Detection of Insect-Specific Flaviviruses in Mosquitoes (Diptera: Culicidae) in Northeastern Regions of South Africa
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area and Mosquito Collection
2.2. Mosquito Processing and RT-PCR
2.3. Molecular Identification of the Mosquito Positive Pools
2.4. Gel Electrophoresis and Sequencing
2.5. Data Analyses
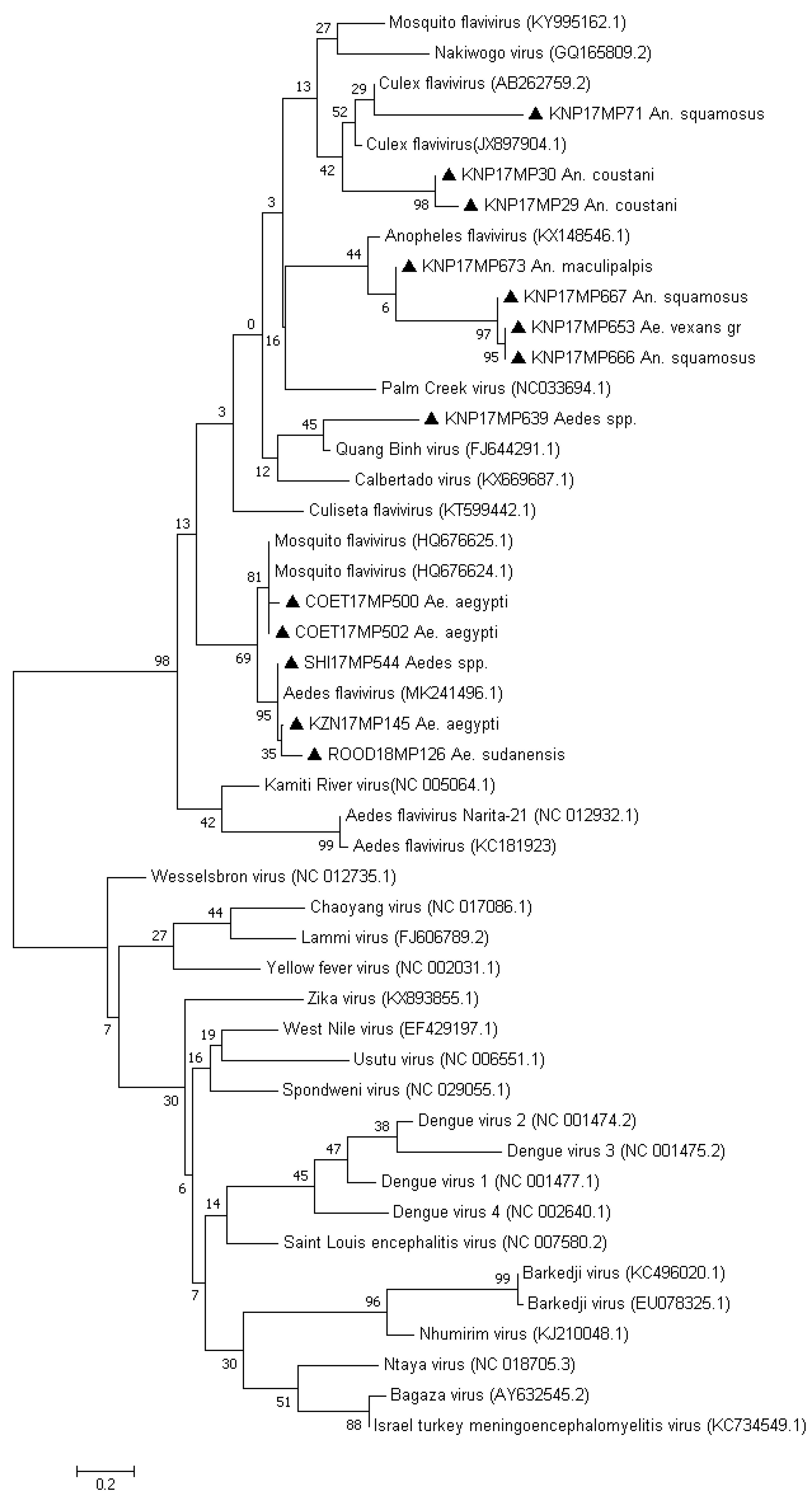
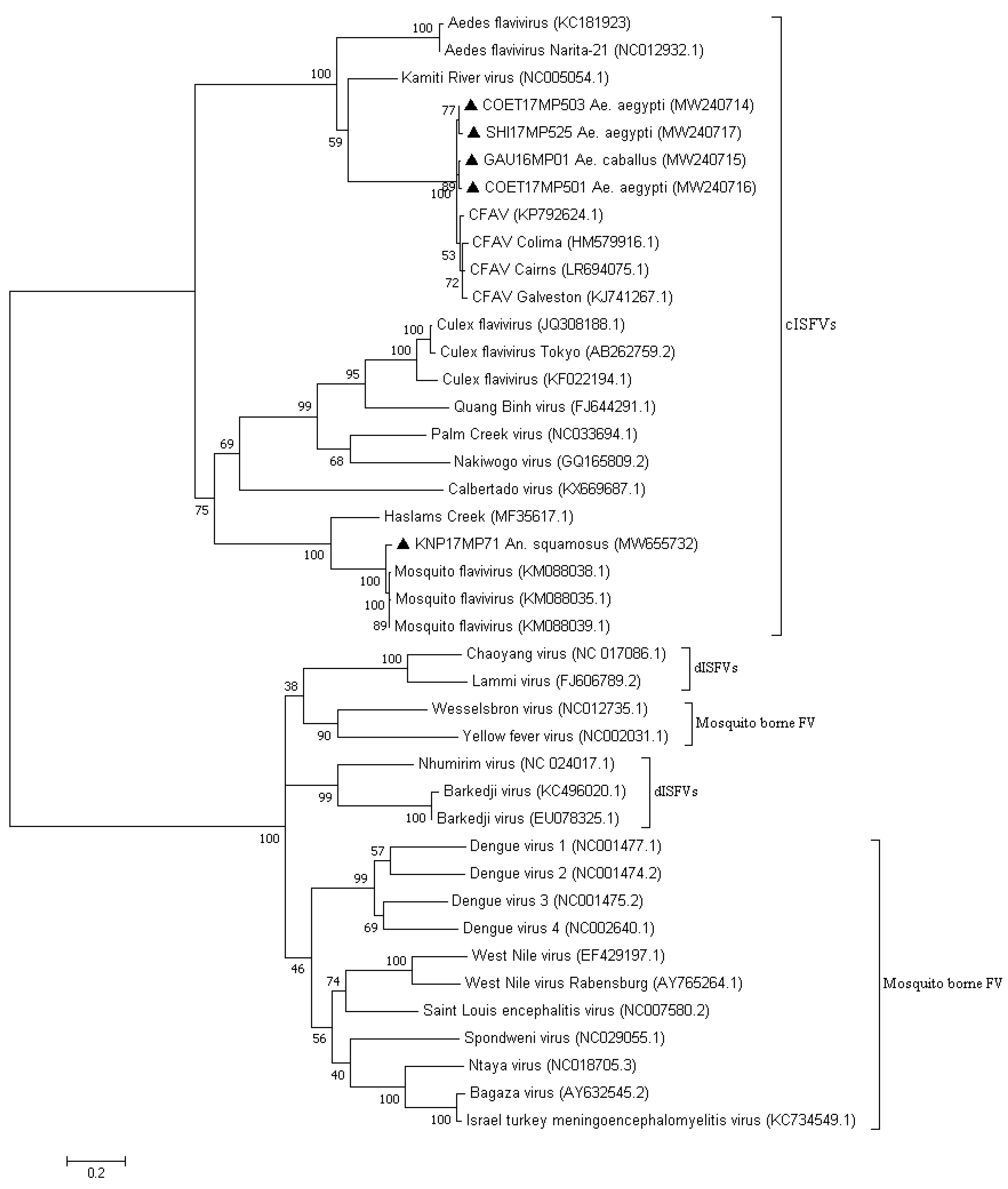
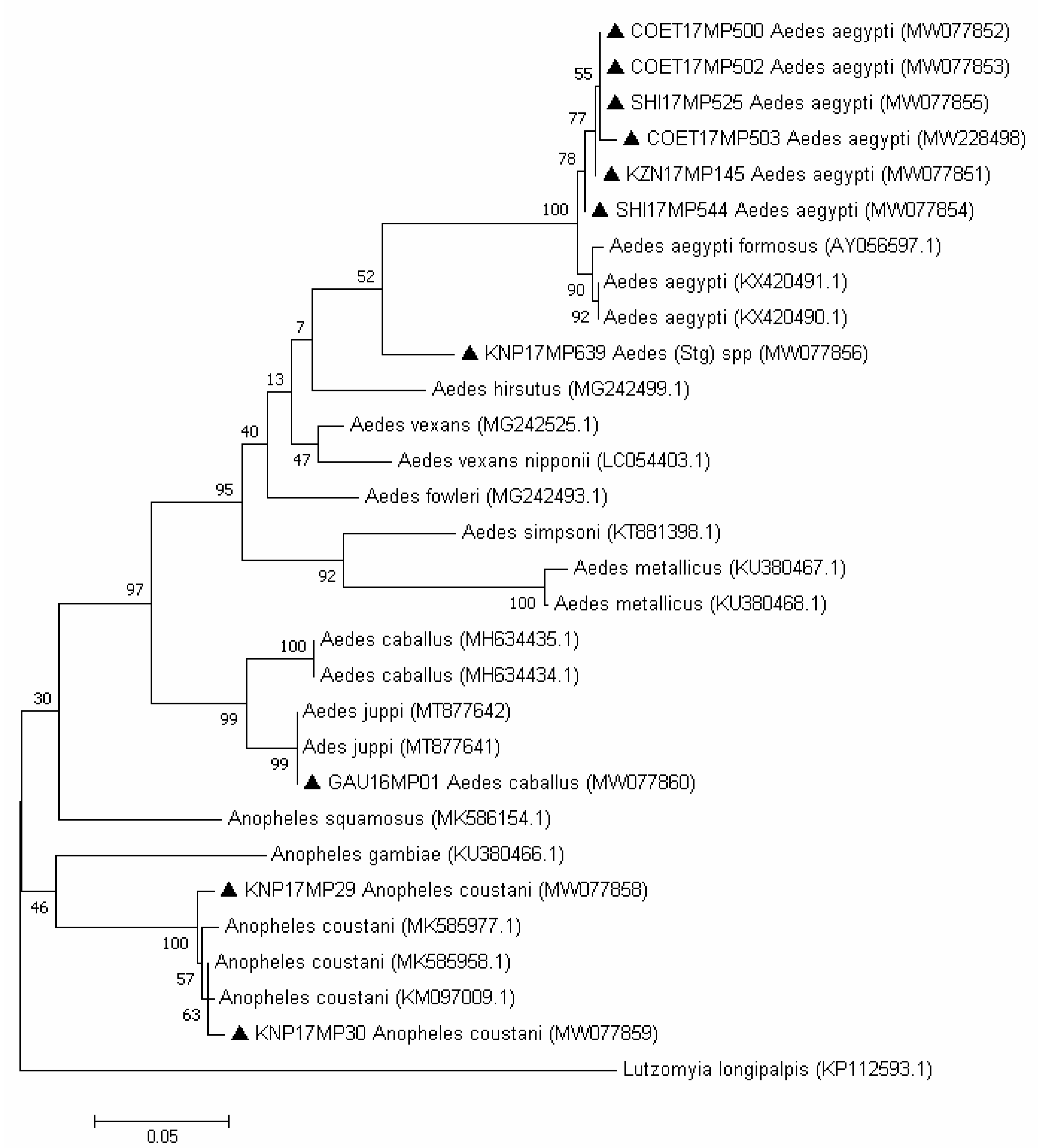
3. Results
3.1. RT-PCR Screening
3.2. Molecular Identification of the Mosquito Positive Pools
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ayers, M.; Adachi, D.; Johnson, G.; Andonova, M.; Drebot, M.; Tellier, R. A single tube RT-PCR assay for the detection of mosquito-borne flaviviruses. J. Virol. Methods 2006, 135, 235–239. [Google Scholar] [CrossRef]
- Roundy, C.M.; Azar, S.R.; Rossi, S.L.; Weaver, S.C.; Vasilakis, N. Insect-Specific Viruses: A Historical Overview and Recent Developments. Adv. Virus Res. 2017, 98, 119–146. [Google Scholar]
- Cook, S.; Bennett, S.N.; Holmes, E.C.; Chesse, R.D.; Moureau, G.; Lamballerie, X.D. Isolation of a new strain of the flavivirus cell fusing agent virus in a natural population from Puerto Rico. J. Gen. Virol. 2006, 87, 735–748. [Google Scholar] [CrossRef] [PubMed]
