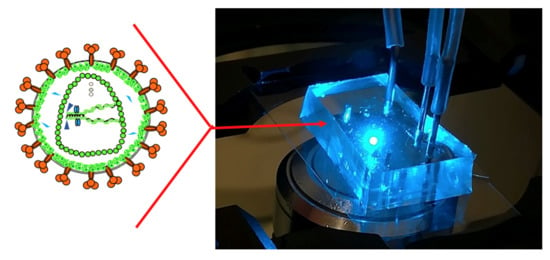
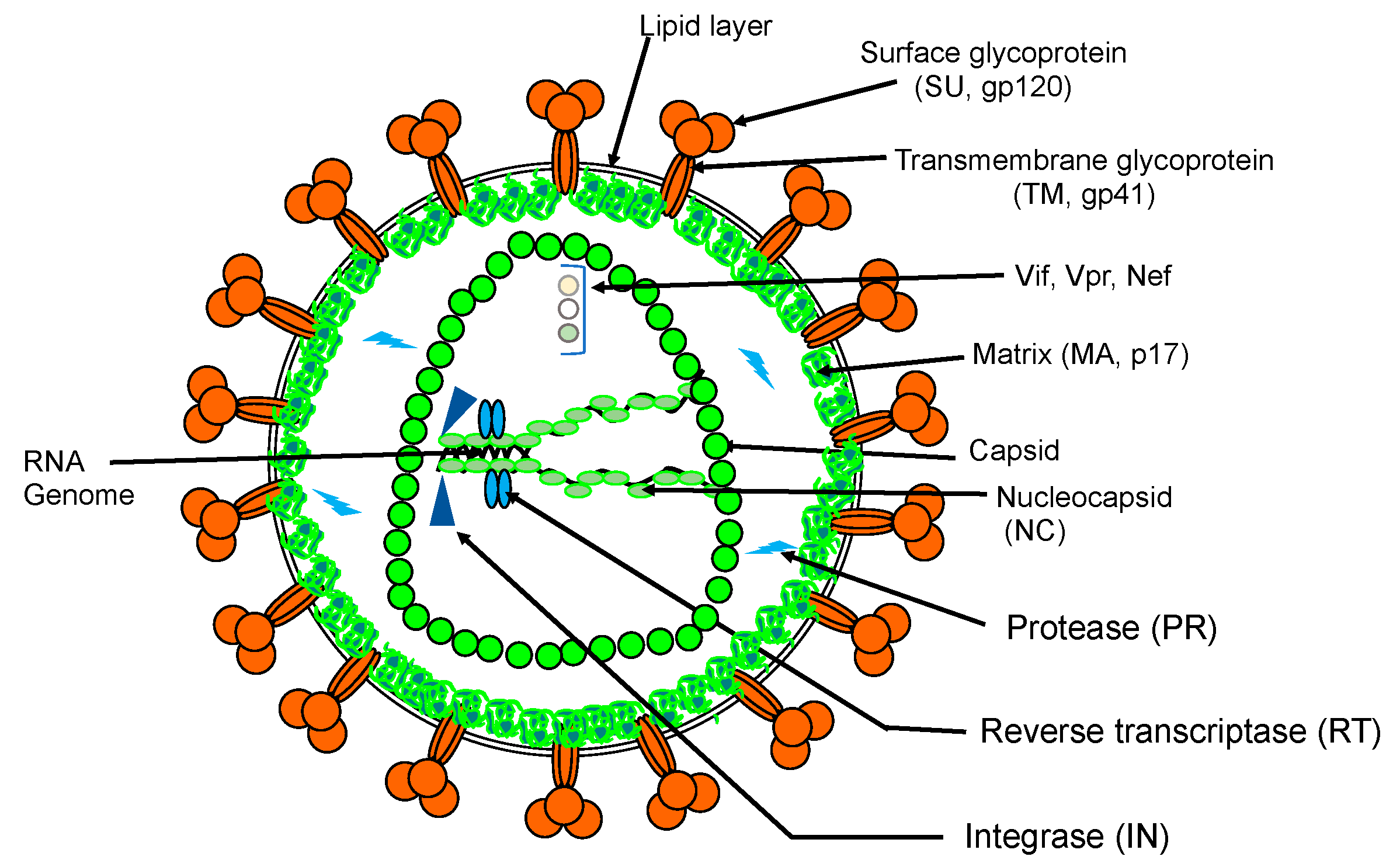
Advances in Continuous Microfluidics-Based Technologies for the Study of HIV Infection
Abstract
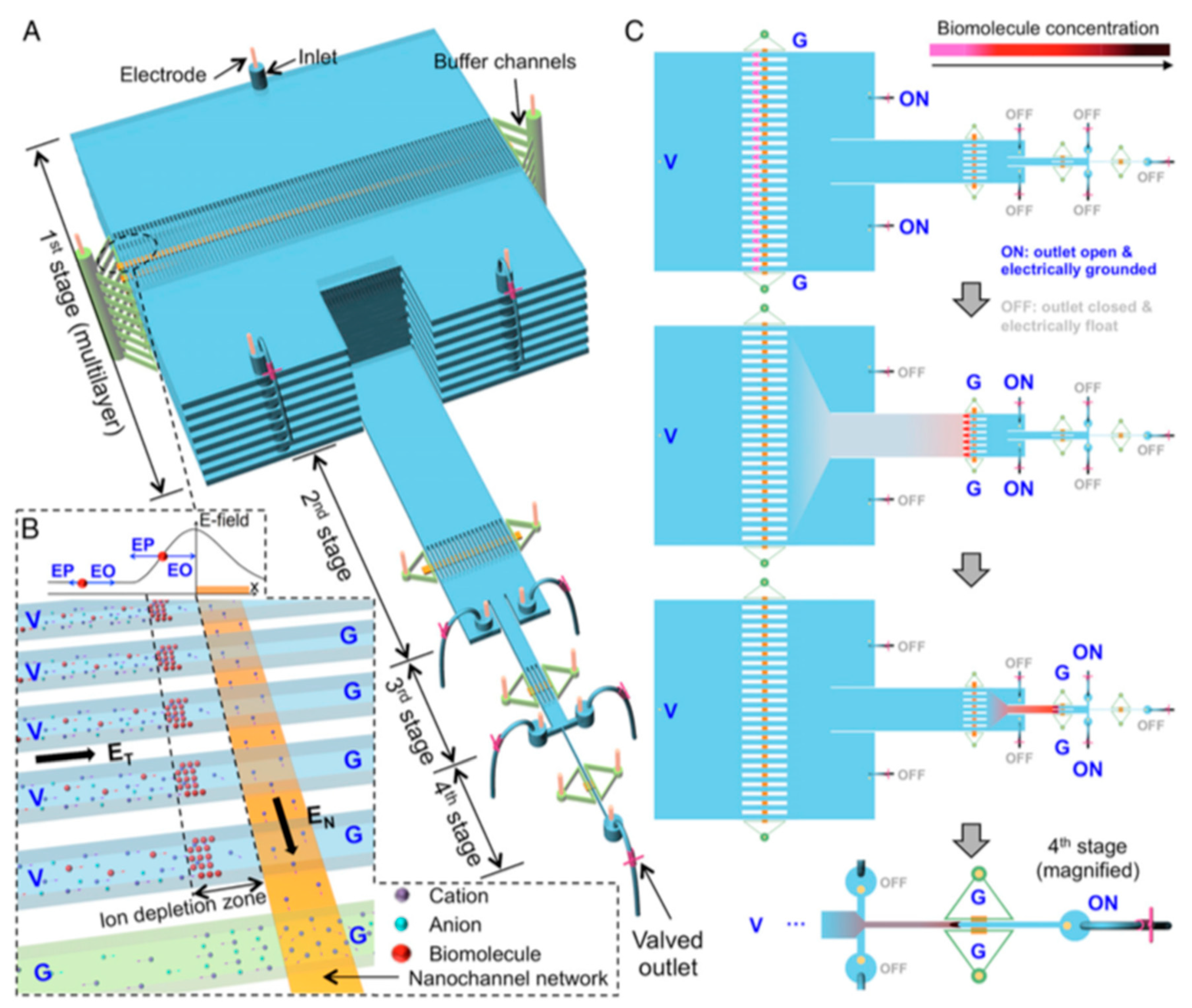
1. Continuous Microfluidics
2. Microfluidics for Studying HIV Infection
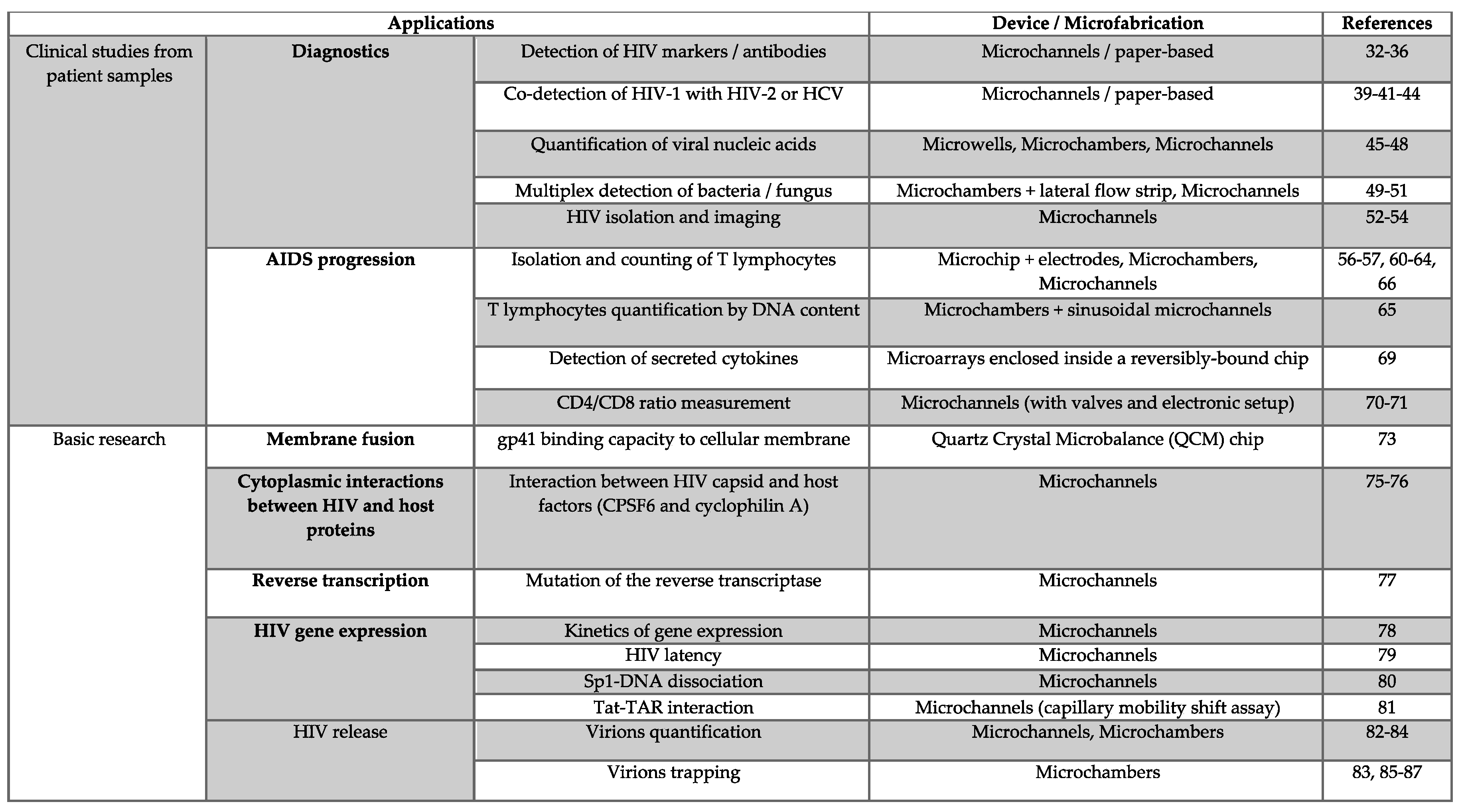
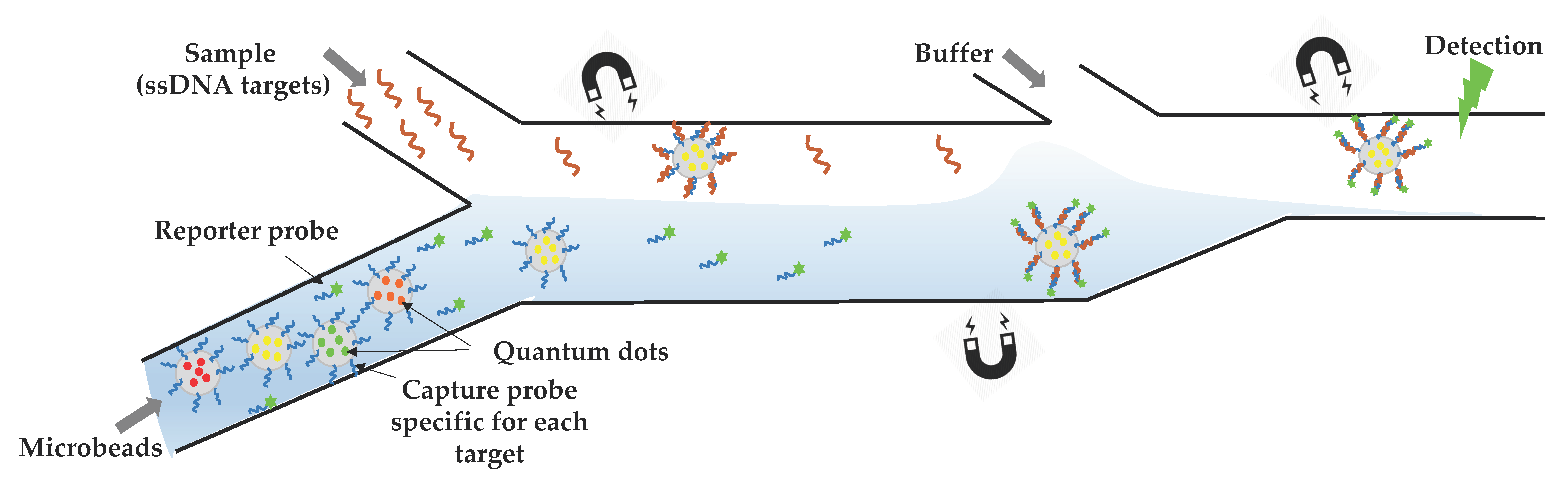
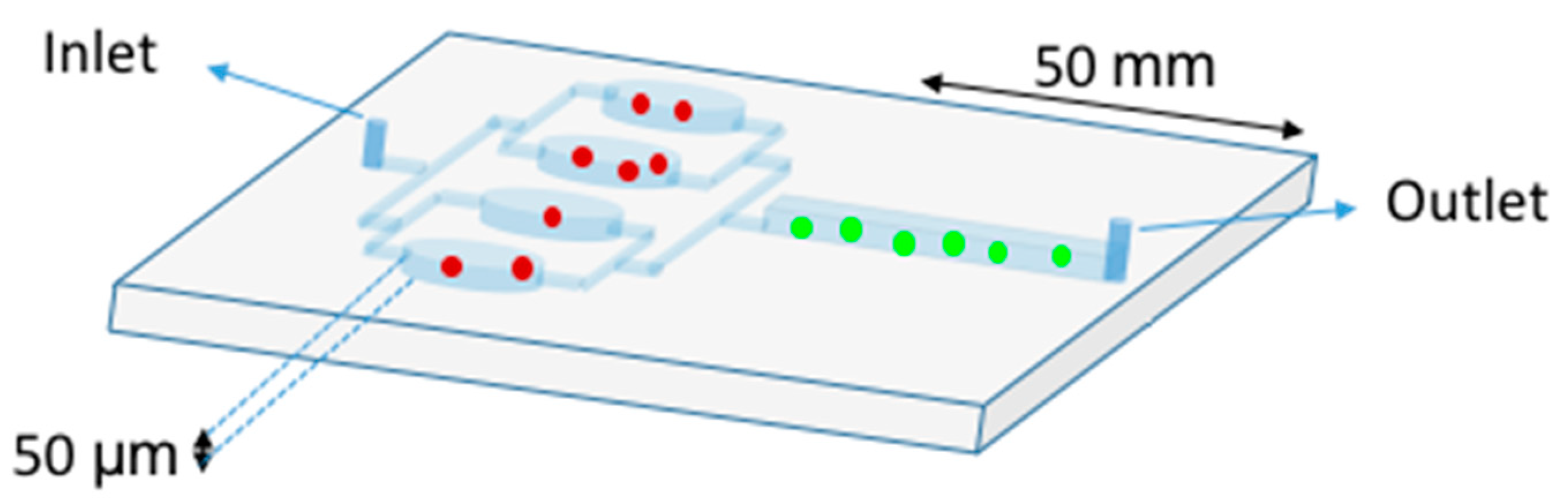
2.1. Microfluidic Tools for Diagnostics
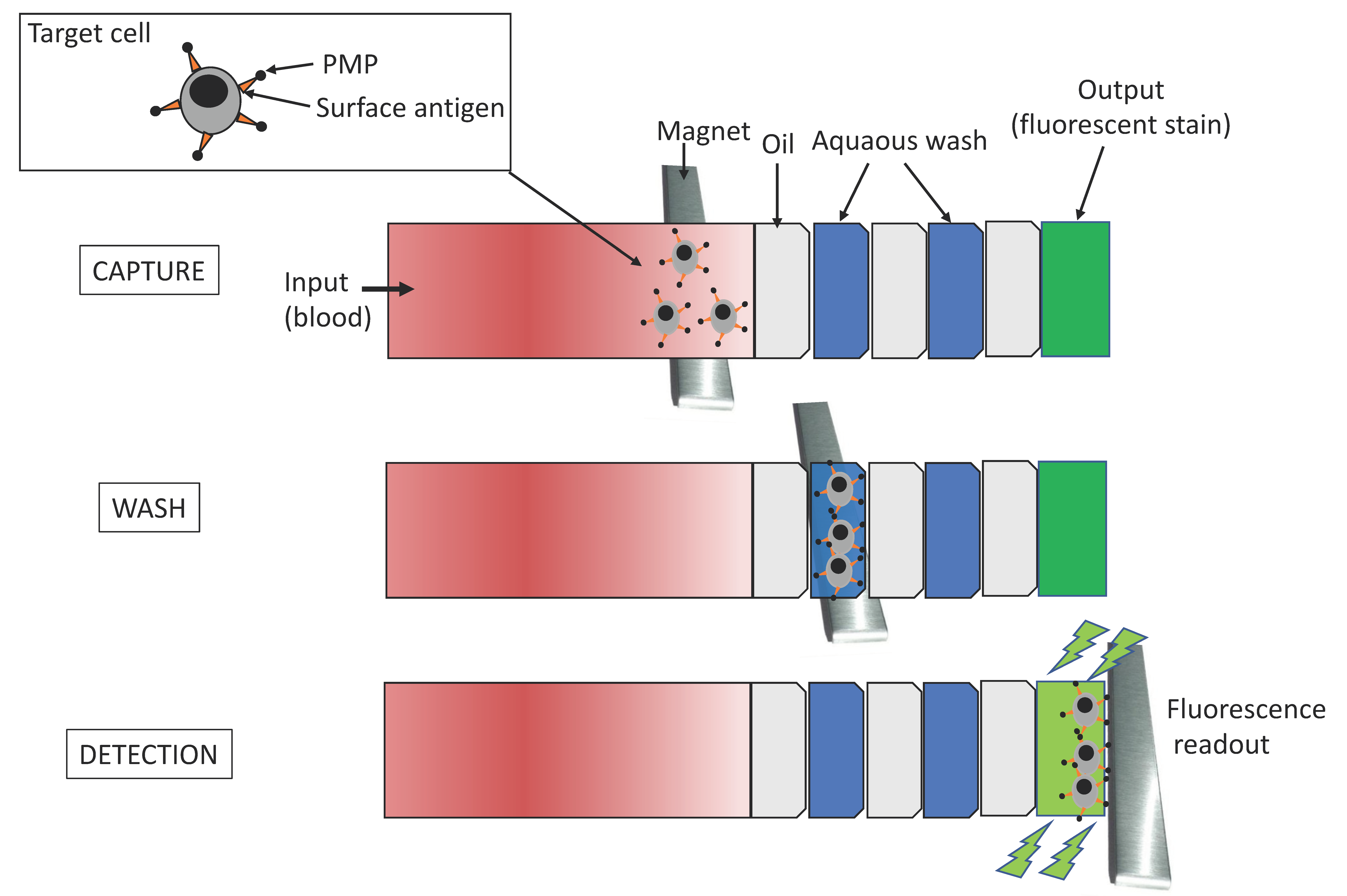
2.2. Microfluidic Tools for Monitoring AIDS Progression
2.3. Microfluidic Applications in HIV-1 Basic Research
3. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Agarwal, A. Digital microfluidics: Techniques, their applications and advantages. J. Bioeng. Biomed. Sci. 2013, 3. [Google Scholar] [CrossRef]
- Chacon, O.L.A.; Baret, J.C. Rapid stabilization of droplets by particles in microfluidics: Role of droplet formation. Chem. Syst. Chem. 2019, 1, 16–24. [Google Scholar] [CrossRef]
- Mashaghi, S.; Abbaspourrad, A.; Weitz, D.A.; van Oijen, A.M. Droplet microfluidics: A tool for biology, chemistry and nanotechnology. TrAC Trends Anal. Chem. 2016, 82, 118–125. [Google Scholar] [CrossRef]
- Malbec, R.; Chami, B.; Aeschbach, L.; Ruiz, B.G.A.; Socol, M.; Joseph, P.; Leichlé, T.; Trofimenko, E.; Bancaud, A.; Dion, V. μ LAS: Sizing of expanded trinucleotide repeats with femtomolar sensitivity in less than 5 minutes. Sci. Rep. 2019, 9, 23. [Google Scholar] [CrossRef] [PubMed]
- Sackmann, E.K.; Fulton, A.L.; Beebe, D.J. The present and future role of microfluidics in biomedical research. Nature 2014, 507, 181–189. [Google Scholar] [CrossRef]
- Socol, M.; Ranchon, H.; Chami, B.; Lesage, A.; Victor, J.M.; Bancaud, A. Contraction and tumbling dynamics of DNA in shear flows under confinement induced by transverse viscoelastic forces. Macromolecules 2019, 52, 1–28. [Google Scholar] [CrossRef]
- Socol, M.; Wang, R.; Jost, D.; Carrivain, P.; Vaillant, C.; Le Cam, E.; Dahirel, V.; Normand, C.; Bystricky, K.; Victor, J.-M.; et al. Rouse model with transient intramolecular contacts on a timescale of seconds recapitulates folding and fluctuation of yeast chromosomes. Nucleic Acids Res. 2019, 47, 6195–6207. [Google Scholar] [CrossRef]
- Bazant, M.Z.; Squires, T.M. Induced-charge electrokinetic phenomena: Theory and microfluidic applications. Phys. Rev. Lett. 2004, 92, 1–4. [Google Scholar] [CrossRef]
- Whitesides, G.M. The origins and the future of microfluidics. Nature 2006, 442, 368–373. [Google Scholar] [CrossRef]
- Gale, B.; Jafek, A.; Lambert, C.; Goenner, B.; Moghimifam, H.; Nze, U.; Kamarapu, S.K. A review of current methods in microfluidic device fabrication and future commercialization prospects. Inventions 2018, 3, 60. [Google Scholar] [CrossRef]
