Functional Characterization of Pepper Vein Banding Virus-Encoded Proteins and Their Interactions: Implications in Potyvirus Infection
Abstract
1. Introduction
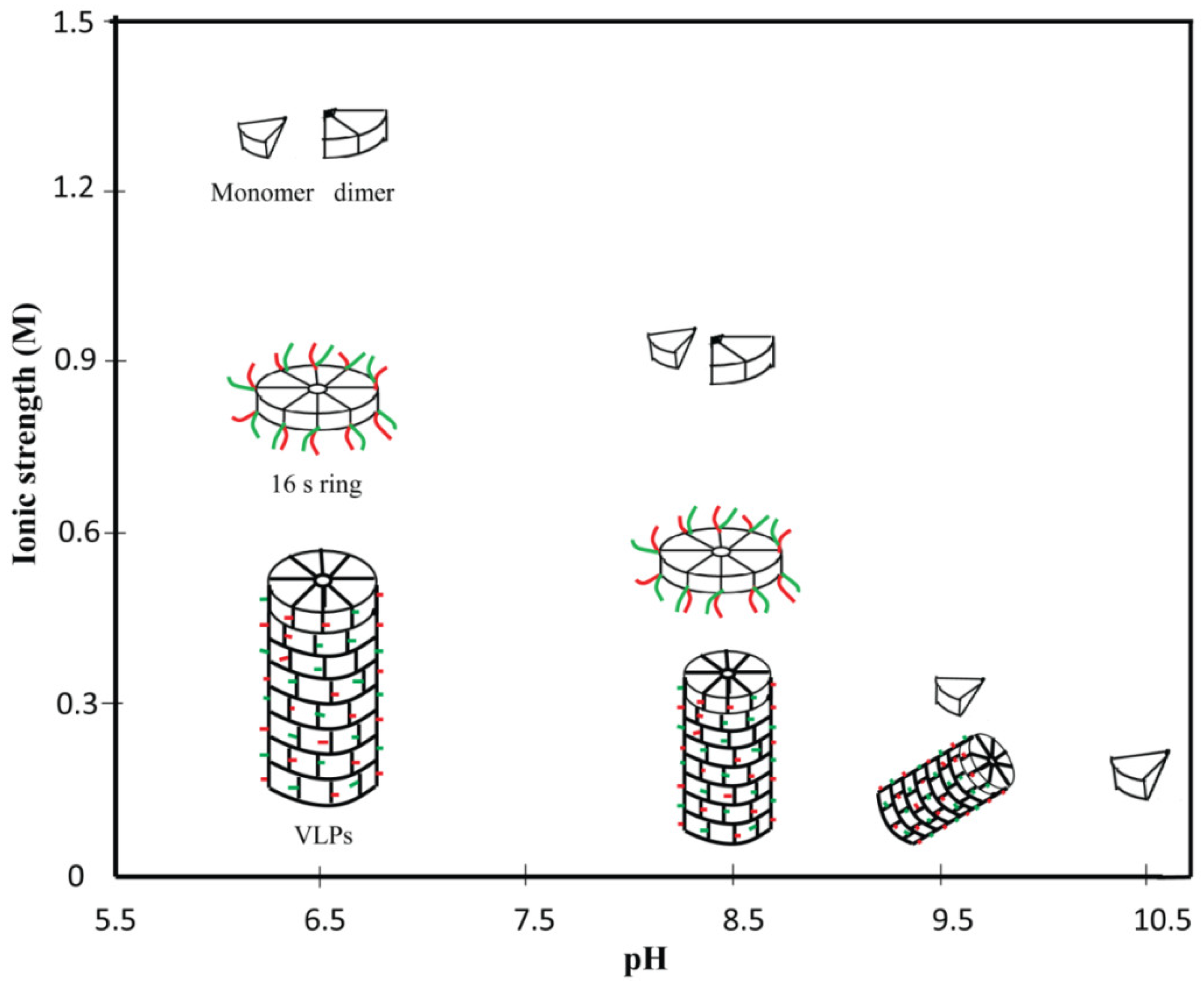
2. Disassembly and Reassembly of PVBV
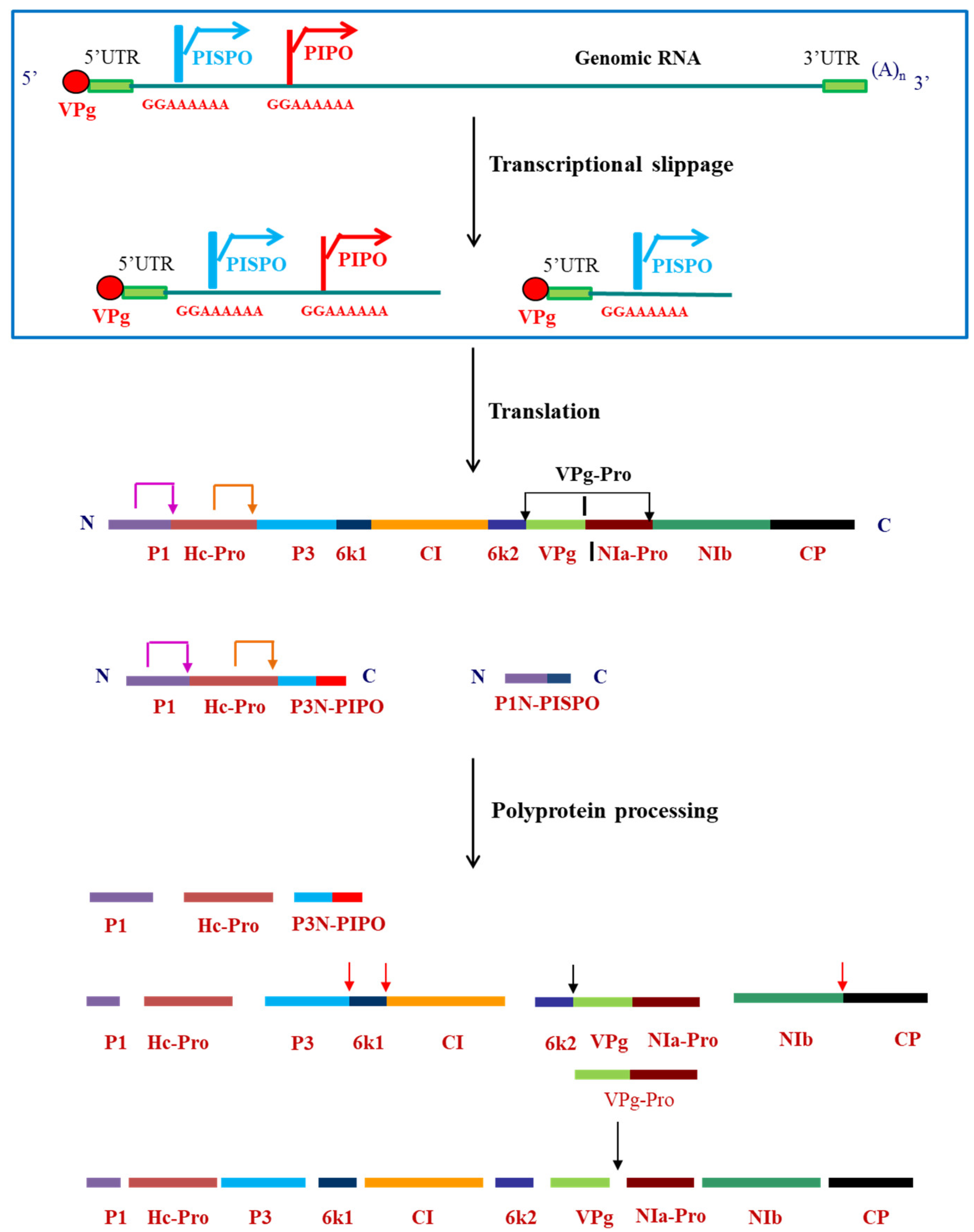
3. Polyprotein Processing: Multifunctional VPg-Pro–the Major Player
4. Replication of PVBV—Highlighting the Multifunctional NIb
5. Regulation of the Potyviral Proteins during Various Events in the Viral Life Cycle
6. Applications of Potyviruses in Biotechnology: Characterization of PVBV VLPs as Nanoparticles and Applications of PVBV Chimeric VLPs
7. Conclusions and Future Prospects
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ward, C.W.; Shukla, D.D. Taxonomy of potyviruses: Current problems and some solutions. Intervirology 1991, 32, 269–296. [Google Scholar] [CrossRef] [PubMed]
- Martínez, F.; Rodrigo, G.; Aragonés, V.; Ruiz, M.; Lodewijk, I.; Fernández, U.; Elena, S.F.; Daròs, J.-A. Interaction network of tobacco etch potyvirus NIa protein with the host proteome during infection. BMC Genom. 2016, 17, 87. [Google Scholar] [CrossRef] [PubMed]
- Revers, F.; García, J.A. Molecular Biology of Potyviruses. Adv. Virus Res. 2015, 92, 101–199. [Google Scholar] [CrossRef] [PubMed]
- Ravi, K.S.; Joseph, J.; Nagaraju, N.; Prasad, S.K.; Reddy, H.R.; Savithri, H.S. Characterization of a Pepper Vein Banding Virus from Chili Pepper in India. Plant Dis. 1997, 81, 673–676. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Roossinck, M.J. Plant Virus Metagenomics: Biodiversity and Ecology. Annu. Rev. Genet. 2012, 46, 359–369. [Google Scholar] [CrossRef]
- Nigam, D.; LaTourrette, K.; Souza, P.F.N.; Garcia-Ruiz, H. Genome-Wide Variation in Potyviruses. Front. Plant Sci. 2019, 10, 1439. [Google Scholar] [CrossRef]
- King, A.M.Q.; Adams, M.J.; Carsten, E.B.; Lefkowitz, E.J. Virus Taxonomy: Classification and Nomenclature of Viruses. Ninth Report of the International Committee on Taxonomy of Viruses; Elsevier Academic Press: San Diego, CA, USA, 2012; ISBN 978-0-12-384684-6. [Google Scholar]
- Valli, A.A.; Gallo, A.; Rodamilans, B.; López-Moya, J.J.; García, J.A. The HCPro from the Potyviridae family: An enviable multitasking Helper Component that every virus would like to have. Mol. Plant Pathol. 2018, 19, 744–763. [Google Scholar] [CrossRef]
- Joseph, J.; Savithri, H.S. Determination of 3′-terminal nucleotide sequence of pepper vein banding virus RNA and expression of its coat protein in Escherichia coli. Arch. Virol. 1999, 144, 1679–1687. [Google Scholar] [CrossRef]
- Anindya, R.; Joseph, J.; Gowri, T.D.S.; Savithri, H.S. Complete genomic sequence of Pepper vein banding virus ( PVBV ): A distinct member of the genus Potyvirus. Arch. Virol. 2004, 149, 625–632. [Google Scholar] [CrossRef]
- Chung, B.Y.; Miller, W.A.; Atkins, J.F.; Firth, A.E. An overlapping essential gene in the Potyviridae. Proc. Natl. Acad. Sci. USA 2008, 105, 5897–5902. [Google Scholar] [CrossRef]
- Untiveros, M.; Olspert, A.; Artola, K.; Firth, A.E.; Kreuze, J.F.; Valkonen, J.P.T. A novel sweet potato potyvirus open reading frame (ORF) is expressed via polymerase slippage and suppresses RNA silencing. Mol. Plant Pathol. 2016, 17, 1111–1123. [Google Scholar] [CrossRef]
