Pregnant Women Infected with Zika Virus Show Higher Viral Load and Immunoregulatory Cytokines Profile with CXCL10 Increase
Abstract
1. Introduction
2. Material and Methods
2.1. Experimental Groups
2.2. ZIKV Infection Confirmation
2.3. Determination of Cytokines and Chemokines in Serum
2.4. Determination of Viral Load
2.5. Statistical Analysis
3. Results
3.1. General Characteristics of Sample Groups
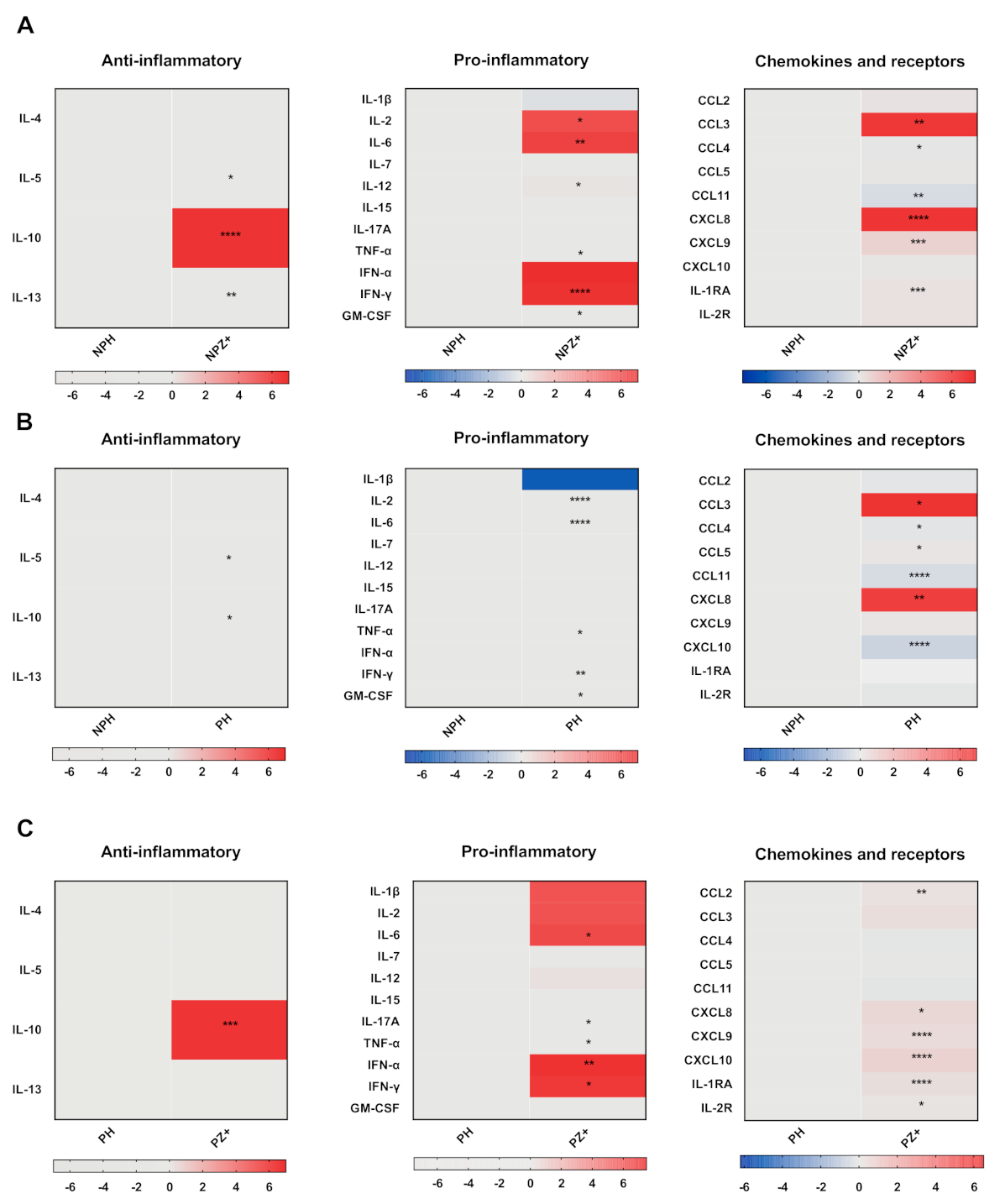
3.2. Cytokine Profile in Nonpregnant Women with ZIKV Infection
3.3. Cytokine Profile in Pregnant Women
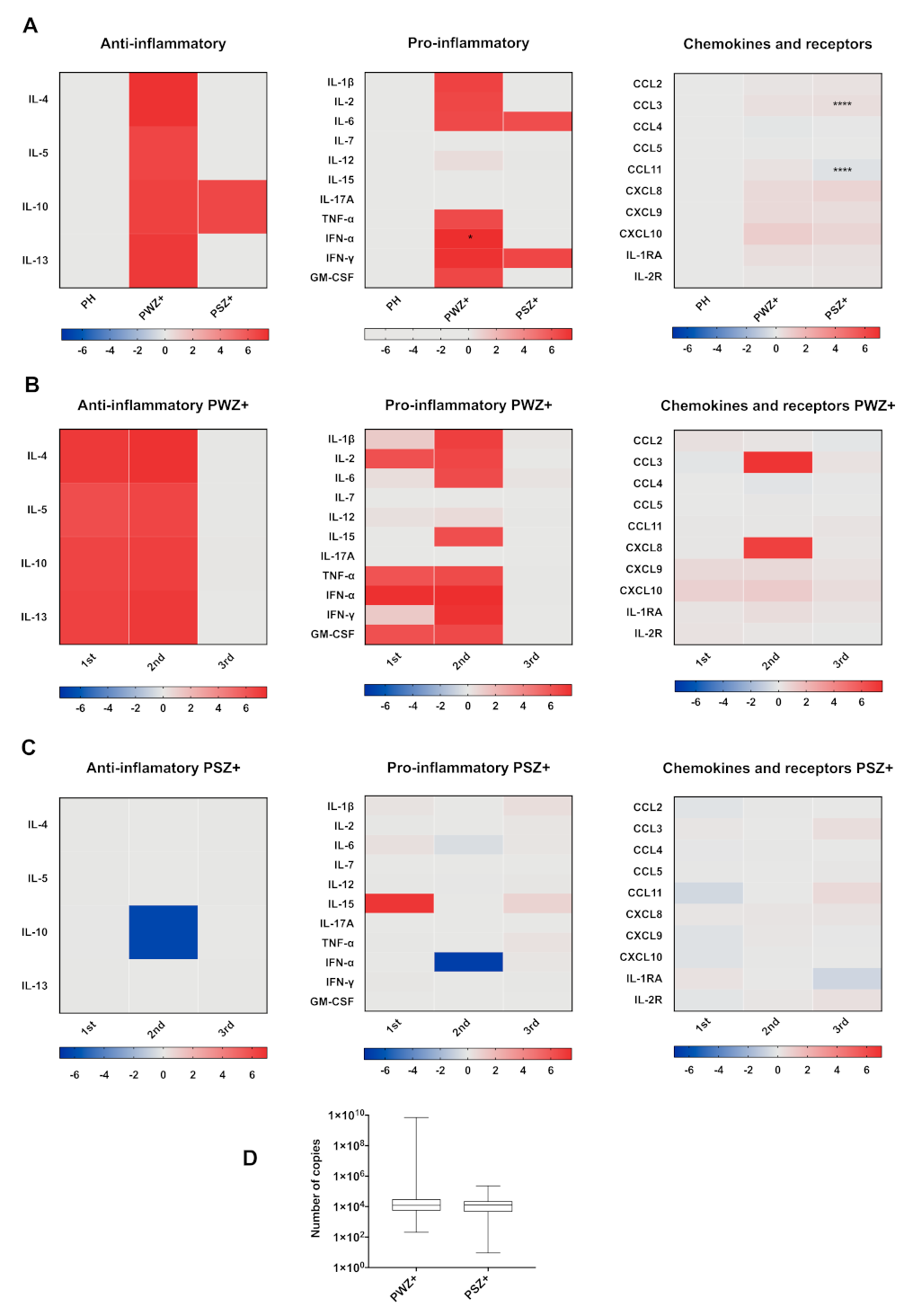
3.4. Cytokines in Pregnant Women Infected with ZIKV
3.5. Cytokine Profiles in Pregnant and Nonpregnant Women Infected with ZIKV
3.6. Cytokine Profile According to Trimester of Pregnancy
3.7. Cytokine Profile According to Days after Disease Onset
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hayes, E.B. Zika Virus outside Africa. Emerg. Infect. Dis. 2009, 15, 1347–1350. [Google Scholar] [CrossRef]
- Kleber de Oliveira, W.; Cortez-Escalante, J.; De Oliveira, W.T.G.H.; do Carmo, G.M.I.; Henriques, C.M.P.; Coelho, G.E.; Araújo de França, G.V. Increase in Reported Prevalence of Microcephaly in Infants Born to Women Living in Areas with Confirmed Zika Virus Transmission During the First Trimester of Pregnancy—Brazil, 2015. Mmwr. Morb. Mortal. Wkly. Rep. 2016, 65, 242–247. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Quiñonez, J.A.; López-Martínez, I.; Torres-Longoria, B.; Vázquez-Pichardo, M.; Cruz-Ramírez, E.; Ramírez-González, J.E.; Ruiz-Matus, C.; Kuri-Morales, P. Evidence of the presence of the Zika virus in Mexico since early 2015. Virus Genes 2016, 52, 855–857. [Google Scholar] [CrossRef] [PubMed]
- Grajales-Muñiz, C.; Borja-Aburto, V.H.; Cabrera-Gaytán, D.A.; Rojas-Mendoza, T.; Arriaga-Nieto, L.; Vallejos-Parás, A. Zika virus: Epidemiological surveillance of the Mexican Institute of Social Security. PLoS ONE 2019, 14, e0212114. [Google Scholar] [CrossRef] [PubMed]
- Faria, N.R.; da Silva Azevedo, R.D.S.; Kraemer, M.U.G.; Souza, R.; Cunha, M.S.; Hill, S.C.; Theze, J.; Bonsall, M.B.; Bowden, T.A.; Rissanen, I.; et al. Zika virus in the Americas: Early epidemiological and genetic findings. Science 2016, 352, 345–349. [Google Scholar] [CrossRef]