- Hamon, M.; Hong, J.W. New tools and new biology: Recent miniaturized systems for molecular and cellular biology. Mol. Cells 2013, 36, 485–506. [Google Scholar] [CrossRef] [PubMed]
- De Jong, J.; Lammertink, R.G.; Wessling, M. Membranes and microfluidics: A review. Lab Chip 2006, 6, 1125–1139. [Google Scholar] [CrossRef] [PubMed]
- Rettig, J.R.; Folch, A. Large-scale single-cell trapping and imaging using microwell arrays. Anal. Chem. 2005, 77, 5628–5634. [Google Scholar] [CrossRef] [PubMed]
- Rudenko, M.I.; Holmes, M.R.; Ermolenko, D.N.; Lunt, E.J.; Gerhardt, S.; Noller, H.F.; Deamer, D.W.; Hawkins, A.; Schmidt, H. Controlled gating and electrical detection of single 50S ribosomal subunits through a solid-state nanopore in a microfluidic chip. Biosens. Bioelectron. 2011, 29, 34–39. [Google Scholar] [CrossRef]
- Jang, J.; Park, J.Y.; Gao, G.; Cho, D.W. Biomaterials-based 3D cell printing for next-generation therapeutics and diagnostics. Biomaterials 2018, 156, 88–106. [Google Scholar] [CrossRef]
- Gong, M.M.; Sinton, D. Turning the page: Advancing paper-based microfluidics for broad diagnostic application. Chem. Rev. 2017, 117, 8447–8480. [Google Scholar] [CrossRef]
- Li, X.; Ballerini, D.R.; Shen, W. A perspective on paper-based microfluidics: Current status and future trends. Biomicrofluidics 2012, 6, 11301–1130113. [Google Scholar] [CrossRef]
- Margolis, D.M.; Archin, N.M.; Cohen, M.S.; Eron, J.J.; Ferrari, G.; Garcia, J.V.; Gay, C.L.; Goonetilleke, N.; Joseph, S.B.; Swanstrom, R.; et al. Curing HIV: Seeking to target and clear persistent infection. Cell 2020, 181, 189–206. [Google Scholar] [CrossRef]
- Mocroft, A.; Vella, S.; Benfield, T.L.; Chiesi, A.; Miller, V.; Gargalianos, P.; d’Arminio, M.A.; Yust, I.; Bruun, J.N.; Phillips, A.N.; et al. Changing patterns of mortality across Europe in patients infected with HIV-1. Lancet 1998, 352, 1725–1730. [Google Scholar] [CrossRef]
- Chupradit, K.; Moonmuang, S.; Nangola, S.; Kitidee, K.; Yasamut, U.; Mougel, M.; Tayapiwatana, C. Current peptide and protein candidates challenging HIV therapy beyond the vaccine era. Viruses 2017, 9, 281. [Google Scholar] [CrossRef]
- Yeo, J.Y.; Goh, G.R.; Su, C.T.; Gan, S.K. The determination of HIV-1 RT mutation rate, its possible allosteric effects, and its implications on drug resistance. Viruses 2020, 12, 297. [Google Scholar] [CrossRef] [PubMed]
- Cohn, L.B.; Chomont, N.; Deeks, S.G. The biology of the HIV-1 latent reservoir and implications for cure strategies. Cell Host Microbe 2020, 27, 519–530. [Google Scholar] [CrossRef]
