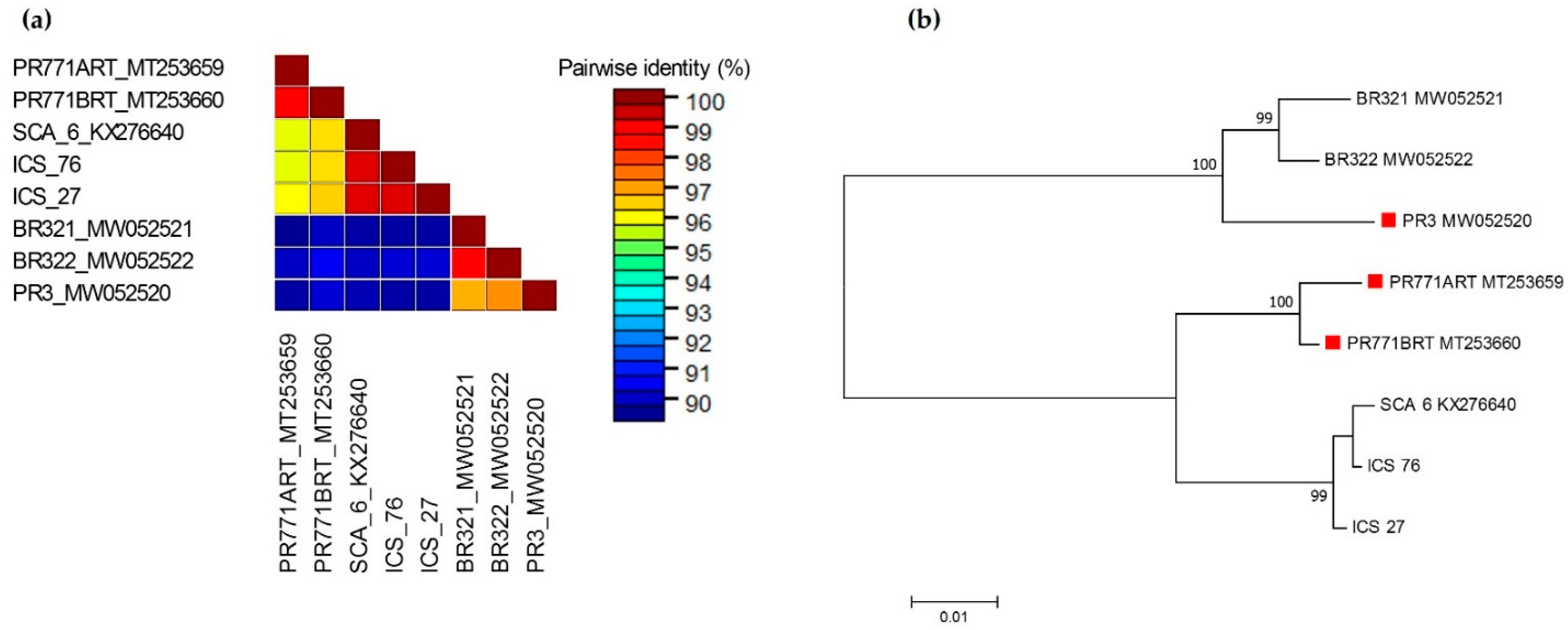
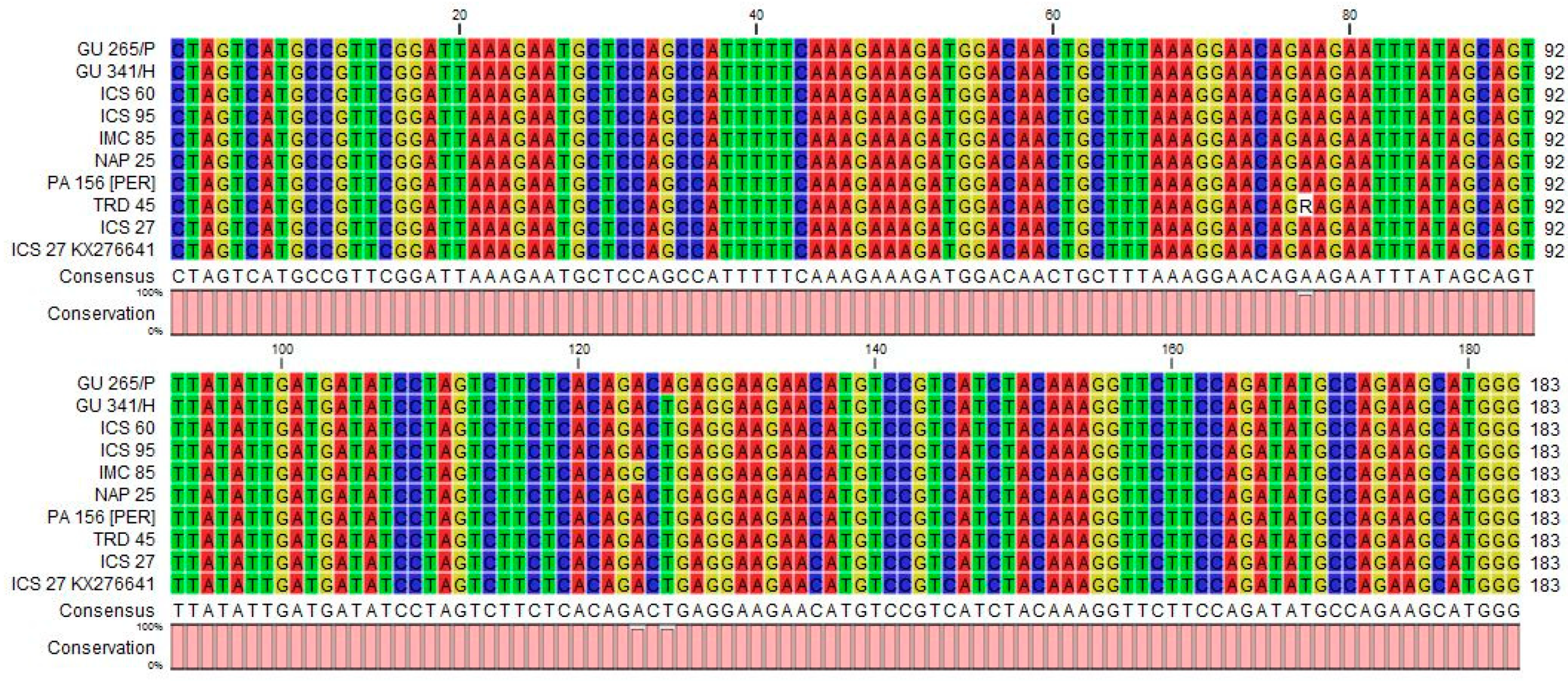
3.2. Sequences of Amplified Fragments and Their Phylogenetic Relationship to Other Isolates
3.3. Screening of ICQC-R Accessions
3.4. Effect of Virus Titre and Leaf Age
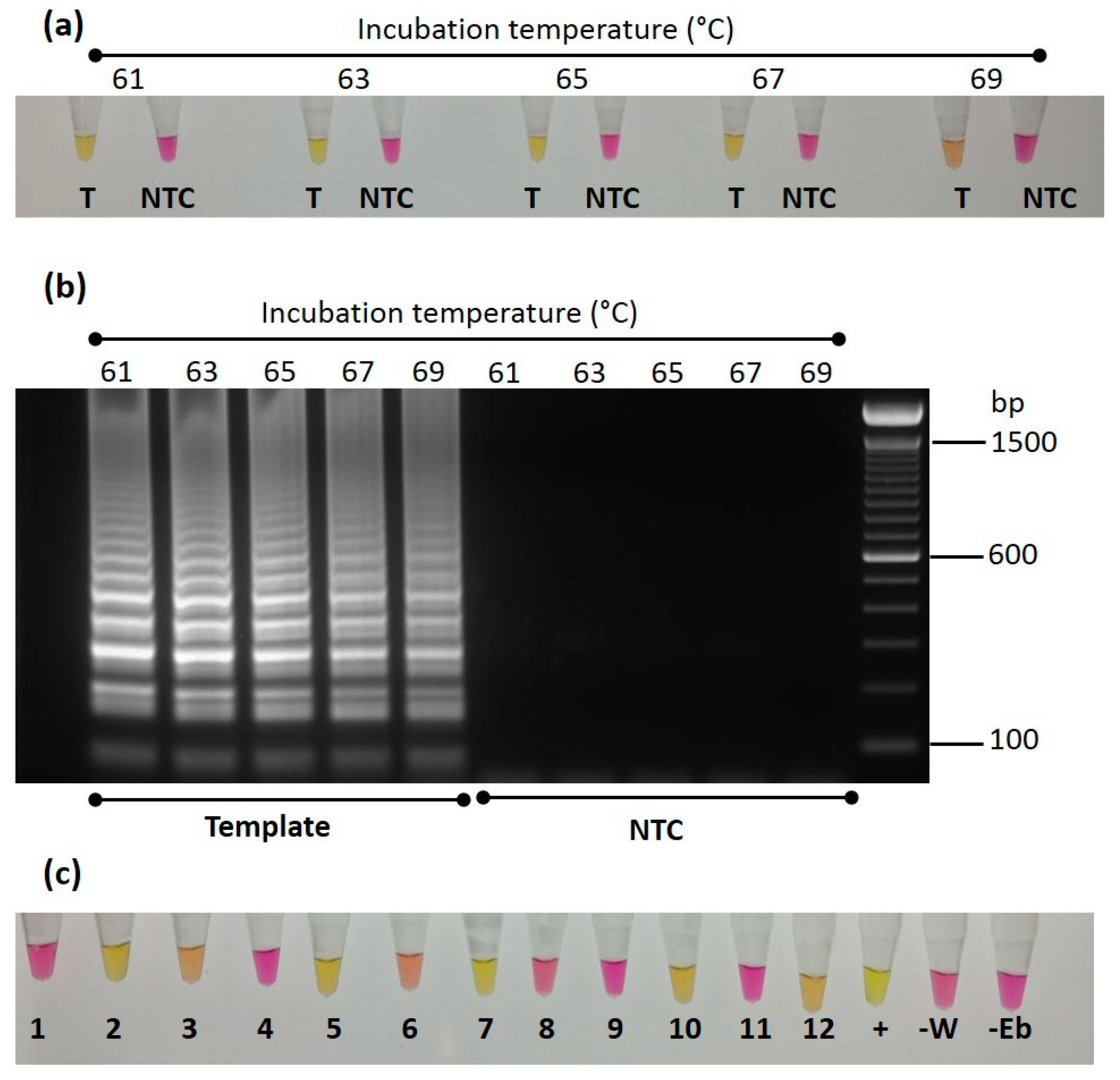
3.5. LAMP Assay
3.6. Identification of CaMMV and CYVBV Viruses in Genomic Sequencing Datasets of Cocoa
3.6.1. Screening of Datasets for CYVBV
3.6.2. Screening of Datasets for CaMMV
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- End, M.J.; Daymond, A.J.; Hadley, P. (Eds.) Technical Guidelines for the Safe Movement of Cacao Germplasm; Revised from the FAO/IPGRI Technical Guidelines No. 20 (Third Update, October 2017); Global Cacao Genetic Resources Network (CacaoNet), Bioversity International: Rome, Italy, 2017. [Google Scholar]
- Muller, E.; Ullah, I.; Dunwell, J.M.; Daymond, A.J.; Richardson, M.; Allainguillaume, J.; Wetten, A. Identification and distribution of novel badnaviral sequences integrated in the genome of cacao (Theobroma cacao). Sci. Rep. 2021, 11, 8270. [Google Scholar] [CrossRef]
- Chingandu, N.; Zia-Ur-Rehman, M.; Sreenivasan, T.N.; Surujdeo-Maharaj, S.; Umaharan, P.; Gutierrez, O.A.; Brown, J.K. Molecular characterization of previously elusive badnaviruses associated with symptomatic cacao in the New World. Arch. Virol. 2017, 162, 1363–1371. [Google Scholar] [CrossRef] [PubMed]
- Kirkpatrick, T.W. Insect transmission of cacao virus disease in Trinidad. Bull. Entomol. Res. 1950, 41, 99–117. [Google Scholar] [CrossRef]
- Swarbrick, J.T. Cacao virus in Trinidad. Trop. Agric. Trin. 1961, 38, 245–249. [Google Scholar]
- Ramos-Sobrinho, R.; Ferro, M.M.M.; Nagata, T.; Puig, A.S.; Von Keith, C.; Britto, D.S.; Gutierrez, O.A.; Marelli, J.-P.; Brown, J.K. Complete genome sequences of three newly discovered cacao mild mosaic virus isolates from Theobroma cacao L. in Brazil and Puerto Rico and evidence for recombination. Arch. Virol. 2021, 166, 2027–2031. [Google Scholar] [CrossRef] [PubMed]