- Blitvich, B.J.; Firth, A.E. Insect-specific Flaviviruses a systematic review of their discovery host Range mode transmission superinfection exclusion potential and genomic organization. Viruses 2015, 7, 1927–1959. [Google Scholar] [CrossRef] [Green Version]
- Bolling, B.G.; Weaver, S.C.; Tesh, R.B.; Vasilakis, N. Insect-Specific Virus Discovery: Significance for the Arbovirus Community. Viruses 2015, 7, 4911–4928. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Öhlund, P.; Lundén, H.; Blomström, A. Insect-specific virus evolution and potential effects on vector competence. Virus Genes 2019, 55, 127–137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stollar, V.; Thomas, V.L. An agent in the Aedes aegypti cell line (Peleg) which causes fusion of Aedes albopictus cells. Virology 1975, 64, 367–377. [Google Scholar] [CrossRef]
- Crochu, S.; Cook, S.; Attoui, H.; Charrel, R.N.; De Chesse, R.; Belhouchet, M.; Lemasson, J.J.; de Micco, P.; de Lamballerie, X. Sequences of flavivirus-related RNA viruses persist in DNA form integrated in the genome of Aedes spp. mosquitoes. J. Gen. Virol. 2004, 85 Pt 7, 1971–1980. [Google Scholar] [CrossRef]
