How HIV-1 Gag Manipulates Its Host Cell Proteins: A Focus on Interactors of the Nucleocapsid Domain
Abstract
1. Introduction
2. Human Immunodeficiency Virus (HIV-1) Group-Specific Antigen (Gag) Structural Polyprotein
2.1. Matrix Domain of HIV-1 Gag
2.2. Capsid Domain of HIV-1 Gag
2.3. The Nucleocapsid (NC) Domain of HIV-1 Gag
2.4. p6 Domain of HIV-1 Gag
2.5. Spacer Peptide (SP1) and SP2 Domains of HIV-1 Gag
3. Interactions Between HIV-1 Gag Protein and Cellular Proteins
4. Interactions Involving the NC Domain of Gag
4.1. Reverse Transcription (RTion)
4.2. Translation
4.3. Viral Assembly
4.4. Viral Budding
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Balasubramaniam, M.; Freed, E.O. New Insights into HIV Assembly and Trafficking. Physiology 2011, 26, 236–251. [Google Scholar] [CrossRef] [PubMed]
- Freed, E.O. HIV-1 assembly, release and maturation. Nat. Rev. Microbiol. 2015, 13, 484–496. [Google Scholar] [CrossRef] [PubMed]
- Sundquist, W.I.; Kräusslich, H.-G. HIV-1 assembly, budding, and maturation. Cold Spring Harb. Perspect. Med. 2012, 2, a006924. [Google Scholar] [CrossRef] [PubMed]
- Jouvenet, N.; Simon, S.M.; Bieniasz, P.D. Imaging the interaction of HIV-1 genomes and Gag during assembly of individual viral particles. Proc. Natl. Acad. Sci. USA 2009, 106, 19114–19119. [Google Scholar] [CrossRef]
- Jouvenet, N.; Neil, S.J.D.; Bess, C.; Johnson, M.C.; Virgen, C.A.; Simon, S.M.; Bieniasz, P.D. Plasma Membrane Is the Site of Productive HIV-1 Particle Assembly. PLoS Biol. 2006, 4, e435. [Google Scholar] [CrossRef]
- Bennett, A.E.; Narayan, K.; Shi, D.; Hartnell, L.M.; Gousset, K.; He, H.; Lowekamp, B.C.; Yoo, T.S.; Bliss, D.; Freed, E.O.; et al. Ion-Abrasion Scanning Electron Microscopy Reveals Surface-Connected Tubular Conduits in HIV-Infected Macrophages. PLoS Pathog. 2009, 5, e1000591. [Google Scholar] [CrossRef]
- Deneka, M.; Pelchen-Matthews, A.; Byland, R.; Ruiz-Mateos, E.; Marsh, M. In macrophages, HIV-1 assembles into an intracellular plasma membrane domain containing the tetraspanins CD81, CD9, and CD53. J. Cell Biol. 2007, 177, 329–341. [Google Scholar] [CrossRef]
- Nkwe, D.O.; Pelchen-Matthews, A.; Burden, J.J.; Collinson, L.M.; Marsh, M. The intracellular plasma membrane-connected compartment in the assembly of HIV-1 in human macrophages. BMC Biol. 2016, 14, 50. [Google Scholar] [CrossRef]
- Pelchen-Matthews, A.; Kramer, B.; Marsh, M. Infectious HIV-1 assembles in late endosomes in primary macrophages. J. Cell Biol. 2003, 162, 443–455. [Google Scholar] [CrossRef]
- Welsch, S.; Keppler, O.T.; Habermann, A.; Allespach, I.; Krijnse-Locker, J.; Kräusslich, H.-G. HIV-1 Buds Predominantly at the Plasma Membrane of Primary Human Macrophages. PLoS Pathog. 2007, 3, e36. [Google Scholar] [CrossRef]
- Welsch, S.; Groot, F.; Krausslich, H.-G.; Keppler, O.T.; Sattentau, Q.J. Architecture and Regulation of the HIV-1 Assembly and Holding Compartment in Macrophages. J. Virol. 2011, 85, 7922–7927. [Google Scholar] [CrossRef] [PubMed]
- Wiegers, K.; Rutter, G.; Kottler, H.; Tessmer, U.; Hohenberg, H.; Kräusslich, H.G. Sequential steps in human immunodeficiency virus particle maturation revealed by alterations of individual Gag polyprotein cleavage sites. J. Virol. 1998, 72, 2846–2854. [Google Scholar] [CrossRef] [PubMed]