- Holmes, M.; Zhang, F.; Bieniasz, P.D. Single-cell and single-cycle analysis of HIV-1 replication. PLoS Pathog. 2015, 11, e1004961. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, P.; Desfarges, S.; Bartha, I.; Joos, B.; Zangger, N.; Munoz, M.; Günthard, H.F.; Beerenwinkel, N.; Telenti, A.; Ciuffi, A. 24 hours in the life of HIV-1 in a T cell line. PLoS Pathog. 2013, 9, e1003161. [Google Scholar] [CrossRef] [PubMed]
- Mougel, M.; Houzet, L.; Darlix, J.L. When is it time for reverse transcription to start and go? Retrovirology 2009, 6, 24. [Google Scholar] [CrossRef]
- Burdick, R.C.; Li, C.; Munshi, M.; Rawson, J.M.O.; Nagashima, K.; Hu, W.S.; Pathak, V.K. HIV-1 uncoats in the nucleus near sites of integration. Proc. Natl. Acad. Sci. USA 2020, 117, 5486–5493. [Google Scholar] [CrossRef] [PubMed]
- Dharan, A.; Bachmann, N.; Talley, S.; Zwikelmaier, V.; Campbell, E.M. Nuclear pore blockade reveals that HIV-1 completes reverse transcription and uncoating in the nucleus. Nat. Microbiol. 2020, 5, 1088–1095. [Google Scholar] [CrossRef]
- Guerrero, S.; Batisse, J.; Libre, C.; Bernacchi, S.; Marquet, R.; Paillart, J.C. HIV-1 replication and the cellular eukaryotic translation apparatus. Viruses 2015, 7, 199–218. [Google Scholar] [CrossRef]
- Ferrer, M.; Clerte, C.; Chamontin, C.; Basyuk, E.; Laine, S.; Hottin, J.; Bertrand, E.; Margeat, E.; Mougel, M. Imaging HIV-1 RNA dimerization in cells by multicolor super-resolution and fluctuation microscopies. Nucleic Acids Res. 2016, 44, 7922–7934. [Google Scholar] [CrossRef]
- Mailler, E.; Bernacchi, S.; Marquet, R.; Paillart, J.C.; Vivet-Boudou, V.; Smyth, R.P. The life-cycle of the HIV-1 gag-RNA complex. Viruses 2016, 8, 248. [Google Scholar] [CrossRef]
- Hurt, C.B.; Nelson, J.A.E.; Hightow-Weidman, L.B.; Miller, W.C. Selecting an HIV test: A narrative review for clinicians and researchers. Sex. Transm. Dis. 2017, 44, 739–746. [Google Scholar] [CrossRef] [PubMed]
- Hughes, A.J.; Herr, A.E. Microfluidic western blotting. Proc. Natl. Acad. Sci. USA 2012, 109, 21450–21455. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Du, B.; Zhang, P.; Haleyurgirisetty, M.; Zhao, J.; Ragupathy, V.; Lee, S.; De Voe, D.L.; Hewlett, I.K. Development of a microchip Europium nanoparticle immunoassay for sensitive point-of-care HIV detection. Biosens. Bioelectron. 2014, 61, 177–183. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, W.; Han, J. Universal amplification-free molecular diagnostics by billion-fold hierarchical nanofluidic concentration. Proc. Natl. Acad. Sci. USA 2019, 116, 16240–16249. [Google Scholar] [CrossRef]