- Vazquez, A.; Sanchez-Seco, M.P.; Palacios, G.; Molero, F.; Reyes, N.; Ruiz, S.; Aranda, C.; Marqués, E.; Escosa, R.; Moreno, J.; et al. Novel flaviviruses detected in different species of mosquitoes in Spain. Vector Borne Zoonotic Dis. 2012, 12, 223–229. [Google Scholar] [CrossRef] [Green Version]
- Aguiar, E.R.G.R.; de Almeida, J.P.P.; Queiroz, L.R.; Oliveira, L.S.; Olmo, R.P.; de Faria, I.J.D.S.; Imler, J.L.; Gruber, A.; Matthews, B.J.; Marques, J.T. A single unidirectional piRNA cluster similar to the flamenco locus is the major source of EVE-derived transcription and small RNAs in Aedes aegypti mosquitoes. RNA 2020, 26, 581–594. [Google Scholar] [CrossRef]
- Palatini, U.; Miesen, P.; Carballar-Lejarazu, R.; Ometto, L.; Rizzo, E.; Tu, Z.; van Rij, R.P.; Bonizzoni, M. Comparative genomics shows that viral integrations are abundant and express piRNAs in the arboviral vectors Aedes aegypti and Aedes albopictus. BMC Genom. 2017, 18, 512. [Google Scholar] [CrossRef] [Green Version]