- De Marco, A.; Müller, B.; Glass, B.; Riches, J.D.; Kräusslich, H.-G.; Briggs, J.A.G. Structural analysis of HIV-1 maturation using cryo-electron tomography. PLoS Pathog. 2010, 6, e1001215. [Google Scholar] [CrossRef] [PubMed]
- Mattei, S.; Anders, M.; Konvalinka, J.; Kräusslich, H.-G.; Briggs, J.A.G.; Müller, B. Induced maturation of human immunodeficiency virus. J. Virol. 2014, 88, 13722–13731. [Google Scholar] [CrossRef] [PubMed]
- Wright, E.R.; Schooler, J.B.; Ding, H.J.; Kieffer, C.; Fillmore, C.; Sundquist, W.I.; Jensen, G.J. Electron cryotomography of immature HIV-1 virions reveals the structure of the CA and SP1 Gag shells. EMBO J. 2007, 26, 2218–2226. [Google Scholar] [CrossRef]
- Briggs, J.A.G.; Riches, J.D.; Glass, B.; Bartonova, V.; Zanetti, G.; Kräusslich, H.-G. Structure and assembly of immature HIV. Proc. Natl. Acad. Sci. USA 2009, 106, 11090–11095. [Google Scholar] [CrossRef]
- Bharat, T.A.M.; Castillo Menendez, L.R.; Hagen, W.J.H.; Lux, V.; Igonet, S.; Schorb, M.; Schur, F.K.M.; Kräusslich, H.-G.; Briggs, J.A.G. Cryo-electron microscopy of tubular arrays of HIV-1 Gag resolves structures essential for immature virus assembly. Proc. Natl. Acad. Sci. USA 2014, 111, 8233–8238. [Google Scholar] [CrossRef]
- Briggs, J.A.G.; Grünewald, K.; Glass, B.; Förster, F.; Kräusslich, H.-G.; Fuller, S.D. The mechanism of HIV-1 core assembly: Insights from three-dimensional reconstructions of authentic virions. Struct. Lond. Engl. 1993 2006, 14, 15–20. [Google Scholar] [CrossRef]
- Briggs, J.A.G.; Simon, M.N.; Gross, I.; Kräusslich, H.-G.; Fuller, S.D.; Vogt, V.M.; Johnson, M.C. The stoichiometry of Gag protein in HIV-1. Nat. Struct. Mol. Biol. 2004, 11, 672–675. [Google Scholar] [CrossRef]
- Mattei, S.; Glass, B.; Hagen, W.J.H.; Kräusslich, H.-G.; Briggs, J.A.G. The structure and flexibility of conical HIV-1 capsids determined within intact virions. Science 2016, 354, 1434–1437. [Google Scholar] [CrossRef]
- Mattei, S.; Tan, A.; Glass, B.; Müller, B.; Kräusslich, H.-G.; Briggs, J.A.G. High-resolution structures of HIV-1 Gag cleavage mutants determine structural switch for virus maturation. Proc. Natl. Acad. Sci. USA 2018, 115, E9401–E9410. [Google Scholar] [CrossRef] [PubMed]
- Pornillos, O.; Ganser-Pornillos, B.K. Maturation of retroviruses. Curr. Opin. Virol. 2019, 36, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Woodward, C.L.; Cheng, S.N.; Jensen, G.J. Electron cryotomography studies of maturing HIV-1 particles reveal the assembly pathway of the viral core. J. Virol. 2015, 89, 1267–1277. [Google Scholar] [CrossRef] [PubMed]
- Benjamin, J.; Ganser-Pornillos, B.K.; Tivol, W.F.; Sundquist, W.I.; Jensen, G.J. Three-dimensional structure of HIV-1 virus-like particles by electron cryotomography. J. Mol. Biol. 2005, 346, 577–588. [Google Scholar] [CrossRef] [PubMed]
- Ott, D.E.; Coren, L.V.; Kane, B.P.; Busch, L.K.; Johnson, D.G.; Sowder, R.C.; Chertova, E.N.; Arthur, L.O.; Henderson, L.E. Cytoskeletal proteins inside human immunodeficiency virus type 1 virions. J. Virol. 1996, 70, 7734–7743. [Google Scholar] [CrossRef]