- Ciferri, R. Una virosis del cacao en Colombia y en la República Dominicana. Rev. Fac. Nac. Agron. Medellín 1948, 8, 79–84. [Google Scholar]
- Posnette, A.F.; Palma, M. Observations on cacao in the Paria Peninsula, Venezuela. Trop. Agric. Trinidad. 1944, 21, 104. [Google Scholar]
- Simangu, H. Gedjala-Gedjala Mosiak Pada Daun Tjoklat; Universitas Gadjah Mada: Jogjakarta, Indonesia, 1961; Volume 2, 13p. [Google Scholar]
- Kenten, R.H.; Woods, R.D. A virus of the Cocoa Swollen Shoot group infecting cocoa in North Sumatra. PANS 1976, 22, 488–490. [Google Scholar] [CrossRef]
- Legg, J.T. Cocoa swollen-shoot disease—Know your enemy. In Proceedings of the 5th International Cocoa Research Conference, Ibadan, Nigeria, 1–9 September 1979; pp. 397–402. [Google Scholar]
- Turner, P.D.; Shepherd, R. Cocoa diseases in Malaysia and Indonesia: Their present and potential importance. In Proceedings of the International Conference Cocoa Coconuts, Kuala Lumpur, Malaysia, 21–24 June 1978; pp. 308–321. [Google Scholar]
- Lockwood, R. MEMORANDUM: Subject: Suspected Cocoa Virus in Malaysia. 1992, pp. 1–4. Available online: http://www.icgd.reading.ac.uk/icqc/data/Lockwood_1992_Report_on_Malaysian_virus_2_Mar1992.pdf (accessed on 17 October 2021).
- Daymond, A.J. Detection of Symptoms Characteristic of Viral Infection on the Clone BR 25; Report from ICQC-Reading. 2018, pp. 1–2. Available online: http://www.icgd.reading.ac.uk/icqc/data/Detection_of_Viral_Symptom_on_the_clone_BR_25.pdf (accessed on 17 October 2021).
- Osorio-Guarín, J.A.; Quackenbush, C.R.; Cornejo, O.E. Ancestry informative alleles captured with reduced representation library sequencing in Theobroma cacao. PLoS ONE 2018, 13, 10. [Google Scholar] [CrossRef] [Green Version]