- Mingot, A.; Valli, A.; Rodamilans, B.; San León, D.; Baulcombe, D.C.; García, J.A.; López-Moya, J.J. The P1N-PISPO Trans-Frame Gene of Sweet Potato Feathery Mottle Potyvirus Is Produced during Virus Infection and Functions as an RNA Silencing Suppressor. J. Virol. 2016, 90, 3543–3557. [Google Scholar] [CrossRef] [PubMed]
- Fink, A.L. Natively Unfolded Proteins. Curr. Opin. Struct. Biol. 2005, 15, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Uversky, V.N. Intrinsically disordered proteins and their “Mysterious” (meta)physics. Front. Phys. 2019, 7, 10. [Google Scholar] [CrossRef]
- Xue, B.; Williams, R.W.; Oldfield, C.J.; Kian-Meng, G.G.; Dunker, A.K.; Uversky, V.N. Viral Disorder or Disordered Viruses: Do Viral Proteins Possess Unique Features? Protein Pept. Lett. 2010, 17, 932–951. [Google Scholar] [CrossRef]
- Uversky, V.N. The Mysterious Unfoldome: Structureless, Underappreciated, Yet Vital Part of Any Given Proteome. J. Biomed. Biotechnol. 2010, 2010, 568068. [Google Scholar] [CrossRef]
- Eliezer, D.; Palmer, A.G. Biophysics: Proteins hunt and gather. Nature 2007, 447, 920–921. [Google Scholar] [CrossRef]
- Uversky, V.N. Intrinsically Disordered Proteins and Their Environment: Effects of Strong Denaturants, Temperature, pH, Counter Ions, Membranes, Binding Partners, Osmolytes, and Macromolecular Crowding. Protein J. 2009, 28, 305–325. [Google Scholar] [CrossRef]
- Wright, P.E.; Dyson, H.J. Linking folding and binding. Curr. Opin. Struct. Biol. 2009, 19, 31–38. [Google Scholar] [CrossRef]
- Makarov, V.V.; Kalinina, N.O. Structure and noncanonical activities of coat proteins of helical plant viruses. Biochemistry 2016, 81, 1–18. [Google Scholar] [CrossRef]
- Ksenofontov, A.L.; Paalme, V.; Arutyunyan, A.M.; Semenyuk, P.I.; Fedorova, N.V.; Rumvolt, R.; Baratova, L.A.; Järvekülg, L.; Dobrov, E.N. Partially Disordered Structure in Intravirus Coat Protein of Potyvirus Potato Virus, A. PLoS ONE 2013, 8, e67830. [Google Scholar] [CrossRef] [PubMed]
- Charon, J.; Theil, S.; Nicaise, V.; Michon, T. Protein intrinsic disorder within the Potyvirus genus: From proteome-wide analysis to functional annotation. Mol. Biosyst. 2016, 12, 634–652. [Google Scholar] [CrossRef] [PubMed]
- Mathur, C.; Jimsheena, V.K.; Banerjee, S.; Makinen, K.; Gowda, L.R.; Savithri, H.S. Functional regulation of PVBV Nuclear Inclusion protein-A protease activity upon interaction with Viral Protein genome-linked and phosphorylation. Virology 2012, 422, 254–264. [Google Scholar] [CrossRef]
- Jiang, J.; Laliberté, J.F. The genome-linked protein VPg of plant viruses—A protein with many partners. Curr. Opin. Virol. 2011, 1, 347–354. [Google Scholar] [CrossRef]
- Grzela, R.; Szolajska, E.; Ebel, C.; Madern, D.; Favier, A.; Wojtal, I.; Zagorski, W.; Chroboczek, J. Virulence factor of potato virus Y, genome-attached terminal protein VPg, is a highly disordered protein. J. Biol. Chem. 2008, 283, 213–221. [Google Scholar] [CrossRef]
- Rantalainen, K.I.; Christensen, P.A.; Hafrén, A.; Otzen, D.E.; Kalkkinen, N.; Mäkinen, K. Interaction of a potyviral VPg with anionic phospholipid vesicles. Virology 2009, 395, 114–120. [Google Scholar] [CrossRef]