- Chertova, E.; Chertov, O.; Coren, L.V.; Roser, J.D.; Trubey, C.M.; Bess, J.W.; Sowder, R.C.; Barsov, E.; Hood, B.L.; Fisher, R.J.; et al. Proteomic and Biochemical Analysis of Purified Human Immunodeficiency Virus Type 1 Produced from Infected Monocyte-Derived Macrophages. J. Virol. 2006, 80, 9039–9052. [Google Scholar] [CrossRef]
- Brass, A.L.; Dykxhoorn, D.M.; Benita, Y.; Yan, N.; Engelman, A.; Xavier, R.J.; Lieberman, J.; Elledge, S.J. Identification of Host Proteins Required for HIV Infection Through a Functional Genomic Screen. Science 2008, 319, 921–926. [Google Scholar] [CrossRef]
- König, R.; Zhou, Y.; Elleder, D.; Diamond, T.L.; Bonamy, G.M.C.; Irelan, J.T.; Chiang, C.; Tu, B.P.; De Jesus, P.D.; Lilley, C.E.; et al. Global Analysis of Host-Pathogen Interactions that Regulate Early-Stage HIV-1 Replication. Cell 2008, 135, 49–60. [Google Scholar] [CrossRef]
- Yeung, M.L.; Houzet, L.; Yedavalli, V.S.R.K.; Jeang, K.-T. A Genome-wide Short Hairpin RNA Screening of Jurkat T-cells for Human Proteins Contributing to Productive HIV-1 Replication. J. Biol. Chem. 2009, 284, 19463–19473. [Google Scholar] [CrossRef]
- Zhou, H.; Xu, M.; Huang, Q.; Gates, A.T.; Zhang, X.D.; Castle, J.C.; Stec, E.; Ferrer, M.; Strulovici, B.; Hazuda, D.J.; et al. Genome-Scale RNAi Screen for Host Factors Required for HIV Replication. Cell Host Microbe 2008, 4, 495–504. [Google Scholar] [CrossRef]
- Pache, L.; König, R.; Chanda, S.K. Identifying HIV-1 host cell factors by genome-scale RNAi screening. Methods 2011, 53, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Bushman, F.D.; Malani, N.; Fernandes, J.; D’Orso, I.; Cagney, G.; Diamond, T.L.; Zhou, H.; Hazuda, D.J.; Espeseth, A.S.; König, R.; et al. Host Cell Factors in HIV Replication: Meta-Analysis of Genome-Wide Studies. PLoS Pathog. 2009, 5, e1000437. [Google Scholar] [CrossRef] [PubMed]
- Murali, T.M.; Dyer, M.D.; Badger, D.; Tyler, B.M.; Katze, M.G. Network-Based Prediction and Analysis of HIV Dependency Factors. PLoS Comput. Biol. 2011, 7, e1002164. [Google Scholar] [CrossRef] [PubMed]
- Jäger, S.; Cimermancic, P.; Gulbahce, N.; Johnson, J.R.; McGovern, K.E.; Clarke, S.C.; Shales, M.; Mercenne, G.; Pache, L.; Li, K.; et al. Global landscape of HIV–human protein complexes. Nature 2012, 481, 365–370. [Google Scholar] [CrossRef]
- Emig-Agius, D.; Olivieri, K.; Pache, L.; Shih, H.L.; Pustovalova, O.; Bessarabova, M.; Young, J.A.T.; Chanda, S.K.; Ideker, T. An Integrated Map of HIV-Human Protein Complexes that Facilitate Viral Infection. PLoS ONE 2014, 9, e96687. [Google Scholar] [CrossRef]
- Le Sage, V.; Cinti, A.; Valiente-Echeverría, F.; Mouland, A.J. Proteomic analysis of HIV-1 Gag interacting partners using proximity-dependent biotinylation. Virol. J. 2015, 12, 138. [Google Scholar] [CrossRef]
- Ritchie, C.; Cylinder, I.; Platt, E.J.; Barklis, E. Analysis of HIV-1 Gag Protein Interactions via Biotin Ligase Tagging. J. Virol. 2015, 89, 3988–4001. [Google Scholar] [CrossRef]