- Kane, N.; Sveinsson, S.; Dempewolf, H.; Yang, J.Y.; Zhang, D.; Engels, J.M.; Cronk, Q. Ultra-barcoding in cacao (Theobroma spp.; Malvaceae) using whole chloroplast genomes and nuclear ribosomal DNA. Am. J. Bot. 2012, 99, 2. [Google Scholar] [CrossRef] [Green Version]
- Cornejo, O.E.; Yee, M.C.; Dominguez, V.; Andrews, M.; Sockell, A.; Strandberg, E.; Livingstone, D., 3rd; Stack, C.; Romero, A.; Umaharan, P.; et al. Population genomic analyses of the chocolate tree, Theobroma cacao L., provide insights into its domestication process. Commun. Biol. 2018, 1, 167. [Google Scholar] [CrossRef] [Green Version]
- Langmead, B.; Salzberg, S.L. Fast gapped-read alignment with Bowtie 2. Nat. Methods 2012, 9, 357–359. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, H.; Handsaker, B.; Wysoker, A.; Fennell, T.; Ruan, J.; Homer, N.; Marth, G.; Abecasis, G.; Durbin, R. The sequence alignment/map format and SAMtools. Bioinformatics 2009, 25, 2078–2079. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Robinson, J.T.; Thorvaldsdottir, H.; Wenger, A.M.; Zehir, A.; Mesirov, J.P. Variant review with the integrative genomics viewer. Cancer Res. 2017, 77, e31–e34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muhire, B.M.; Varsani, A.; Martin, D.P. SDT: A virus classification tool based on pairwise sequence alignment and identity calculation. PLoS ONE 2014, 9, e108277. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kimura, M. A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. J. Mol. Evol. 1980, 16, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Puig, A.; Ramos-Sobrinho, R.; Keith, C.; Kitchen, N.; Gutierrez, O.; Goenaga, R.; Brown, J.K. First report of Cacao mild mosaic virus (CaMMV) associated with symptomatic commercial cacao (Theobroma cacao L.) trees in Puerto Rico. Plant Dis. 2020, 104, 3089. [Google Scholar] [CrossRef]