- Sia, S.K.; Linder, V.; Parviz, B.A.; Siegel, A.; Whitesides, G.M. An integrated approach to a portable and low-cost immunoassay for resource-poor settings. Angew. Chem. Int. Ed. 2004, 43, 498–502. [Google Scholar] [CrossRef]
- Li, X.; Liu, X. A microfluidic paper-based origami nanobiosensor for label-free, ultrasensitive immunoassays. Adv. Healthc. Mater. 2016, 5, 1326–1335. [Google Scholar] [CrossRef] [PubMed]
- Chew, K.W.; Bhattacharya, D. Virologic and immunologic aspects of HIV-hepatitis C virus coinfection. AIDS 2016, 30, 2395–2404. [Google Scholar] [CrossRef]
- Requena, S.; Caballero, E.; Lozano, A.B.; Rios-Villegas, M.J.; Benito, R.; Rojo, S.; Cabezas, T.; Macià, M.D.; Nieto, M.D.C.; Soriano, V.; et al. Treatment outcome in dually HIV-1 and HIV-2 coinfected patients living in Spain. AIDS 2019, 33, 2167–2172. [Google Scholar] [CrossRef]
- Corstjens, P.L.A.M.; Chen, Z.; Zuiderwijk, M.; Bau, H.H.; Abrams, W.R.; Malamud, D.; Niedbala, R.S.; Tanke, H.J. Rapid assay format for multiplex detection of humoral immune responses to infectious disease pathogens (HIV, HCV, and TB). Ann. N. Y. Acad. Sci. 2007, 1098, 437–445. [Google Scholar] [CrossRef]
- Klostranec, J.M.; Xiang, Q.; Farcas, G.A.; Lee, J.A.; Rhee, A.; Lafferty, E.I.; Perrault, S.D.; Kain, K.C.; Chan, W.C.W. Convergence of quantum dot barcodes with microfluidics and signal processing for multiplexed high-throughput infectious disease diagnostics. Nano Lett. 2007, 7, 2812–2818. [Google Scholar] [CrossRef]
- Zhao, C.; Liu, X. A portable paper-based microfluidic platform for multiplexed electrochemical detection of human immunodeficiency virus and hepatitis C virus antibodies in serum. Biomicrofluidics 2016, 10, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Mendoza, C.; Lozano, A.B.; Caballero, E.; Cabezas, T.; Ramos, J.M.; Soriano, V. Antiretroviral therapy for HIV-2 infection in non-endemic regions. AIDS Rev. 2020, 22, 44–56. [Google Scholar] [CrossRef] [PubMed]
- Nsagha, D.S.; Njunda, A.L.; Kamga, H.L.; Assob, J.C.; Bongkem, E.A. HIV-1/HIV-2 co-infection among voluntary counselling and testing subjects at a regional hospital in Cameroon. Afr. Health Sci. 2012, 12, 276–281. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Sun, J.; Zou, Y.; Chen, W.; Zhang, W.; Xi, J.J.; Jiang, X. Barcoded microchips for biomolecular assays. Anal. Chem. 2015, 87, 900–906. [Google Scholar] [CrossRef] [PubMed]
- Selck, D.A.; Karymov, M.A.; Sun, B.; Ismagilov, R.F. Increased robustness of single-molecule counting with microfluidics, digital isothermal amplification, and a mobile phone versus real-time kinetic measurements. Anal. Chem. 2013, 85, 11129–11136. [Google Scholar] [CrossRef] [PubMed]