- Sabharwal, P.; Srinivas, S.; Savithri, H.S. Mapping the domain of interaction of PVBV VPg with NIa-Pro: Role of N-terminal disordered region of VPg in the modulation of structure and function. Virology 2018, 524, 2. [Google Scholar] [CrossRef]
- Katuwawala, A.; Peng, Z.; Yang, J.; Kurgan, L. Computational Prediction of MoRFs, Short Disorder-to-order Transitioning Protein Binding Regions. Comput. Struct. Biotechnol. J. 2019, 17, 454–462. [Google Scholar] [CrossRef]
- Klug, A. The tobacco mosaic virus particle: Structure and assembly. Philos. Trans. R. Soc. Lond. B 1999, 354, 1–6. [Google Scholar] [CrossRef]
- Anindya, R.; Savithri, H.S. Surface-exposed amino- and carboxy-terminal residues are crucial for the initiation of assembly in Pepper vein banding virus: A flexuous rod-shaped virus. Virology 2003, 316, 325–336. [Google Scholar] [CrossRef]
- McDonald, M.; Kendall, A.; Bian, W.; McCullough, I.; Lio, E.; Havens, W.M.; Ghabrial, S.A.; Stubbs, G. Architecture of the potyviruses. Virology 2010, 405, 309–313. [Google Scholar] [CrossRef] [PubMed]
- Ivanov, K.I.; Puustinen, P.; Merits, A.; Saarma, M.; Mäkinen, K. Phosphorylation down-regulates the RNA binding function of the coat protein of potato virus A. J. Biol. Chem. 2001, 276, 13530–13540. [Google Scholar] [CrossRef] [PubMed]
- Hervás, M.; Navajas, R.; Chagoyen, M.; García, J.A.; Martínez-Turiño, S. Phosphorylation-related crosstalk between distant regions of the core region of the coat protein contributes to virion assembly of plum pox virus. Mol. Plant Microbe Interact. 2020, 33, 653–667. [Google Scholar] [CrossRef] [PubMed]
- Comer, F.I.; Hart, G.W. O-glycosylation of nuclear and cytosolic proteins. Dynamic interplay between O-GlcNAc and O-phosphate. J. Biol. Chem. 2000, 275, 29179–29182. [Google Scholar] [CrossRef] [PubMed]
- Hervás, M.; Ciordia, S.; Navajas, R.; García, J.A.; Martínez-Turiño, S. Common and Strain-Specific Post-Translational Modifications of the Potyvirus Plum pox virus Coat Protein in Different Hosts. Viruses 2020, 12, 308. [Google Scholar] [CrossRef] [PubMed]
- Pasin, F.; Simón-Mateo, C.; García, J.A. The Hypervariable Amino-Terminus of P1 Protease Modulates Potyviral Replication and Host Defense Responses. PLoS Pathog. 2014, 10, e1003985. [Google Scholar] [CrossRef]
- Merits, A.; Lindholm, P.; Runeberg-Roos, P.; Kekarainen, T.; Rajamäki, M.-L.; Puustinen, P.; Mäkeläinen, K.; Saarma, M. Proteolytic processing and membrane-association of potyviral proteins in insect cells. Enviado Virol. 2000, 83, 1211–1221. [Google Scholar] [CrossRef]
- Urcuqui-Inchima, S.; Haenni, A.L.; Bernardi, F. Potyvirus proteins: A wealth of functions. Virus Res. 2001, 74, 157–175. [Google Scholar] [CrossRef]
- Dougherty, W.G.; Parks, T.D. Post-translational processing of the tobacco etch virus 49-kDa small nuclear inclusion polyprotein: Identification of an internal cleavage site and delimitation of VPg and proteinase domains. Virology 1991, 183, 449–456. [Google Scholar] [CrossRef]
- Joseph, J.; Savithri, H.S. Mutational analysis of the NIa protease from pepper vein banding potyvirus. Arch. Virol. 2000, 145, 2493–2502. [Google Scholar] [CrossRef][Green Version]