- Pecman, A.; Kutnjak, D.; Gutiérrez-Aguirre, I.; Adams, I.; Fox, A.; Boonham, N.; Ravnikar, M. Next generation sequencing for detection and discovery of plant viruses and Viroids: Comparison of two approaches. Front. Microbiol. 2017, 8, 1998. [Google Scholar] [CrossRef] [Green Version]
- Glasa, M.; Hančinský, R.; Šoltys, K.; Predajňa, L.; Tomašechová, J.; Hauptvogel, P.; Mrkvová, M.; Mihálik, D.; Candresse, T. Molecular characterization of Potato Virus Y (PVY) using high-throughput sequencing: Constraints on full genome reconstructions imposed by mixed infection involving recombinant PVY strains. Plants 2021, 10, 753. [Google Scholar] [CrossRef]
- Sukal, A.C.; Kidanemariam, D.B.; Dale, J.J.; Harding, R.M.; James, A.P. Assessment and optimization of rolling circle amplification protocols for the detection and characterization of badnaviruses. Virology 2019, 529, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Baker, R.E.; Dale, W.T. Notes on a virus disease of cacao. Ann. Appl. Biol. 1947, 34, 60–65. [Google Scholar] [CrossRef] [PubMed]
- Staginnus, C.; Richert-Pöggeler, K.R. Endogenous pararetroviruses: Two-faced travelers in the plant genome. Trends Plant Sci. 2006, 11, 485–491. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Saito, N.; Encabo, J.R.; Yamada, K.; Choi, I.-R.; Kishima, Y. Ancient endogenous pararetroviruses in oryza genomes provide insights into the heterogeneity of viral gene macroevolution. Genome Biol. Evol. 2018, 10, 2686–2696. [Google Scholar] [CrossRef]
- Chabannes, M.; Gabriel, M.; Aksa, A.; Galzi, S.; Dufayard, J.-F.; Iskra-Caruana, M.-L.; Muller, E. Badnaviruses and banana genomes: A long association sheds light on Musa phylogeny and origin. Mol. Plant Pathol. 2021, 22, 216–230. [Google Scholar] [CrossRef] [PubMed]
- Serfraz, S.; Sharma, V.; Maumus, F.; Aubriot, X.; Geering, A.D.W.; Teycheney, F.-Y. Insertion of badnaviral DNA in the late blight resistance gene (R1a) of brinjal eggplant (Solanum melongena). Front. Plant Sci. 2021. [Google Scholar] [CrossRef]