- Engeland, C.E.; Brown, N.P.; Börner, K.; Schümann, M.; Krause, E.; Kaderali, L.; Müller, G.A.; Kräusslich, H.-G. Proteome analysis of the HIV-1 Gag interactome. Virology 2014, 460–461, 194–206. [Google Scholar] [CrossRef]
- Li, Y.; Frederick, K.M.; Haverland, N.A.; Ciborowski, P.; Belshan, M. Investigation of the HIV-1 matrix interactome during virus replication. PROTEOMICS - Clin. Appl. 2016, 10, 156–163. [Google Scholar] [CrossRef]
- Alfadhli, A.; Barklis, E. The roles of lipids and nucleic acids in HIV-1 assembly. Front. Microbiol. 2014, 5. [Google Scholar] [CrossRef]
- Bukrinskaya, A. HIV-1 matrix protein: A mysterious regulator of the viral life cycle. Virus Res. 2007, 124, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Chukkapalli, V.; Ono, A. Molecular Determinants that Regulate Plasma Membrane Association of HIV-1 Gag. J. Mol. Biol. 2011, 410, 512–524. [Google Scholar] [CrossRef] [PubMed]
- Dick, R.A.; Vogt, V.M. Membrane interaction of retroviral Gag proteins. Front. Microbiol. 2014, 5. [Google Scholar] [CrossRef]
- Ghanam, R.H.; Samal, A.B.; Fernandez, T.F.; Saad, J.S. Role of the HIV-1 Matrix Protein in Gag Intracellular Trafficking and Targeting to the Plasma Membrane for Virus Assembly. Front. Microbiol. 2012, 3. [Google Scholar] [CrossRef] [PubMed]
- Jouvenet, N.; Lainé, S.; Pessel-Vivares, L.; Mougel, M. Cell biology of retroviral RNA packaging. RNA Biol. 2011, 8, 572–580. [Google Scholar] [CrossRef]
- Lingappa, J.R.; Reed, J.C.; Tanaka, M.; Chutiraka, K.; Robinson, B.A. How HIV-1 Gag assembles in cells: Putting together pieces of the puzzle. Virus Res. 2014, 193, 89–107. [Google Scholar] [CrossRef]
- Maldonado, J.O.; Martin, J.L.; Mueller, J.D.; Zhang, W.; Mansky, L.M. New insights into retroviral Gag-Gag and Gag-membrane interactions. Front. Microbiol. 2014, 5, 302. [Google Scholar] [CrossRef]
- Olety, B.; Ono, A. Roles played by acidic lipids in HIV-1 Gag membrane binding. Virus Res. 2014, 193, 108–115. [Google Scholar] [CrossRef]
- Olson, E.D.; Musier-Forsyth, K. Retroviral Gag protein–RNA interactions: Implications for specific genomic RNA packaging and virion assembly. Semin. Cell Dev. Biol. 2019, 86, 129–139. [Google Scholar] [CrossRef]
- Parent, L.J.; Gudleski, N. Beyond Plasma Membrane Targeting: Role of the MA domain of Gag in Retroviral Genome Encapsidation. J. Mol. Biol. 2011, 410, 553–564. [Google Scholar] [CrossRef]
- Bryant, M.; Ratner, L. Myristoylation-dependent replication and assembly of human immunodeficiency virus 1. Proc. Natl. Acad. Sci. USA 1990, 87, 523–527. [Google Scholar] [CrossRef] [PubMed]
- Gottlinger, H.G.; Sodroski, J.G.; Haseltine, W.A. Role of capsid precursor processing and myristoylation in morphogenesis and infectivity of human immunodeficiency virus type 1. Proc. Natl. Acad. Sci. USA 1989, 86, 5781–5785. [Google Scholar] [CrossRef]
- Ono, A.; Freed, E.O. Binding of Human Immunodeficiency Virus Type 1 Gag to Membrane: Role of the Matrix Amino Terminus. J. Virol. 1999, 73, 4136–4144. [Google Scholar] [CrossRef] [PubMed]
- Saad, J.S.; Miller, J.; Tai, J.; Kim, A.; Ghanam, R.H.; Summers, M.F. Structural basis for targeting HIV-1 Gag proteins to the plasma membrane for virus assembly. Proc. Natl. Acad. Sci. USA 2006, 103, 11364–11369. [Google Scholar] [CrossRef] [PubMed]