- Myers, F.B.; Henrikson, R.H.; Bone, J.; Lee, L.P. A handheld point-of-care genomic diagnostic system. PLoS ONE 2013, 8, e70266. [Google Scholar] [CrossRef]
- Prakash, R.; Kaler, K.V.I.S. An integrated genetic analysis microfluidic platform with valves and a PCR chip reusability method to avoid contamination. Microfluidics Nanofluidics 2007, 177–187. [Google Scholar] [CrossRef]
- Banerjee, I.; Aralaguppe, S.G.; Lapins, N.; Zhang, W.; Kazemzadeh, A.; Sonnerborg, A.; Neogi, U.; Russom, A. Microfluidic centrifugation assisted precipitation based DNA quantification. Lab Chip 2019, 19, 1657–1664. [Google Scholar] [CrossRef]
- Abrams, W.R.; Barber, C.A.; McCann, K.; Tong, G.; Chen, Z.; Mauk, M.G.; Neogi, U.; Russom, A. Development of a microfluidic device for detection of pathogens in oral samples using upconverting phosphor technology (UPT). Ann. N. Y. Acad. Sci. 2007, 1098, 375–388. [Google Scholar] [CrossRef]
- Gao, Y.; Lam, A.W.; Chan, W.C. Automating quantum dot barcode assays using microfluidics and magnetism for the development of a point-of-care device. ACS Appl. Mater. Interfaces 2013, 5, 2853–2860. [Google Scholar] [CrossRef]
- Xu, L.; Kong, J. A multiplexed nucleic acid microsystem for point-of-care detection of HIV co-infection with MTB and PCP. Talanta 2013, 117, 532–535. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.G.; Moon, S.; Kuritzkes, D.R.; Demirci, U. Quantum dot-based HIV capture and imaging in a microfluidic channel. Biosens. Bioelectron. 2009, 25, 253–258. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Esfahani, M.; Gurkan, U.A.; Inci, F.; Kuritzkes, D.R.; Demirci, U. Efficient on-chip isolation of HIV subtypes. Lab Chip 2012, 12, 1508–1515. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Ip, A.; Xu, F.; Giguel, F.F.; Moon, S.; Akay, A.; Kuritzkes, D.R.; Demirci, U. Development of a microfluidic system for measuring HIV-1 viral load. Proc. SPIE Int. Soc. Opt. Eng. 2010, 7666, 76661H. [Google Scholar] [CrossRef]
- Cossarizza, A.; Chang, H.D.; Radbruch, A.; Acs, A.; Adam, D.; Adam-Klages, S.; Agace, W.W.; Aghaeepour, N.; Akdis, M.; Allez, M.; et al. Guidelines for the use of flow cytometry and cell sorting in immunological studies. Eur. J. Immunol. 2017, 47, 1584–1797. [Google Scholar] [CrossRef]
- Cheung, K.; Gawad, S.; Renaud, P. Impedance spectroscopy flow cytometry: On-chip label-free cell differentiation. Cytom. A J. Int. Soc. Anal. Cytol. 2005, 65, 124–132. [Google Scholar] [CrossRef]
- Cheng, X.; Gupta, A.; Chen, C.; Tompkins, R.G.; Rodriguez, W.; Toner, M. Enhancing the performance of a point-of-care CD4+ T-cell counting microchip through monocyte depletion for HIV/AIDS diagnostics. Lab Chip 2009, 9, 1357–1364. [Google Scholar] [CrossRef]