- Kim, D.; Kang, B.H.; Han, J.S.; Choi, K.Y. Temperature and salt effects on proteolytic function of turnip mosaic potyvirus nuclear inclusion protein a exhibiting a low-temperature optimum activity. Biochim. Biophys. Acta 2000, 1480, 29–40. [Google Scholar] [CrossRef]
- Rorrer, K.; Parks, T.D.; Scheffler, B.; Bevan, M.; Dougherty, W.G. Autocatalytic activity of the tobacco etch virus NIa proteinase in viral and foreign protein sequences. J. Gen. Virol. 1992, 73, 775–783. [Google Scholar] [CrossRef] [PubMed]
- Schaad, M.C.; Haldeman-Cahill, R.; Cronin, S.; Carrington, J.C. Analysis of the VPg-proteinase (NIa) encoded by tobacco etch potyvirus: Effects of mutations on subcellular transport, proteolytic processing, and genome amplification. J. Virol. 1996, 70, 7039–7048. [Google Scholar] [CrossRef] [PubMed]
- Carrington, J.C.; Haldeman, R.; Dolja, V.V.; Restrepo-Hartwig, M.A. Internal cleavage and trans-proteolytic activities of the VPg-proteinase (NIa) of tobacco etch potyvirus in vivo. J. Virol. 1993, 67, 6995–7000. [Google Scholar] [CrossRef]
- Carrington, J.C.; Cary, S.M.; Parks, T.D.; Dougherty, W.G. A second proteinase encoded by a plant potyvirus genome. EMBO J. 1989, 8, 365–370. [Google Scholar] [CrossRef]
- Ghabrial, S.A.; Smith, H.A.; Parks, T.D.; Dougherty, W.G. Molecular genetic analyses of the soybean mosaic virus NIa protease. J. Gen. Virol. 1990, 71, 1921–1927. [Google Scholar] [CrossRef]
- Dougherty, W.G.; Carrington, J.C. Expression and Function of Potyviral Gene Products. Annu. Rev. Phytopathol. 1988, 26, 123–143. [Google Scholar] [CrossRef]
- Phan, J.; Zdanov, A.; Evdokimov, A.G.; Tropea, J.E.; Iii, H.K.P.; Kapust, R.B.; Li, M.; Wlodawer, A.; Waugh, D.S. Structural Basis for the Substrate Specificity of Tobacco Etch Virus Protease. J. Biol. Chem. 2002, 277, 50564–50572. [Google Scholar] [CrossRef]
- Sun, P.; Austin, B.P.; Tözsér, J.; Waugh, D.S. Structural determinants of tobacco vein mottling virus protease substrate specificity. Protein Sci. 2010, 19, 2240–2251. [Google Scholar] [CrossRef]
- Chouard, T. Structural biology: Breaking the protein rules. Nature 2011, 471, 151–153. [Google Scholar] [CrossRef] [PubMed]
- Mathur, C.; Savithri, H.S. Novel ATPase activity of the polyprotein intermediate, Viral Protein genome-linked-Nuclear Inclusion—A protease, of Pepper vein banding potyvirus. Biochem. Biophys. Res. Commun. 2012, 427, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Eskelin, K.; Hafren, A.; Rantalainen, K.I.; Makinen, K. Potyviral VPg Enhances Viral RNA Translation and Inhibits Reporter mRNA Translation In Planta. J. Virol. 2011, 85, 9210–9221. [Google Scholar] [CrossRef] [PubMed]
- Rajamäki, M.-L.; Valkonen, J.P.T. Control of nuclear and nucleolar localization of nuclear inclusion protein a of picorna-like Potato virus A in Nicotiana species. Plant Cell 2009, 21, 2485–2502. [Google Scholar] [CrossRef] [PubMed]
- Rantalainen, K.I.; Eskelin, K.; Tompa, P.; Mäkinen, K. Structural flexibility allows the functional diversity of potyvirus genome-linked protein VPg. J. Virol. 2011, 85, 2449–2457. [Google Scholar] [CrossRef]