- Spearman, P.; Horton, R.; Ratner, L.; Kuli-Zade, I. Membrane binding of human immunodeficiency virus type 1 matrix protein in vivo supports a conformational myristyl switch mechanism. J. Virol. 1997, 71, 6582–6592. [Google Scholar] [CrossRef]
- Tang, C.; Loeliger, E.; Luncsford, P.; Kinde, I.; Beckett, D.; Summers, M.F. Entropic switch regulates myristate exposure in the HIV-1 matrix protein. Proc. Natl. Acad. Sci. USA 2004, 101, 517–522. [Google Scholar] [CrossRef]
- Dick, R.A.; Kamynina, E.; Vogt, V.M. Effect of Multimerization on Membrane Association of Rous Sarcoma Virus and HIV-1 Matrix Domain Proteins. J. Virol. 2013, 87, 13598–13608. [Google Scholar] [CrossRef]
- Fledderman, E.L.; Fujii, K.; Ghanam, R.H.; Waki, K.; Prevelige, P.E.; Freed, E.O.; Saad, J.S. Myristate Exposure in the Human Immunodeficiency Virus Type 1 Matrix Protein Is Modulated by pH. Biochemistry 2010, 49, 9551–9562. [Google Scholar] [CrossRef]
- Campbell, S.; Fisher, R.J.; Towler, E.M.; Fox, S.; Issaq, H.J.; Wolfe, T.; Phillips, L.R.; Rein, A. Modulation of HIV-like particle assembly in vitro by inositol phosphates. Proc. Natl. Acad. Sci. USA 2001, 98, 10875–10879. [Google Scholar] [CrossRef]
- Murray, P.S.; Li, Z.; Wang, J.; Tang, C.L.; Honig, B.; Murray, D. Retroviral Matrix Domains Share Electrostatic Homology: Models for Membrane Binding Function throughout the Viral Life Cycle. Structure 2005, 13, 1521–1531. [Google Scholar] [CrossRef]
- Zhou, W.; Parent, L.J.; Wills, J.W.; Resh, M.D. Identification of a membrane-binding domain within the amino-terminal region of human immunodeficiency virus type 1 Gag protein which interacts with acidic phospholipids. J. Virol. 1994, 68, 2556–2569. [Google Scholar] [CrossRef] [PubMed]
- Alfadhli, A.; Barklis, R.L.; Barklis, E. HIV-1 matrix organizes as a hexamer of trimers on membranes containing phosphatidylinositol-(4,5)-bisphosphate. Virology 2009, 387, 466–472. [Google Scholar] [CrossRef] [PubMed]
- Alfadhli, A.; Still, A.; Barklis, E. Analysis of Human Immunodeficiency Virus Type 1 Matrix Binding to Membranes and Nucleic Acids. J. Virol. 2009, 83, 12196–12203. [Google Scholar] [CrossRef] [PubMed]
- Chukkapalli, V.; Hogue, I.B.; Boyko, V.; Hu, W.-S.; Ono, A. Interaction between the human immunodeficiency virus type 1 Gag matrix domain and phosphatidylinositol-(4,5)-bisphosphate is essential for efficient gag membrane binding. J. Virol. 2008, 82, 2405–2417. [Google Scholar] [CrossRef] [PubMed]
- Mercredi, P.Y.; Bucca, N.; Loeliger, B.; Gaines, C.R.; Mehta, M.; Bhargava, P.; Tedbury, P.R.; Charlier, L.; Floquet, N.; Muriaux, D.; et al. Structural and Molecular Determinants of Membrane Binding by the HIV-1 Matrix Protein. J. Mol. Biol. 2016, 428, 1637–1655. [Google Scholar] [CrossRef]
- Shkriabai, N.; Datta, S.A.K.; Zhao, Z.; Hess, S.; Rein, A.; Kvaratskhelia, M. Interactions of HIV-1 Gag with Assembly Cofactors. Biochemistry 2006, 45, 4077–4083. [Google Scholar] [CrossRef]
- Ono, A.; Ablan, S.D.; Lockett, S.J.; Nagashima, K.; Freed, E.O. Phosphatidylinositol (4,5) bisphosphate regulates HIV-1 Gag targeting to the plasma membrane. Proc. Natl. Acad. Sci. USA 2004, 101, 14889–14894. [Google Scholar] [CrossRef]