- Whitfield, Z.J.; Dolan, P.T.; Kunitomi, M.; Tassetto, M.; Seetin, M.G.; Oh, S.; Oh, S.; Heiner, C.; Paxinos, E.; Andino, R. The Diversity, Structure, and Function of Heritable Adaptive Immunity Sequences in the Aedes aegypti Genome. Curr. Biol. 2017, 27, 3511–3519.e7. [Google Scholar] [CrossRef] [Green Version]
- Hoshino, K.; Isawa, H.; Tsuda, Y.; Sawabe, K.; Kobayashi, M. Isolation and characterization of a new insect flavivirus from Aedes albopictus and Aedes flavopictus mosquitoes in Japan. Virology 2009, 391, 119–129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Agboli, E.; Leggewie, M.; Altinli, M.; Schnettler, E. Mosquito-Specific Viruses-Transmission and Interaction. Viruses 2019, 11, 873. [Google Scholar] [CrossRef] [Green Version]
- Edwards, F.W. Mosquitoes of the Ethiopian Region. III-Culicine Adults and Pupae; The Oxford University Press: Oxford, UK, 1941. [Google Scholar]
- Gillies, M.T.; Coetzee, M. A Supplement to the Anophelinae of South of the Sahara (Afrotropical Region); The South African Institute for Medical Research: Johannesburg, South Africa, 1987. [Google Scholar]
- Jupp, P.G. Mosquitoes of Southern Africa. Culicinae and Toxorhynchitinae; Ekogilde Publishers: Hartebeespoort, South Africa, 1996. [Google Scholar]