- Subissi, L.; Daudens-Vaysse, E.; Cassadou, S.; Ledrans, M.; Bompard, P.; Gustave, J.; Aubry, M.; Cao-Lormeau, V.M.; Mallet, H.P. Revising rates of asymptomatic Zika virus infection based on sentinel surveillance data from French Overseas Territories. Int. J. Infect. Dis. 2017, 65, 116–118. [Google Scholar] [CrossRef]
- Duffy, M.R.; Chen, T.-H.; Hancock, W.T.; Powers, A.M.; Kool, J.L.; Lanciotti, R.S.; Pretrick, M.; Marfel, M.; Holzbauer, S.; Dubray, C.; et al. Zika Virus Outbreak on Yap Island, Federated States of Micronesia. N. Engl. J. Med. 2009, 360, 2536–2543. [Google Scholar] [CrossRef]
- Barros, J.B.; Silva, P.A.; Koga, R.D.; Gonzalez-Dias, P.; Carmo Filho, J.R.; Nagib, P.R.; Coelho, V.; Nakaya, H.I.; Fonseca, S.G.; Pfrimer, I.A. Acute Zika Virus Infection in an Endemic Area Shows Modest Proinflammatory Systemic Immunoactivation and Cytokine-Symptom Associations. Front. Immunol. 2018, 9. [Google Scholar] [CrossRef]
- Mayer, S.V.; Tesh, R.B.; Vasilakis, N. The emergence of arthropod-borne viral diseases: A global prospective on dengue, chikungunya and zika fevers. Acta Trop. 2017, 166, 155–163. [Google Scholar]
- Wheeler, A.C. Development of Infants with Congenital Zika Syndrome: What Do We Know and What Can We Expect? Pediatrics 2018, 141, S154–S160. [Google Scholar] [CrossRef]
- Elong Ngono, A.; Shresta, S. Immune Response to Dengue and Zika. Annu. Rev. Immunol. 2018, 36, 279–308. [Google Scholar] [CrossRef] [PubMed]
- Seong, R.-K.; Lee, J.K.; Shin, O.S. Zika Virus-Induction of the Suppressor of Cytokine Signaling 1/3 Contributes to the Modulation of Viral Replication. Pathogens 2020, 9, 163. [Google Scholar] [CrossRef] [PubMed]
- Priyamvada, L.; Suthar, M.S.; Ahmed, R.; Wrammert, J. Humoral Immune Responses Against Zika Virus Infection and the Importance of Preexisting Flavivirus Immunity. J. Infect. Dis. 2017, 216, S906–S911. [Google Scholar] [CrossRef]
- Balsitis, S.J.; Williams, K.L.; Lachica, R.; Flores, D.; Kyle, J.L.; Mehlhop, E.; Johnson, S.; Diamond, M.S.; Beatty, P.R.; Harris, E. Lethal Antibody Enhancement of Dengue Disease in Mice Is Prevented by Fc Modification. PLoS Pathog. 2010, 6, e1000790. [Google Scholar] [CrossRef] [PubMed]