- Monde, K.; Chukkapalli, V.; Ono, A. Assembly and Replication of HIV-1 in T Cells with Low Levels of Phosphatidylinositol-(4,5)-Bisphosphate. J. Virol. 2011, 85, 3584–3595. [Google Scholar] [CrossRef]
- Barros, M.; Heinrich, F.; Datta, S.A.K.; Rein, A.; Karageorgos, I.; Nanda, H.; Lösche, M. Membrane Binding of HIV-1 Matrix Protein: Dependence on Bilayer Composition and Protein Lipidation. J. Virol. 2016, 90, 4544–4555. [Google Scholar] [CrossRef]
- Ono, A.; Waheed, A.A.; Freed, E.O. Depletion of cellular cholesterol inhibits membrane binding and higher-order multimerization of human immunodeficiency virus type 1 Gag. Virology 2007, 360, 27–35. [Google Scholar] [CrossRef]
- Alfadhli, A.; McNett, H.; Tsagli, S.; Bächinger, H.P.; Peyton, D.H.; Barklis, E. HIV-1 Matrix Protein Binding to RNA. J. Mol. Biol. 2011, 410, 653–666. [Google Scholar] [CrossRef] [PubMed]
- Ott, D.E.; Chertova, E.N.; Busch, L.K.; Coren, L.V.; Gagliardi, T.D.; Johnson, D.G. Mutational Analysis of the Hydrophobic Tail of the Human Immunodeficiency Virus Type 1 p6Gag Protein Produces a Mutant That Fails To Package Its Envelope Protein. J. Virol. 1999, 73, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Bieniasz, P.; Telesnitsky, A. Multiple, Switchable Protein:RNA Interactions Regulate Human Immunodeficiency Virus Type 1 Assembly. Annu. Rev. Virol. 2018, 5, 165–183. [Google Scholar] [CrossRef]
- Mailler, E.; Bernacchi, S.; Marquet, R.; Paillart, J.-C.; Vivet-Boudou, V.; Smyth, R. The Life-Cycle of the HIV-1 Gag–RNA Complex. Viruses 2016, 8, 248. [Google Scholar] [CrossRef]
- Chukkapalli, V.; Inlora, J.; Todd, G.C.; Ono, A. Evidence in Support of RNA-Mediated Inhibition of Phosphatidylserine-Dependent HIV-1 Gag Membrane Binding in Cells. J. Virol. 2013, 87, 7155–7159. [Google Scholar] [CrossRef] [PubMed]
- Chukkapalli, V.; Oh, S.J.; Ono, A. Opposing mechanisms involving RNA and lipids regulate HIV-1 Gag membrane binding through the highly basic region of the matrix domain. Proc. Natl. Acad. Sci. USA 2010, 107, 1600–1605. [Google Scholar] [CrossRef] [PubMed]
- Kutluay, S.B.; Zang, T.; Blanco-Melo, D.; Powell, C.; Jannain, D.; Errando, M.; Bieniasz, P.D. Global Changes in the RNA Binding Specificity of HIV-1 Gag Regulate Virion Genesis. Cell 2014, 159, 1096–1109. [Google Scholar] [CrossRef]
- Carlson, L.-A.; Bai, Y.; Keane, S.C.; Doudna, J.A.; Hurley, J.H. Reconstitution of selective HIV-1 RNA packaging in vitro by membrane-bound Gag assemblies. eLife 2016, 5, e14663. [Google Scholar] [CrossRef]
- Gaines, C.R.; Tkacik, E.; Rivera-Oven, A.; Somani, P.; Achimovich, A.; Alabi, T.; Zhu, A.; Getachew, N.; Yang, A.L.; McDonough, M.; et al. HIV-1 Matrix Protein Interactions with tRNA: Implications for Membrane Targeting. J. Mol. Biol. 2018, 430, 2113–2127. [Google Scholar] [CrossRef]
- Thornhill, D.; Olety, B.; Ono, A. Relationships between MA-RNA Binding in Cells and Suppression of HIV-1 Gag Mislocalization to Intracellular Membranes. J. Virol. 2019, 93, 19. [Google Scholar] [CrossRef]