- Catoni, M.; Noris, E.; Vaira, A.M.; Jonesman, T.; Matić, S.; Soleimani, R.; Behjatnia, S.A.A.; Vinals, N.; Paszkowski, J.; Accotto, G.P. Virus-mediated export of chromosomal DNA in plants. Nat. Commun. 2018, 9, 5308. [Google Scholar] [CrossRef]
- Kreuze, J.F.; Perez, A.; Gargurevich, M.G.; Cuellar, W.J. Badnaviruses of sweet potato: Symptomless coinhabitants on a global scale. Front. Plant Sci. 2020, 11, 313. [Google Scholar] [CrossRef]
- Marais, A.; Umber, M.; Filloux, D.; Gomez, R.M.; Faure, C.; Pavis, C.; Julian, C.; Roumagnac, P.; Acina-Mambole, I.; Bonheur, L.; et al. Yam asymptomatic virus 1, a novel virus infecting yams (Dioscorea spp.) with significant prevalence in a germplasm collection. Arch. Virol. 2020, 165, 2653–2657. [Google Scholar] [CrossRef] [PubMed]
- Marshall, P.L.; King, J.L.; Budowle, B. Utility of amplification enhancers in low copy number DNA analysis. Int. J. Legal Med. 2015, 129, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Armani, A.; Giusti, A.; Guardone, L.; Castigliego, L.; Gianfaldoni, D.; Guidi, A. Universal primers used for species identification of foodstuff of animal origin: Effects of oligonucleotide tails on PCR amplification and sequencing performance. Food Anal. Methods 2016, 9, 1199–1209. [Google Scholar] [CrossRef]
- Bartley, B.G.D. The Genetic Diversity of Cacao and Its Utilization; CABI Publishing: Wallingford, UK, 2005; p. 341. ISBN 0851996191. [Google Scholar]
- Mahas, A.; Hassan, N.; Aman, R.; Marsic, T.; Wang, Q.; Ali, Z.; Mahfouz, M.M. LAMP-Coupled CRISPR–Cas12a module for rapid and sensitive detection of plant DNA viruses. Viruses 2021, 13, 466. [Google Scholar] [CrossRef] [PubMed]
- Sherrill-Mix, S.; Hwang, Y.; Roche, A.M.; Glascock, A.; Weiss, S.R.; Li, Y.; Haddad, L.; Deraska, P.; Monahan, C.; Kromer, A.; et al. Detection of SARS-CoV-2 RNA using RT-LAMP and molecular beacons. Genome Biol. 2021, 22, 169. [Google Scholar] [CrossRef] [PubMed]