- Wen, J.; Shresta, S. T Cell Immunity to Zika and Dengue Viral Infections. J. Interf. Cytokine Res. 2017, 37, 475–479. [Google Scholar] [CrossRef]
- Zuñiga, J.; Choreño-Parra, J.A.; Jiménez-Alvarez, L.; Cruz-Lagunas, A.; Márquez-García, J.E.; Ramírez-Martínez, G.; Goodina, A.; Hernández-Montiel, E.; Fernández-López, L.A.; Cabrera-Cornejo, M.F.; et al. A unique immune signature of serum cytokine and chemokine dynamics in patients with Zika virus infection from a tropical region in Southern Mexico. Int. J. Infect. Dis. 2020, 94, 4–11. [Google Scholar] [CrossRef]
- Naveca, F.G.; Pontes, G.S.; Chang, A.Y.; Silva, G.A.; Nascimento, V.A.; Monteiro, D.C.; Silva, M.S.; Abdalla, L.F.; Santos, J.H.; Almeida, T.A.; et al. Analysis of the immunological biomarker profile during acute Zika virus infection reveals the overexpression of CXCL10, a chemokine linked to neuronal damage. Mem. Inst. Oswaldo Cruz 2018, 113. [Google Scholar] [CrossRef]
- Lum, F.M.; Lye, D.C.B.; Tan, J.J.L.; Lee, B.; Chia, P.Y.; Chua, T.K.; Amrun, S.N.; Kam, Y.W.; Yee, W.X.; Ling, W.P.; et al. Longitudinal study of cellular and systemic cytokine signatures to define the dynamics of a balanced immune environment during disease manifestation in zika virus–infected patients. J. Infect. Dis. 2018, 218, 814–824. [Google Scholar] [CrossRef]
- Cumming, H.E.; Bourke, N.M. Type I IFNs in the female reproductive tract: The first line of defense in an ever-changing battleground. J. Leukoc. Biol. 2019, 105, 353–361. [Google Scholar] [CrossRef]
- Robertson, S.A.; Petroff, M.G.; Hunt, J.S. Immunology of Pregnancy. In Knobil and Neill’s Physiology of Reproduction; Elsevier: Amsterdam, The Netherlands, 2015; Volume 52, pp. 1835–1874. ISBN 9780123977694. [Google Scholar]
- Racicot, K.; Kwon, J.-Y.; Aldo, P.; Silasi, M.; Mor, G. Understanding the Complexity of the Immune System during Pregnancy. Am. J. Reprod. Immunol. 2014, 72, 107–116. [Google Scholar] [CrossRef]
- Ruiz, R.J.; Fullerton, J.; Dudley, D.J. The Interrelationship of Maternal Stress, Endocrine Factors and Inflammation on Gestational Length. Obs. Gynecol. Surv. 2003, 58, 415–428. [Google Scholar] [CrossRef]