- Service, M.W. Handbook to the Afrotropical Toxorhynchitine and Culicine Mosquitoes, Excepting Aedes and Culex; British Museum (Natural History): London, UK, 1990. [Google Scholar]
- Zaayman, D.; Human, S.; Venter, M. A highly sensitive method for the detection and genotyping of West Nile virus by real-time PCR. J. Virol. Methods 2009, 157, 155–160. [Google Scholar] [CrossRef] [PubMed]
- Kuno, G.; Chang, G.J.; Tsuchyia, R.; Karabatsos, N.; Cropp, B. Phylogeny of the Genus Flavivirus. J. Virol. 1998, 72, 73–83. [Google Scholar] [CrossRef] [Green Version]
- Folmer, O.; Black, M.; Hoeh, W.; Lutz, R.; Vrijenhoek, R. DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrate. Mol. Mar. Biol. Biotechnol. 1994, 3, 294–299. [Google Scholar] [PubMed]
- CLC Genomics Workbench 8.0.1: Qiagen Bioinformatics. Available online: https://www.qiagenbioinformatics.com (accessed on 2 December 2020).
- Ratnasigham, S.; Herbert, P.D.N. BOLD: The Barcode of Life Data System (www.barcodinglife.org). Mol. Ecol. Notes 2007, 7, 355–364. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Medicine Nlo. National Center for Biotechnology Information (NCBI) Bethesda (US). 1988. Available online: https://www.ncbi.nlm.nih.gov/ (accessed on 2 December 2020).