| Sr Number | Name | Accession Number | Donor Genebank |
|---|---|---|---|
| CaMMV | |||
| 1 | GEBP 584/A-F [ADI] | RUQ 1643 | ICG-T |
| 2 | PNG 138 | RUQ 1354 | CIRAD |
| 3 | SCA 12 | RUQ 1689 | ICG-T |
| 4 | WA 40 [DR] | RUQ 1283 | CIRAD |
| CYVBV | |||
| 1 | GU 144/C | RUQ 191 | CIRAD |
| 2 | GU 219/F | RUQ 768 | ICG-T |
| 3 | GU 265/P | RUQ 890 | ICG-T |
| 4 | GU 341/H | RUQ 231 | CIRAD |
| 5 | IMC 85 | RUQ 1666 | ICG-T |
| 6 | JA 10/12 [POU] | RUQ 456 | ICG-T |
| 7 | ICS 60 | RUQ 959 | ICG-T |
| 8 | ICS 95 | RUQ 1144 | CIRAD |
| 9 | NAP 25 | RUQ 1547 | INIAP |
| 10 | PA 156 [PER] | RUQ 1531 | ICG-T |
| 11 | TRD 45 | RUQ 1441 | ICG-T |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ullah, I.; Daymond, A.J.; Hadley, P.; End, M.J.; Umaharan, P.; Dunwell, J.M. Identification of Cacao Mild Mosaic Virus (CaMMV) and Cacao Yellow Vein-Banding Virus (CYVBV) in Cocoa (Theobroma cacao) Germplasm. Viruses 2021, 13, 2152. https://doi.org/10.3390/v13112152
Ullah I, Daymond AJ, Hadley P, End MJ, Umaharan P, Dunwell JM. Identification of Cacao Mild Mosaic Virus (CaMMV) and Cacao Yellow Vein-Banding Virus (CYVBV) in Cocoa (Theobroma cacao) Germplasm. Viruses. 2021; 13(11):2152. https://doi.org/10.3390/v13112152
Chicago/Turabian StyleUllah, Ihsan, Andrew J. Daymond, Paul Hadley, Michelle J. End, Pathmanathan Umaharan, and Jim M. Dunwell. 2021. "Identification of Cacao Mild Mosaic Virus (CaMMV) and Cacao Yellow Vein-Banding Virus (CYVBV) in Cocoa (Theobroma cacao) Germplasm" Viruses 13, no. 11: 2152. https://doi.org/10.3390/v13112152
APA StyleUllah, I., Daymond, A. J., Hadley, P., End, M. J., Umaharan, P., & Dunwell, J. M. (2021). Identification of Cacao Mild Mosaic Virus (CaMMV) and Cacao Yellow Vein-Banding Virus (CYVBV) in Cocoa (Theobroma cacao) Germplasm. Viruses, 13(11), 2152. https://doi.org/10.3390/v13112152