- Inlora, J.; Collins, D.R.; Trubin, M.E.; Chung, J.Y.J.; Ono, A. Membrane binding and subcellular localization of retroviral Gag proteins are differentially regulated by MA interactions with phosphatidylinositol-(4,5)-bisphosphate and RNA. mBio 2014, 5, e02202. [Google Scholar] [CrossRef] [PubMed]
- Checkley, M.A.; Luttge, B.G.; Freed, E.O. HIV-1 envelope glycoprotein biosynthesis, trafficking, and incorporation. J. Mol. Biol. 2011, 410, 582–608. [Google Scholar] [CrossRef] [PubMed]
- Tedbury, P.R.; Freed, E.O. The role of matrix in HIV-1 envelope glycoprotein incorporation. Trends Microbiol. 2014, 22, 372–378. [Google Scholar] [CrossRef] [PubMed]
- Ganser, B.K.; Li, S.; Klishko, V.Y.; Finch, J.T.; Sundquist, W.I. Assembly and analysis of conical models for the HIV-1 core. Science 1999, 283, 80–83. [Google Scholar] [CrossRef]
- Pornillos, O.; Ganser-Pornillos, B.K.; Yeager, M. Atomic-level modelling of the HIV capsid. Nature 2011, 469, 424–427. [Google Scholar] [CrossRef]
- Gamble, T.R.; Vajdos, F.F.; Yoo, S.; Worthylake, D.K.; Houseweart, M.; Sundquist, W.I.; Hill, C.P. Crystal structure of human cyclophilin A bound to the amino-terminal domain of HIV-1 capsid. Cell 1996, 87, 1285–1294. [Google Scholar] [CrossRef]
- Gitti, R.K.; Lee, B.M.; Walker, J.; Summers, M.F.; Yoo, S.; Sundquist, W.I. Structure of the Amino-Terminal Core Domain of the HIV-1 Capsid Protein. Science 1996, 273, 231–235. [Google Scholar] [CrossRef]
- Gamble, T.R. Structure of the Carboxyl-Terminal Dimerization Domain of the HIV-1 Capsid Protein. Science 1997, 278, 849–853. [Google Scholar] [CrossRef]
- Von Schwedler, U.K. Proteolytic refolding of the HIV-1 capsid protein amino-terminus facilitates viral core assembly. EMBO J. 1998, 17, 1555–1568. [Google Scholar] [CrossRef]
- Gres, A.T.; Kirby, K.A.; KewalRamani, V.N.; Tanner, J.J.; Pornillos, O.; Sarafianos, S.G. X-ray crystal structures of native HIV-1 capsid protein reveal conformational variability. Science 2015, 349, 99–103. [Google Scholar] [CrossRef]
- Carlson, L.-A.; Briggs, J.A.G.; Glass, B.; Riches, J.D.; Simon, M.N.; Johnson, M.C.; Müller, B.; Grünewald, K.; Kräusslich, H.-G. Three-dimensional analysis of budding sites and released virus suggests a revised model for HIV-1 morphogenesis. Cell Host Microbe 2008, 4, 592–599. [Google Scholar] [CrossRef] [PubMed]
- Schur, F.K.M.; Hagen, W.J.H.; Rumlová, M.; Ruml, T.; Müller, B.; Kräusslich, H.-G.; Briggs, J.A.G. Structure of the immature HIV-1 capsid in intact virus particles at 8.8 Å resolution. Nature 2015, 517, 505–508. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wu, B.; Musier-Forsyth, K.; Mansky, L.M.; Mueller, J.D. Fluorescence fluctuation spectroscopy on viral-like particles reveals variable gag stoichiometry. Biophys. J. 2009, 96, 1961–1969. [Google Scholar] [CrossRef] [PubMed]
- Novikova, M.; Zhang, Y.; Freed, E.O.; Peng, K. Multiple Roles of HIV-1 Capsid during the Virus Replication Cycle. Virol. Sin. 2019, 34, 119–134. [Google Scholar] [CrossRef] [PubMed]
- Mattei, S.; Schur, F.K.; Briggs, J.A. Retrovirus maturation-an extraordinary structural transformation. Curr. Opin. Virol. 2016, 18, 27–35. [Google Scholar] [CrossRef]