- Murphy, F.J.; Reen, D.J. Differential expression of function-related antigens on newborn and adult monocyte subpopulations. Immunology 1996, 89, 587–591. [Google Scholar] [CrossRef]
- Wintergerst, E.S.; Jelk, J.; Asmis, R. Differential expression of CD14, CD36 and the LDL receptor on human monocyte-derived macrophages. A novel cell culture system to study macrophage differentiation and heterogeneity. Histochem. Cell Biol. 1998, 110, 231–241. [Google Scholar] [CrossRef]
- Moon, S.; Gurkan, U.A.; Blander, J.; Fawzi, W.W.; Aboud, S.; Mugusi, F.; Kuritzkes, D.R.; Demirci, U. Enumeration of CD4+ T-cells using a portable microchip count platform in Tanzanian HIV-infected patients. PLoS ONE 2011, 6, e21409. [Google Scholar] [CrossRef]
- Moon, S.; Keles, H.O.; Ozcan, A.; Khademhosseini, A.; Haeggstrom, E.; Kuritzkes, D.; Demirci, U. Integrating microfluidics and lensless imaging for point-of-care testing. Biosens. Bioelectron. 2009, 24, 3208–3214. [Google Scholar] [CrossRef] [PubMed]
- Jokerst, J.V.; Floriano, P.N.; Christodoulides, N.; Simmons, G.W.; McDevitt, J.T. Integration of semiconductor quantum dots into nano-bio-chip systems for enumeration of CD4+ T cell counts at the point-of-need. Lab Chip 2008, 8, 2079–2090. [Google Scholar] [CrossRef] [PubMed]
- Glynn, M.; Kirby, D.; Chung, D.; Kinahan, D.J.; Kijanka, G.; Ducree, J. Centrifugo-magnetophoretic purification of CD4+ cells from whole blood toward future HIV/AIDS point-of-care applications. J. Lab. Autom. 2014, 19, 285–296. [Google Scholar] [CrossRef] [PubMed]
- Howard, A.L.; Pezzi, H.M.; Beebe, D.J.; Berry, S.M. Exclusion-based capture and enumeration of CD4+ T cells from whole blood for low-resource settings. J. Lab. Autom. 2014, 19, 313–321. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Liu, Q.; Chernish, A.; Du, V.J.A.; Ouyang, Y.; Li, J.; Qian, Q.; Bazydlo, L.A.L.; Haverstick, D.M.; Landers, J.P. The ARTμS: A novel microfluidic CD4+ T-cell enumeration system for monitoring antiretroviral therapy in HIV patients. Lab Chip 2016, 16, 506–514. [Google Scholar] [CrossRef]
- Wasserberg, D.; Zhang, X.; Breukers, C.; Connell, B.J.; Baeten, E.; van Blink, D.; Benet, E.S.; Bloem, A.C.; Nijhuis, M.; Wensing, A.M.J.; et al. All-printed cell counting chambers with on-chip sample preparation for point-of-care CD4 counting. Biosens. Bioelectron. 2018, 117, 659–668. [Google Scholar] [CrossRef]
- Pantaleo, G.; Harari, A. Functional signatures in antiviral T-cell immunity for monitoring virus-associated diseases. Nat. Rev. Immunol. 2006, 6, 417–423. [Google Scholar] [CrossRef]
- Pantaleo, G.; Koup, R.A. Correlates of immune protection in HIV-1 infection: What we know, what we don’t know, what we should know. Nat. Med. 2004, 10, 806–810. [Google Scholar] [CrossRef]