- Katoh, K.; Rozewicki, J.; Yamada, K.D. MAFFT online service: Multiple sequence alignment, interactive sequence choice and visualization. Brief Bioinform. 2019, 20, 1160–1166. [Google Scholar] [CrossRef] [Green Version]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef] [Green Version]
- Guarido, M.M.; Riddin, M.A.; Johnson, T.; Braack, L.E.O.; Schrama, M.; Gorsich, E.E.; Brooke, B.D.; Almeida, A.P.G.; Venter, M. Aedes species (Diptera: Culicidae) ecological and host feeding patterns in the north-eastern parts of South Africa, 2014-2018. Parasit. Vectors 2021, 14, 339. [Google Scholar] [CrossRef]
- Nei, M.; Kumar, S. Molecular Evolution and Phylogenetics; Oxford University Press: New York, NY, USA, 2001. [Google Scholar]
- Feselsntein, J. Phylogenies and the Comparative Method. Am. Nat. 1985, 125, 1–15. [Google Scholar] [CrossRef]
- Cook, S.; Moureau, G.; Harbach, R.E.; Mukwaya, L.G.; Goodger, K.; Ssenfuka, F.; Gould, E.; Holmes, E.C.; de Lamballerie, X. Isolation of a novel species of flavivirus and a new strain of Culex flavivirus from a natural mosquito population in Uganda. J. Gen. Virol. 2009, 90, 2669–2678. [Google Scholar] [CrossRef]
- Colmant, A.M.G.; Hobson-Peters, J.; Bielefeldt-Ohmann, H.; van Den Hurk, A.F.; Hall-Mendelin, S.; Chow, W.K.; Johansen, C.A.; Fros, J.; Simmonds, P.; Watterson, D.; et al. A new clade of Insect specific flaviviruses from Australian Anopheles mosquitoes displays species specific host restriction. mSphere 2017, 2, e00262-17. [Google Scholar] [CrossRef] [Green Version]
- Fauver, J.R.; Grubaugh, N.D.; Krajacich, B.J.; Weger-Lucarelli, J.; Lakin, S.M.; Fakoli, L.S., 3rd; Bolay, F.K.; Diclaro, J.W., 2nd; Dabiré, K.R.; Foy, B.D.; et al. West African Anopheles gambiae mosquitoes harbor a taxonomically diverse virome including new insect-specific flaviviruses, mononegaviruses, and totiviruses. Virology 2016, 498, 288–299. [Google Scholar] [CrossRef] [Green Version]
- Villinger, J.; Mbaya, M.K.; Ouso, D.; Kipanga, P.N.; Lutomiah, J.; Masiga, D.K. Arbovirus and insect-specific virus discovery in Kenya by novel six genera multiplex high-resolution melting analysis. Mol. Ecol. Resour. 2017, 17, 466–480. [Google Scholar] [CrossRef] [PubMed]
- Espinoza-Gomez, F.; Lopez-Lemus, A.U.; Rodriguez-Sanchez, I.P.; Martinez-Fierro, M.L.; Newton-Sanchez, O.A.; Chavez-Flores, E.; Delgado-Enciso, I. Detection of sequences from a potentially novel strain of cell fusing agent virus in Mexican Stegomyia (Aedes) aegypti mosquitoes. Arch. Virol. 2011, 156, 1263–1267. [Google Scholar] [CrossRef]
- Martin, E.; Tang, W.; Briggs, C.; Hopson, H.; Juarez, J.G.; Garcia-Luna, S.M.; de Valdez, M.W.; Badillo-Vargas, I.E.; Borucki, M.K.; Frank, M.; et al. Cell fusing agent virus (Flavivirus) infection in Aedes aegypti in Texas: Seasonality, comparison by trap type, and individual viral loads. Arch. Virol. 2020, 165, 1769–1776. [Google Scholar] [CrossRef]
- Yamanaka, A.; Thongrungkiat, S.; Ramasoota, P.; Konishi, E. Genetic and evolutionary analysis of cell-fusing agent virus based on Thai strains isolated in 2008 and 2012. Infect. Genet. Evol. 2013, 19, 188–194. [Google Scholar] [CrossRef] [PubMed]