- Christiaens, I.; Zaragoza, D.B.; Guilbert, L.; Robertson, S.A.; Mitchell, B.F.; Olson, D.M. Inflammatory processes in preterm and term parturition. J. Reprod. Immunol. 2008, 79, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Avelino-Silva, V.I.; Mayaud, P.; Tami, A.; Miranda, M.C.; Rosenberger, K.D.; Alexander, N.; Nacul, L.; Segurado, A.; Pohl, M.; Bethencourt, S.; et al. Study protocol for the multicentre cohorts of Zika virus infection in pregnant women, infants, and acute clinical cases in Latin America and the Caribbean: The ZIKAlliance consortium. BMC Infect. Dis. 2019, 19, 1081. [Google Scholar] [CrossRef] [PubMed]
- Lanciotti, R.S.; Kosoy, O.L.; Laven, J.J.; Velez, J.O.; Lambert, A.J.; Johnson, A.J.; Stanfield, S.M.; Duffy, M.R. Genetic and Serologic Properties of Zika Virus Associated with an Epidemic, Yap State, Micronesia. Emerg. Infect. Dis. 2008, 14, 1232–1239. [Google Scholar] [CrossRef] [PubMed]
- InDRE-Secretaría de Salud Lineamientos de Vigilancia por Laboratorio. Dengue y Otras Arbovirosis. Available online: https://www.gob.mx/cms/uploads/attachment/file/506677/Lineamientos_Dengue_Arb_V2_2019.pdf (accessed on 1 December 2020).
- Orsi, N.M.; Tribe, R.M. Cytokine Networks and the Regulation of Uterine Function in Pregnancy and Parturition. J. Neuroendocr. 2008, 20, 462–469. [Google Scholar] [CrossRef]
- Brasil, P.; Pereira, J.P.; Moreira, M.E.; Ribeiro Nogueira, R.M.; Damasceno, L.; Wakimoto, M.; Rabello, R.S.; Valderramos, S.G.; Halai, U.-A.; Salles, T.S.; et al. Zika Virus Infection in Pregnant Women in Rio de Janeiro. N. Engl. J. Med. 2016, 375, 2321–2334. [Google Scholar] [CrossRef]
- Marzi, M.; Vigano, A.; Trabattoni, D.; Villa, M.L.; Salvaggio, A.; Clerici, E.; Clerici, M. Characterization of type 1 and type 2 cytokine production profile in physiologic and pathologic human pregnancy. Clin. Exp. Immunol. 1996, 106, 127–133. [Google Scholar] [CrossRef]