- Zhu, H.; Stybayeva, G.; Macal, M.; Ramanculov, E.; George, M.D.; Dandekar, S.; Revzin, A. A microdevice for multiplexed detection of T-cell-secreted cytokines. Lab Chip 2008, 8, 2197–2205. [Google Scholar] [CrossRef]
- Li, W.; Gao, Y.; Pappas, D. A complementary method to CD4 counting: Measurement of CD4+/CD8+ T lymphocyte ratio in a tandem affinity microfluidic system. Biomed. Microdevices 2015, 17, 1–9. [Google Scholar] [CrossRef]
- Hassan, U.; Watkins, N.N.; Reddy, B., Jr.; Damhorst, G.; Bashir, R. Microfluidic differential immunocapture biochip for specific leukocyte counting. Nat. Protoc. 2016, 11, 714–726. [Google Scholar] [CrossRef]
- Chen, B. Molecular mechanism of HIV-1 entry. Trends Microbiol. 2019, 27, 878–891. [Google Scholar] [CrossRef]
- Lu, C.H.; Zhang, Y.; Tang, S.F.; Fang, Z.B.; Yang, H.H.; Chen, X.; Chen, G.-N. Sensing HIV related protein using epitope imprinted hydrophilic polymer coated quartz crystal microbalance. Biosens. Bioelectron. 2012, 31, 439–444. [Google Scholar] [CrossRef] [PubMed]
- Novikova, M.; Zhang, Y.; Freed, E.O.; Peng, K. Multiple roles of HIV-1 capsid during the virus replication cycle. Virol. Sin. 2019, 34, 119–134. [Google Scholar] [CrossRef] [PubMed]
- Lau, D.; Walsh, J.C.; Peng, W.; Shah, V.B.; Turville, S.; Jacques, D.A.; Bocking, T. Fluorescence biosensor for real-time interaction dynamics of host proteins with HIV-1 capsid tubes. ACS Appl. Mater. Interfaces 2019, 11, 34586–34594. [Google Scholar] [CrossRef] [PubMed]
- Peng, W.; Shi, J.; Marquez, C.L.; Lau, D.; Walsh, J.; Faysal, K.M.R.; Byeon, C.H.; Byeon, I.-J.L.; Aiken, C.; Bocking, T. Functional analysis of the secondary HIV-1 capsid binding site in the host protein cyclophilin A. Retrovirology 2019, 16, 10. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, J.; Roebelen, J.; Tripathi, A. A simple microfluidic assay for the detection of ligation product. Mol. Diagn. Ther. 2015, 19, 59–64. [Google Scholar] [CrossRef]
- Razooky, B.S.; Gutierrez, E.; Terry, V.H.; Spina, C.A.; Groisman, A.; Weinberger, L.S. Microwell devices with finger-like channels for long-term imaging of HIV-1 expression kinetics in primary human lymphocytes. Lab Chip 2012, 12, 4305–4312. [Google Scholar] [CrossRef]
- Ramji, R.; Wong, V.C.; Chavali, A.K.; Gearhart, L.M.; Miller-Jensen, K. A passive-flow microfluidic device for imaging latent HIV activation dynamics in single T cells. Integr. Biol. 2015, 7, 998–1010. [Google Scholar] [CrossRef]
- Yeh, H.C.; Puleo, C.M.; Lim, T.C.; Ho, Y.P.; Giza, P.E.; Huang, R.C.; Wang, T.-H. A microfluidic-FCS platform for investigation on the dissociation of Sp1-DNA complex by doxorubicin. Nucleic Acids Res. 2006, 34, 1–9. [Google Scholar] [CrossRef]