- Dunoyer, P.; Thomas, C.; Harrison, S.; Revers, F.; Maule, A. A Cysteine-Rich Plant Protein Potentiates Potyvirus Movement through an Interaction with the Virus Genome-Linked Protein VPg. J. Virol. 2004, 78, 2301–2309. [Google Scholar] [CrossRef]
- Grzela, R.; Strokovska, L.; Andrieu, J.P.; Dublet, B.; Zagorski, W.; Chroboczek, J. Potyvirus terminal protein VPg, effector of host eukaryotic initiation factor eIF4E. Biochimie 2006, 88, 887–896. [Google Scholar] [CrossRef]
- Léonard, S.; Plante, D.; Wittmann, S.; Daigneault, N.; Fortin, M.G.; Laliberté, J.F. Complex formation between potyvirus VPg and translation eukaryotic initiation factor 4E correlates with virus infectivity. J. Virol. 2000, 74, 7730–7737. [Google Scholar] [CrossRef]
- Hébrard, E.; Bessin, Y.; Michon, T.; Longhi, S.; Uversky, V.N.; Delalande, F.; Van Dorsselaer, A.; Romero, P.; Walter, J.; Declerck, N.; et al. Intrinsic disorder in Viral Proteins Genome-Linked: Experimental and predictive analyses. Virol. J. 2009, 6, 23. [Google Scholar] [CrossRef]
- Satheshkumar, P.S.; Gayathri, P.; Prasad, K.; Savithri, H.S. “Natively unfolded” VPg is essential for Sesbania mosaic virus serine protease activity. J. Biol. Chem. 2005, 280, 30291–30300. [Google Scholar] [CrossRef]
- Schein, C.H.; Oezguen, N.; Volk, D.E.; Garimella, R.; Paul, A.; Braun, W. NMR structure of the viral peptide linked to the genome (VPg) of poliovirus. Peptides 2006, 27, 1676–1684. [Google Scholar] [CrossRef]
- Fernández, A.; Guo, H.S.; Sáenz, P.; Simón-buela, L.; Cedrón, M.G.; De García, J.A. The motif V of plum pox potyvirus CI RNA helicase is involved in NTP hydrolysis and is essential for virus RNA replication. Nucleic Acids Res. 1997, 25, 4474–4480. [Google Scholar] [CrossRef] [PubMed]
- Guo, D.; Rajamäki, M.L.; Saarma, M.; Valkonen, J.P.T. Towards a protein interaction map of potyviruses: Protein interaction matrixes of two potyviruses based on the yeast two-hybrid system. J. Gen. Virol. 2001, 82, 935–939. [Google Scholar] [CrossRef] [PubMed]
- Klein, P.G.; Klein, R.R.; Rodríguez-Cerezo, E.; Hunt, A.G.; Shaw, J.G. Mutational Analysis of the Tobacco Vein Mottling Virus Genome. Virology 1994, 204, 759–769. [Google Scholar] [CrossRef] [PubMed]
- Riechmann, J.L.; Lain, S.; Garcia, J.A. Highlights and prospects of potyvirus molecular biology. J. Gen. Virol. 1992, 73, 1–16. [Google Scholar] [CrossRef]
- Hong, Y.; Hunt, A.G. RNA Polymerase Activity Catalyzed by a Potyvirus-Encoded RNA-Dependent RNA Polymerase. Virology 1996, 226, 146–151. [Google Scholar] [CrossRef]
- Anindya, R.; Chittori, S.; Savithri, H.S. Tyrosine 66 of Pepper vein banding virus genome-linked protein is uridylylated by RNA-dependent RNA polymerase. Virology 2005, 336, 154–162. [Google Scholar] [CrossRef]
- Puustinen, P.; Mäkinen, K. Uridylylation of the potyvirus VPg by viral replicase NIb correlates with the nucleotide binding capacity of VPg. J. Biol. Chem. 2004, 279, 38103–38110. [Google Scholar] [CrossRef]
- Carette, J.E.; Kujawa, A.; Gühl, K.; Verver, J.; Wellink, J.; Van Kammen, A. Mutational Analysis of the Genome-Linked Protein of Cowpea Mosaic Virus. Virology 2001, 290, 21–29. [Google Scholar] [CrossRef]