- Wira, C.R.; Rodriguez-Garcia, M.; Patel, M.V. The role of sex hormones in immune protection of the female reproductive tract. Nat. Rev. Immunol. 2015, 15, 217–230. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, M.D.; Lippes, J.; Srivastava, B.I.S. Cytokines of the Human Reproductive Tract. Am. J. Reprod. Immunol. 1996, 36, 157–166. [Google Scholar] [CrossRef] [PubMed]
- Southcombe, J.H.; Redman, C.W.G.; Sargent, I.L.; Granne, I. Interleukin-1 family cytokines and their regulatory proteins in normal pregnancy and pre-eclampsia. Clin. Exp. Immunol. 2015, 181, 480–490. [Google Scholar] [CrossRef] [PubMed]
- Keogh, R.J.; Harris, L.K.; Freeman, A.; Baker, P.N.; Aplin, J.D.; Whitley, G.S.; Cartwright, J.E. Fetal-Derived Trophoblast Use the Apoptotic Cytokine Tumor Necrosis Factor-α–Related Apoptosis-Inducing Ligand to Induce Smooth Muscle Cell Death. Circ. Res. 2007, 100, 834–841. [Google Scholar] [CrossRef] [PubMed]
- Hanna, J.; Goldman-Wohl, D.; Hamani, Y.; Avraham, I.; Greenfield, C.; Natanson-Yaron, S.; Prus, D.; Cohen-Daniel, L.; Arnon, T.I.; Manaster, I.; et al. Decidual NK cells regulate key developmental processes at the human fetal-maternal interface. Nat. Med. 2006, 12, 1065–1074. [Google Scholar] [CrossRef]
- Sakowicz, A. The role of NF κ B in the three stages of pregnancy—implantation, maintenance, and labour: A review article. BJOG Int. J. Obs. Gynaecol. 2018, 125, 1379–1387. [Google Scholar] [CrossRef] [PubMed]
- Reyes-Lagos, J.J.; Peña-Castillo, M.Á.; Echeverría, J.C.; Pérez-Sánchez, G.; Álvarez-Herrera, S.; Becerril-Villanueva, E.; Pavón, L.; Ayala-Yáñez, R.; González-Camarena, R.; Pacheco-López, G. Women serum concentrations of the IL-10 family of cytokines and IFN-γ decrease from the third trimester of pregnancy to active labor. Neuroimmunomodulation 2017, 24, 162–170. [Google Scholar] [CrossRef] [PubMed]
- Osman, I. Leukocyte density and pro-inflammatory cytokine expression in human fetal membranes, decidua, cervix and myometrium before and during labour at term. Mol. Hum. Reprod. 2003, 9, 41–45. [Google Scholar] [CrossRef]
- Faas, M.M.; Spaans, F.; De Vos, P. Monocytes and Macrophages in Pregnancy and Pre-Eclampsia. Front. Immunol. 2014, 5, 1–11. [Google Scholar] [CrossRef]