- Fourtounis, J.; Falgueyret, J.P.; Sayegh, C.E. Assessing protein-RNA interactions using microfluidic capillary mobility shift assays. Anal. Biochem. 2011, 411, 161–163. [Google Scholar] [CrossRef] [PubMed]
- Vu, H.N.; Li, Y.; Casali, M.; Irimia, D.; Megeed, Z.; Yarmush, M.L. A microfluidic bioreactor for increased active retrovirus output. Lab Chip 2008, 8, 75–80. [Google Scholar] [CrossRef] [PubMed]
- Pang, Y.; Song, H.; Kim, J.H.; Hou, X.; Cheng, W. Optical trapping of individual human immunodeficiency viruses in culture fluid reveals heterogeneity with single-molecule resolution. Nat. Nanotechnol. 2014, 9, 624–630. [Google Scholar] [CrossRef] [PubMed]
- Steppert, P.; Burgstaller, D.; Klausberger, M.; Tover, A.; Berger, E.; Jungbauer, A. Quantification and characterization of virus-like particles by size-exclusion chromatography and nanoparticle tracking analysis. J. Chromatogr. A 2017, 1487, 89–99. [Google Scholar] [CrossRef]
- Surawathanawises, K.; Kundrod, K.; Cheng, X. Microfluidic devices with templated regular macroporous structures for HIV viral capture. Analyst 2016, 141, 1669–1677. [Google Scholar] [CrossRef]
- Shafiee, H.; Jahangir, M.; Inci, F.; Wang, S.; Willenbrecht, R.B.; Giguel, F.F.; Tsibris, A.M.N.; Kuritzkes, D.R.; Demirci, U. Acute on-chip HIV detection through label-free electrical sensing of viral nano-lysate. Small 2013, 9, 2553–2563. [Google Scholar] [CrossRef]
- Shafiee, H.; Kanakasabapathy, M.K.; Juillard, F.; Keser, M.; Sadasivam, M.; Yuksekkaya, M.; Hanhauser, E.; Henrich, T.J.; Kuritzkes, D.R.; Kaye, K.M.; et al. Printed flexible plastic microchip for viral load measurement through quantitative detection of viruses in plasma and saliva. Sci. Rep. 2015, 5, 9919. [Google Scholar] [CrossRef]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eid, J.; Mougel, M.; Socol, M. Advances in Continuous Microfluidics-Based Technologies for the Study of HIV Infection. Viruses 2020, 12, 982. https://doi.org/10.3390/v12090982
Eid J, Mougel M, Socol M. Advances in Continuous Microfluidics-Based Technologies for the Study of HIV Infection. Viruses. 2020; 12(9):982. https://doi.org/10.3390/v12090982
Chicago/Turabian StyleEid, Joëlle, Marylène Mougel, and Marius Socol. 2020. "Advances in Continuous Microfluidics-Based Technologies for the Study of HIV Infection" Viruses 12, no. 9: 982. https://doi.org/10.3390/v12090982
APA StyleEid, J., Mougel, M., & Socol, M. (2020). Advances in Continuous Microfluidics-Based Technologies for the Study of HIV Infection. Viruses, 12(9), 982. https://doi.org/10.3390/v12090982