- Murray, K.E.; Barton, D.J. Poliovirus CRE-dependent VPg uridylylation is required for positive-strand RNA synthesis but not for negative-strand RNA synthesis. J. Virol. 2003, 77, 4739–4750. [Google Scholar] [CrossRef]
- Paul, A.V.; van Boom, J.H.; Filippov, D.; Wimmer, E. Protein-Primed RNA synthesis by purified poliovirus RNA polymerase. Nature 1998, 393, 280–284. [Google Scholar] [CrossRef]
- Cheng, X.; Xiong, R.; Li, Y.; Li, F.; Zhou, X.; Wang, A. Sumoylation of turnip mosaic virus RNA polymerase promotes viral infection by counteracting the host NPR1-Mediated immune response. Plant Cell 2017, 29, 508–525. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Zhang, C.; Li, Y.; Wu, G.; Hou, X.; Zhou, X.; Wang, A. Beclin1 restricts RNA virus infection in plants through suppression and degradation of the viral polymerase. Nat. Commun. 2018, 9, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Wang, A. The Potyvirus Silencing Suppressor Protein VPg Mediates Degradation of SGS3 via Ubiquitination and Autophagy Pathways. J. Virol. 2017, 91, 1. [Google Scholar] [CrossRef] [PubMed]
- Kushwaha, N.K.; Hafrén, A.; Hofius, D. Autophagy-virus interplay in plants: From antiviral recognition to proviral manipulation. Mol. Plant Pathol. 2019, 20, 1211–1216. [Google Scholar] [CrossRef] [PubMed]
- Langenberg, W.G.; Zhang, L. Immunocytology shows the presence of tobacco etch virus P3 protein in nuclear inclusions. J. Struct. Biol. 1997, 118, 243–247. [Google Scholar] [CrossRef]
- Anindya, R.; Savithri, H.S. Potyviral NIa Proteinase, a Proteinase with Novel Deoxyribonuclease Activity. J. Biol. Chem. 2004, 279, 32159–32169. [Google Scholar] [CrossRef]
- Riechmann, J.L.; Cervera, M.T.; Garcia, J.A. Processing of the plum pox virus polyprotein at the P3-6K1 junction is not required for virus viability. J. Gen. Virol. 1995, 76, 951–956. [Google Scholar] [CrossRef]
- Hafren, A.; Hofius, D.; Ronnholm, G.; Sonnewald, U.; Makinen, K. HSP70 and Its Cochaperone CPIP Promote Potyvirus Infection in Nicotiana benthamiana by Regulating Viral Coat Protein Functions. Plant Cell 2010, 22, 523–535. [Google Scholar] [CrossRef]
- Besong-Ndika, J.; Ivanov, K.I.; Hafrèn, A.; Michon, T.; Mäkinen, K. Cotranslational Coat Protein-Mediated Inhibition of Potyviral RNA Translation. J. Virol. 2015, 89, 4237–4248. [Google Scholar] [CrossRef]
- Puustinen, P.; Rajamäki, M.-L.; Ivanov, K.I.; Valkonen, J.P.T.; Mäkinen, K. Detection of the potyviral genome-linked protein VPg in virions and its phosphorylation by host kinases. J. Virol. 2002, 76, 12703–12711. [Google Scholar] [CrossRef]
- Ivanov, K.I.; Puustinen, P.; Gabrenaite, R.; Vihinen, H.; Rönnstrand, L.; Valmu, L.; Kalkkinen, N.; Mäkinen, K. Phosphorylation of the potyvirus capsid protein by protein kinase CK2 and its relevance for virus infection. Plant Cell 2003, 15, 2124–2139. [Google Scholar] [CrossRef] [PubMed]
- Xiong, R.; Wang, A. SCE1, the SUMO-Conjugating Enzyme in Plants That Interacts with NIb, the RNA-Dependent RNA Polymerase of Turnip Mosaic Virus, Is Required for Viral Infection. J. Virol. 2013, 87, 4704–4715. [Google Scholar] [CrossRef] [PubMed]