- Kam, Y.W.; Leite, J.A.; Lum, F.M.; Tan, J.J.; Lee, B.; Judice, C.C.; Teixeira, D.A.; Andreata-Santos, R.; Vinolo, M.A.; Angerami, R.; et al. Specific Biomarkers Associated With Neurological Complications and Congenital Central Nervous System Abnormalities From Zika Virus–Infected Patients in Brazil. J. Infect. Dis. 2017, 216, 172–181. [Google Scholar] [CrossRef]
- Kumar, A.; Devi, S.G.; Kar, P.; Agarwal, S.; Husain, S.A.; Gupta, R.K.; Sharma, S. Association of cytokines in hepatitis E with pregnancy outcome. Cytokine 2014, 65, 95–104. [Google Scholar] [CrossRef]
- Tobler, L.H.; Cameron, M.J.; Lanteri, M.C.; Prince, H.E.; Danesh, A.; Persad, D.; Lanciotti, R.S.; Norris, P.J.; Kelvin, D.J.; Busch, M.P. Interferon and Interferon-Induced Chemokine Expression Is Associated with Control of Acute Viremia in West Nile Virus-Infected Blood Donors. J. Infect. Dis. 2008, 198, 979–983. [Google Scholar] [CrossRef]
- Kurane, I.; Innis, B.L.; Nimmannitya, S.; Nisalak, A.; Ennis, F.A.; Meager, A. High Levels of Interferon Alpha in the Sera of Children with Dengue Virus Infection. Am. J. Trop. Med. Hyg. 1993, 48, 222–229. [Google Scholar] [CrossRef]
- Foo, S.-S.; Chen, W.; Chan, Y.; Lee, W.-S.; Lee, S.-A.; Cheng, G.; Nielsen-Saines, K.; Brasil, P.; Jung, J.U. Biomarkers and immunoprofiles associated with fetal abnormalities of ZIKV-positive pregnancies. JCI Insight 2018, 3, e124152. [Google Scholar] [CrossRef]
- Yockey, L.J.; Iwasaki, A. Interferons and Proinflammatory Cytokines in Pregnancy and Fetal Development. Immunity 2018, 49, 397–412. [Google Scholar] [CrossRef] [PubMed]
- O’Garra, A.; Vieira, P.L.; Vieira, P.; Goldfeld, A.E. IL-10–producing and naturally occurring CD4+ Tregs: Limiting collateral damage. J. Clin. Investig. 2004, 114, 1372–1378. [Google Scholar]
- Gonçalves Pereira, M.H.; Figueiredo, M.M.; Queiroz, C.P.; Magalhães, T.V.; Mafra, A.; Diniz, L.M.; da Costa, Ú.L.; Gollob, K.J.; Antonelli, L.R.; Santiago, H.D. T-cells producing multiple combinations of IFNγ, TNF and IL10 are associated with mild forms of dengue infection. Immunology 2020, 160, 90–102. [Google Scholar] [CrossRef] [PubMed]
- Ornelas, A.M.M.; Pezzuto, P.; Silveira, P.P.; Melo, F.O.; Ferreira, T.A.; Oliveira-Szejnfeld, P.S.; Leal, J.I.; Amorim, M.M.R.; Hamilton, S.; Rawlinson, W.D.; et al. Immune activation in amniotic fluid from Zika virus-associated microcephaly. Ann. Neurol. 2017, 81, 152–156. [Google Scholar] [CrossRef] [PubMed]