- Gravina, H.D.; Suzukawa, A.A.; Zanluca, C.; Cardozo Segovia, F.M.; Tscha, M.K.; Martins da Silva, A.; Faoro, H.; da Silva Ribeiro, R.; Torres, L.P.M.; Rojas, A.; et al. Identification of insect-specific flaviviruses in areas of Brazil and Paraguay experiencing endemic arbovirus transmission and the description of a novel flavivirus infecting Sabethes belisarioi. Virology 2019, 527, 98–106. [Google Scholar] [CrossRef] [PubMed]
- Miranda, J.; Mattar, S.; Gonzalez, M.; Hoyos-Lopez, R.; Aleman, A.; Aponte, J. First report of Culex flavivirus infection from Culex coronator (Diptera: Culicidae), Colombia. Virol. J. 2019, 16, 1. [Google Scholar] [CrossRef] [PubMed]
| Culicidae Species | Site | ID Number | Pool Size | Virus | Virus Accession Number | Coi Accession Number |
|---|---|---|---|---|---|---|
| An. coustani | KNP | KNP17MP29 | 39 | Cx. flavivirus-like | NA < 200 bp | MW077858 |
| An. coustani | KNP | KNP17MP30 | 9 | Cx. flavivirus-like | NA < 200 bp | MW077859 |
| An. squamosus | KNP | KNP17MP71 | 15 | Mosq. Flavivirus | MW655732 | NA |
| An. maculipalpis | KNP | KNP17MP673 | 1 | An. Flavivirus | NA < 200 bp | NA |
| An. squamosus | KNP | KNP17MP666 | 1 | Mosq. Flavivirus | NA < 200 bp | NA |
| An. squamosus | KNP | KNP17MP667 | 9 | Mosq. Flavivirus | NA < 200 bp | NA |
| Ae. aegypti | Jozini | KZN17MP145 | 15 | Mosq. Flavivirus | NA < 200 bp | MW077851 |
| Ae. aegypti | Pretoria | COET17MP500 | 4 | Mosq. Flavivirus | NA < 200 bp | MW077852 |
| Ae. aegypti | Pretoria | COET17MP502 | 21 | Mosq. Flavivirus | NA < 200 bp | MW077853 |
| Aedes spp | KNP-SHI | SHI17MP544 | 7 | Mosq. Flavivirus | NA < 200 bp | MW077854 |
| Aedes spp | KNP | KNP17MP639 | 4 | Mosq. Flavivirus | NA < 200 bp | MW077856 |
| Aedes vexans gr. | KNP | KNP17MP653 | 3 | Mosq. Flavivirus | NA < 200 bp | NA |
| Ae. sudanensis | Roodeplat | ROOD18MP126 | 1 | Ae. Flavivirus | NA < 200 bp | NA |
| Ae. aegypti | Pretoria | COET17MP503 | 35 | CFAV | MW240714 | MW228498 |
| Ae. aegypti | Pretoria | COET17MP501 | 20 | CFAV | MW240716 | NA |
| Ae. caballus | Boschkop | GAU16MP01 | 9 | CFAV | MW240715 | MW077860 |
| Ae. aegypti | KNP-SHI | SHI17MP525 | 50 | CFAV | MW240717 | MW077855 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guarido, M.M.; Govender, K.; Riddin, M.A.; Schrama, M.; Gorsich, E.E.; Brooke, B.D.; Almeida, A.P.G.; Venter, M. Detection of Insect-Specific Flaviviruses in Mosquitoes (Diptera: Culicidae) in Northeastern Regions of South Africa. Viruses 2021, 13, 2148. https://doi.org/10.3390/v13112148
Guarido MM, Govender K, Riddin MA, Schrama M, Gorsich EE, Brooke BD, Almeida APG, Venter M. Detection of Insect-Specific Flaviviruses in Mosquitoes (Diptera: Culicidae) in Northeastern Regions of South Africa. Viruses. 2021; 13(11):2148. https://doi.org/10.3390/v13112148
Chicago/Turabian StyleGuarido, Milehna M., Kamini Govender, Megan A. Riddin, Maarten Schrama, Erin E. Gorsich, Basil D. Brooke, Antonio Paulo Gouveia Almeida, and Marietjie Venter. 2021. "Detection of Insect-Specific Flaviviruses in Mosquitoes (Diptera: Culicidae) in Northeastern Regions of South Africa" Viruses 13, no. 11: 2148. https://doi.org/10.3390/v13112148
APA StyleGuarido, M. M., Govender, K., Riddin, M. A., Schrama, M., Gorsich, E. E., Brooke, B. D., Almeida, A. P. G., & Venter, M. (2021). Detection of Insect-Specific Flaviviruses in Mosquitoes (Diptera: Culicidae) in Northeastern Regions of South Africa. Viruses, 13(11), 2148. https://doi.org/10.3390/v13112148