- Rajamäki, M.L.; Kelloniemi, J.; Alminaite, A.; Kekarainen, T.; Rabenstein, F.; Valkonen, J.P. A novel insertion site inside the potyvirus P1 cistron allows expression of heterologous proteins and suggests some P1 functions. Virology 2005, 342, 88–101. [Google Scholar] [CrossRef] [PubMed]
- Dolja, V.V.; McBride, H.J.; Carrington, J.C. Tagging of plant potyvirus replication and movement by insertion of beta-glucuronidase into the viral polyprotein. Proc. Natl. Acad. Sci. USA 1992, 89, 10208–10212. [Google Scholar] [CrossRef] [PubMed]
- Varrelmann, M.; Palkovics, L.; Maiss, E. Transgenic or plant expression vector-mediated recombination of Plum Pox Virus. J. Virol. 2000, 74, 7462–7469. [Google Scholar] [CrossRef] [PubMed]
- Parks, T.D.; Leuther, K.K.; Howard, E.D.; Johnston, S.A.; Dougherty, W.G. Release of Proteins and Peptides from Fusion Proteins Using a Recombinant Plant Virus Proteinase. Anal. Biochem. 1994, 216, 413–417. [Google Scholar] [CrossRef]
- Kapust, R.B.; Tözsér, J.; Fox, J.D.; Anderson, D.E.; Cherry, S.; Copeland, T.D.; Waugh, D.S. Tobacco etch virus protease: Mechanism of autolysis and rational design of stable mutants with wild-type catalytic proficiency. Protein Eng. 2001, 14, 993–1000. [Google Scholar] [CrossRef]
- Zheng, N.; de Jesús Pérez, J.; Zhang, Z.; Domínguez, E.; Garcia, J.A.; Xie, Q. Specific and efficient cleavage of fusion proteins by recombinant plum pox virus NIa protease. Protein Expr. Purif. 2008, 57, 153–162. [Google Scholar] [CrossRef]
- Nallamsetty, S.; Kapust, R.B.; Tözsér, J.; Cherry, S.; Tropea, J.E.; Copeland, T.D.; Waugh, D.S. Efficient site-specific processing of fusion proteins by tobacco vein mottling virus protease in vivo and in vitro. Protein Expr. Purif. 2004, 38, 108–115. [Google Scholar] [CrossRef]
- Alemzadeh, E.; Dehshahri, A.; Izadpanah, K.; Ahmadi, F. Plant virus nanoparticles: Novel and robust nanocarriers for drug delivery and imaging. Colloids Surf. B. Biointerfaces 2018, 167, 20–27. [Google Scholar] [CrossRef]
- Fernández-Fernández, M.R.; Martínez-Torrecuadrada, J.L.; Casal, J.I.; García, J.A. Development of an antigen presentation system based on plum pox potyvirus. FEBS Lett. 1998, 427, 229–235. [Google Scholar] [CrossRef]
- Sanchez, F.; Saez, M.; Lunello, P.; Ponz, F. Plant viral elongated nanoparticles modified for log-increases of foreign peptide immunogenicity and specific antibody detection. J. Biotechnol. 2013, 168, 409–415. [Google Scholar] [CrossRef] [PubMed]
- Besong-Ndika, J.; Wahlsten, M.; Cardinale, D.; Pille, J.; Walter, J.; Michon, T.; Mäkinen, K. Toward the Reconstitution of a Two-Enzyme Cascade for Resveratrol Synthesis on Potyvirus Particles. Front. Plant Sci. 2016, 7, 89. [Google Scholar] [CrossRef] [PubMed]
- Sabharwal, P.; Amritha, C.K.; Sushmitha, C.; Natraj, U.; Savithri, H.S. Intracellular trafficking and endocytic uptake pathway of Pepper vein banding virus-like particles in epithelial cells. Nanomedicine 2019, 14, 1247–1265. [Google Scholar] [CrossRef]
- Sahay, G.; Alakhova, D.Y.; Kabanov, A.V. Endocytosis of Nanomedicines. J. Control. Release 2010, 145, 182–195. [Google Scholar] [CrossRef]
- Du, N.; Cong, H.; Tian, H.; Zhang, H.; Zhang, W.; Song, L.; Tien, P. Cell Surface Vimentin Is an Attachment Receptor for Enterovirus 71. J. Virol. 2014, 88, 5816–5833. [Google Scholar] [CrossRef]