- Mamik, M.K.; Ghorpade, A. Chemokine CXCL8 Promotes HIV-1 Replication in Human Monocyte-Derived Macrophages and Primary Microglia via Nuclear Factor-κB Pathway. PLoS ONE 2014, 9, e92145. [Google Scholar] [CrossRef]
- Jovanović, M.; Stefanoska, I.; Radojčić, L.; Vićovac, L. Interleukin-8 (CXCL8) stimulates trophoblast cell migration and invasion by increasing levels of matrix metalloproteinase (MMP)2 and MMP9 and integrins α5 and β1. Reproduction 2010, 139, 789–798. [Google Scholar] [CrossRef]
- Galazios, G.; Tsoulou, S.; Zografou, C.; Tripsianis, G.; Koutlaki, N.; Papazoglou, D.; Tsikouras, P.; Maltezos, E.; Liberis, V. The role of cytokines IL-6 and IL-8 in the pathogenesis of spontaneous abortions. J. Matern. Neonatal Med. 2011, 24, 1283–1285. [Google Scholar] [CrossRef]
- Langerak, T.; Mumtaz, N.; Tolk, V.I.; van Gorp, E.C.M.; Martina, B.E.; Rockx, B.; Koopmans, M.P.G. The possible role of cross-reactive dengue virus antibodies in Zika virus pathogenesis. Plos Pathog. 2019, 15, e1007640. [Google Scholar] [CrossRef]



| Clinical Parameters | NPH | NPZ+ | PH | PZ+ | |
|---|---|---|---|---|---|
| PWZ+ | PSZ+ | ||||
| Number of samples | 14 | 22 | 30 | 19 | 25 |
| Age | 26.2 (19–45) | 30.6 (20–41) | 27.1 (17–42) | 30.1 (20–41) | 26.86 (20–37) |
| Pregnancy status (weeks) | |||||
| First trimester (0–13) | N/A | N/A | 10 (33.3%) | 6 (31.6%) | 5 (20%) |
| Second trimester (14–26) | N/A | N/A | 15 (50%) | 10 (52.6%) | 17 (68%) |
| Third trimester (>27) | N/A | N/A | 5 (16.6%) | 3 (15.8%) | 3 (12%) |
| Days after disease onset (mean) | N/A | 3 | N/A | 3 | 4 |
| Symptomatology, no. (% total) | |||||
| Fever (>38 °C) | 0.0 | 45.5 | 3.3 | 63.2 | 56 |
| Headache | 0.0 | 77.3 | 6.7 | 47.4 | 80 |
| Myalgia | 0.0 | 77.3 | 6.7 | 36.8 | 68 |
| Arthralgia | 0.0 | 77.3 | 6.7 | 36.8 | 56 |
| Retro-orbital pain | 0.0 | 36.4 | 6.7 | 36.8 | 52 |
| Pruritus | 0.0 | 72.7 | 6.7 | 68.4 | 64 |
| Conjunctivitis | 0.0 | 59.1 | 3.3 | 52.6 | 32 |
| Edema | 0.0 | 9.1 | 0.0 | 0.0 | 4 |
| Exanthema | 0.0 | 95.5 | 6.7 | 77.3 | |
| Respiratory symptoms or other | 0.0 | 0.0 | 6.7 | 0.0 | 0.0 |
| qRT-PCR (%) | |||||
| ZIKV | N/A | 100 | 0 | 100 | 100 |
| DENV | N/A | 0 | 0 | 0 | 0 |
| CHIKV | N/A | 0 | 0 | 0 | 0 |
| Serological tests | |||||
| ZIKV IgM | N/D | 18.2 | 0 | 15.8 | 20.0 |
| ZIKV IgG | N/D | 22.7 | 0 | 15.8 | 8.0 |
| DENV IgM | N/D | - | 0 | 5.3 | 0.0 |
| DENV IgG | N/D | 27.3 | 0 | 15.8 | 36.0 |
| CHIKV IgM | N/D | 0 | 0 | 0 | 0.0 |
| Location | Mexico City | Endemic areas of Mexico | Endemic areas of Mexico | Endemic areas of Mexico | Endemic areas of Mexico |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Camacho-Zavala, E.; Santacruz-Tinoco, C.; Muñoz, E.; Chacón-Salinas, R.; Salazar-Sanchez, M.I.; Grajales, C.; González-Ibarra, J.; Borja-Aburto, V.H.; Jaenisch, T.; Gonzalez-Bonilla, C.R. Pregnant Women Infected with Zika Virus Show Higher Viral Load and Immunoregulatory Cytokines Profile with CXCL10 Increase. Viruses 2021, 13, 80. https://doi.org/10.3390/v13010080
Camacho-Zavala E, Santacruz-Tinoco C, Muñoz E, Chacón-Salinas R, Salazar-Sanchez MI, Grajales C, González-Ibarra J, Borja-Aburto VH, Jaenisch T, Gonzalez-Bonilla CR. Pregnant Women Infected with Zika Virus Show Higher Viral Load and Immunoregulatory Cytokines Profile with CXCL10 Increase. Viruses. 2021; 13(1):80. https://doi.org/10.3390/v13010080
Chicago/Turabian StyleCamacho-Zavala, Elizabeth, Clara Santacruz-Tinoco, Esteban Muñoz, Rommel Chacón-Salinas, Ma Isabel Salazar-Sanchez, Concepción Grajales, Joaquin González-Ibarra, Victor Hugo Borja-Aburto, Thomas Jaenisch, and Cesar R. Gonzalez-Bonilla. 2021. "Pregnant Women Infected with Zika Virus Show Higher Viral Load and Immunoregulatory Cytokines Profile with CXCL10 Increase" Viruses 13, no. 1: 80. https://doi.org/10.3390/v13010080
APA StyleCamacho-Zavala, E., Santacruz-Tinoco, C., Muñoz, E., Chacón-Salinas, R., Salazar-Sanchez, M. I., Grajales, C., González-Ibarra, J., Borja-Aburto, V. H., Jaenisch, T., & Gonzalez-Bonilla, C. R. (2021). Pregnant Women Infected with Zika Virus Show Higher Viral Load and Immunoregulatory Cytokines Profile with CXCL10 Increase. Viruses, 13(1), 80. https://doi.org/10.3390/v13010080