- Nédellec, P.; Vicart, P.; Laurent-Winter, C.; Martinat, C.; Prévost, M.C.; Brahic, M. Interaction of Theiler’s virus with intermediate filaments of infected cells. J. Virol. 1998, 72, 9553–9560. [Google Scholar] [CrossRef]
- Koudelka, K.J.; Destito, G.; Plummer, E.M.; Trauger, S.A.; Siuzdak, G.; Manchester, M. Endothelial Targeting of Cowpea Mosaic Virus (CPMV) via Surface Vimentin. PLoS Pathog. 2009, 5, e1000417. [Google Scholar] [CrossRef]
- Wu, G.; Cui, X.; Dai, Z.; He, R.; Li, Y.; Yu, K.; Bernards, M.; Chen, X.; Wang, A. A plant RNA virus hijacks endocytic proteins to establish its infection in plants. Plant J. 2019, 101, 384–400. [Google Scholar] [CrossRef]
- Wu, G.; Cui, X.; Chen, H.; Renaud, J.B.; Yu, K.; Chen, X.; Wang, A. Dynamin-Like Proteins of Endocytosis in Plants Are Coopted by Potyviruses To Enhance Virus Infection. J. Virol. 2018, 92. [Google Scholar] [CrossRef]
- Slastnikova, T.A.; Ulasov, A.V.; Rosenkranz, A.A.; Sobolev, A.S. Targeted Intracellular Delivery of Antibodies: The State of the Art. Front. Pharmacol. 2018, 9, 1208. [Google Scholar] [CrossRef] [PubMed]
- Sabharwal, P.; Sushmitha, C.; Amritha, C.K.; Natraj, U.; Murthy, M.R.N.; Savithri, H.S. Development of pepper vein banding virus chimeric virus-like particles for potential diagnostic and therapeutic applications. Arch. Virol. 2020, 165, 1163–1176. [Google Scholar] [CrossRef] [PubMed]
- Shen, W.; Shi, Y.; Dai, Z.; Wang, A. The RNA-dependent RNA polymerase NiB of potyviruses plays multifunctional, contrasting roles during viral infection. Viruses 2020, 12, 77. [Google Scholar] [CrossRef] [PubMed]
- Zamora, M.; Méndez-López, E.; Agirrezabala, X.; Cuesta, R.; Lavín, J.L.; Sánchez-Pina, M.A.; Aranda, M.A.; Valle, M. Potyvirus virion structure shows conserved protein fold and RNA binding site in ssRNA viruses. Sci. Adv. 2017, 3, eaao2182. [Google Scholar] [CrossRef] [PubMed]
- Kežar, A.; Kavčič, L.; Polák, M.; Nováček, J.; Gutiérrez-Aguirre, I.; Žnidarič, M.T.; Coll, A.; Stare, K.; Gruden, K.; Ravnikar, M.; et al. Structural basis for the multitasking nature of the potato virus Y coat protein. Sci. Adv. 2019, 5, eaaw3808. [Google Scholar] [CrossRef] [PubMed]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sabharwal, P.; Savithri, H.S. Functional Characterization of Pepper Vein Banding Virus-Encoded Proteins and Their Interactions: Implications in Potyvirus Infection. Viruses 2020, 12, 1037. https://doi.org/10.3390/v12091037
Sabharwal P, Savithri HS. Functional Characterization of Pepper Vein Banding Virus-Encoded Proteins and Their Interactions: Implications in Potyvirus Infection. Viruses. 2020; 12(9):1037. https://doi.org/10.3390/v12091037
Chicago/Turabian StyleSabharwal, Pallavi, and Handanahal S. Savithri. 2020. "Functional Characterization of Pepper Vein Banding Virus-Encoded Proteins and Their Interactions: Implications in Potyvirus Infection" Viruses 12, no. 9: 1037. https://doi.org/10.3390/v12091037
APA StyleSabharwal, P., & Savithri, H. S. (2020). Functional Characterization of Pepper Vein Banding Virus-Encoded Proteins and Their Interactions: Implications in Potyvirus Infection. Viruses, 12(9), 1037. https://doi.org/10.3390/v12091037