- Kim, K.; Dauphin, A.; Komurlu, S.; McCauley, S.M.; Yurkovetskiy, L.; Carbone, C.; Diehl, W.E.; Strambio-De-Castillia, C.; Campbell, E.M.; Luban, J. Cyclophilin A protects HIV-1 from restriction by human TRIM5α. Nat. Microbiol. 2019, 4, 2044–2051. [Google Scholar] [CrossRef]
- Fernandez, J.; Portilho, D.M.; Danckaert, A.; Munier, S.; Becker, A.; Roux, P.; Zambo, A.; Shorte, S.; Jacob, Y.; Vidalain, P.-O.; et al. Microtubule-associated proteins 1 (MAP1) promote human immunodeficiency virus type I (HIV-1) intracytoplasmic routing to the nucleus. J. Biol. Chem. 2015, 290, 4631–4646. [Google Scholar] [CrossRef]
- Dharan, A.; Opp, S.; Abdel-Rahim, O.; Keceli, S.K.; Imam, S.; Diaz-Griffero, F.; Campbell, E.M. Bicaudal D2 facilitates the cytoplasmic trafficking and nuclear import of HIV-1 genomes during infection. Proc. Natl. Acad. Sci. USA 2017, 114, E10707–E10716. [Google Scholar] [CrossRef]
- Malikov, V.; da Silva, E.S.; Jovasevic, V.; Bennett, G.; de Souza Aranha Vieira, D.A.; Schulte, B.; Diaz-Griffero, F.; Walsh, D.; Naghavi, M.H. HIV-1 capsids bind and exploit the kinesin-1 adaptor FEZ1 for inward movement to the nucleus. Nat. Commun. 2015, 6, 6660. [Google Scholar] [CrossRef]
- Carnes, S.K.; Zhou, J.; Aiken, C. HIV-1 Engages a Dynein-Dynactin-BICD2 Complex for Infection and Transport to the Nucleus. J. Virol. 2018, 92, e00358-18. [Google Scholar] [CrossRef]
- Dharan, A.; Talley, S.; Tripathi, A.; Mamede, J.I.; Majetschak, M.; Hope, T.J.; Campbell, E.M. KIF5B and Nup358 Cooperatively Mediate the Nuclear Import of HIV-1 during Infection. PLoS Pathog. 2016, 12, e1005700. [Google Scholar] [CrossRef] [PubMed]
- Di Nunzio, F.; Danckaert, A.; Fricke, T.; Perez, P.; Fernandez, J.; Perret, E.; Roux, P.; Shorte, S.; Charneau, P.; Diaz-Griffero, F.; et al. Human Nucleoporins Promote HIV-1 Docking at the Nuclear Pore, Nuclear Import and Integration. PLoS ONE 2012, 7, e46037. [Google Scholar] [CrossRef] [PubMed]
- Matreyek, K.A.; Engelman, A. The Requirement for Nucleoporin NUP153 during Human Immunodeficiency Virus Type 1 Infection Is Determined by the Viral Capsid. J. Virol. 2011, 85, 7818–7827. [Google Scholar] [CrossRef] [PubMed]
- Price, A.J.; Jacques, D.A.; McEwan, W.A.; Fletcher, A.J.; Essig, S.; Chin, J.W.; Halambage, U.D.; Aiken, C.; James, L.C. Host Cofactors and Pharmacologic Ligands Share an Essential Interface in HIV-1 Capsid That Is Lost upon Disassembly. PLoS Pathog. 2014, 10, e1004459. [Google Scholar] [CrossRef] [PubMed]
- Di Nunzio, F.; Fricke, T.; Miccio, A.; Valle-Casuso, J.C.; Perez, P.; Souque, P.; Rizzi, E.; Severgnini, M.; Mavilio, F.; Charneau, P.; et al. Nup153 and Nup98 bind the HIV-1 core and contribute to the early steps of HIV-1 replication. Virology 2013, 440, 8–18. [Google Scholar] [CrossRef]
- Koh, Y.; Wu, X.; Ferris, A.L.; Matreyek, K.A.; Smith, S.J.; Lee, K.; KewalRamani, V.N.; Hughes, S.H.; Engelman, A. Differential Effects of Human Immunodeficiency Virus Type 1 Capsid and Cellular Factors Nucleoporin 153 and LEDGF/p75 on the Efficiency and Specificity of Viral DNA Integration. J. Virol. 2013, 87, 648–658. [Google Scholar] [CrossRef]
- Bhattacharya, A.; Alam, S.L.; Fricke, T.; Zadrozny, K.; Sedzicki, J.; Taylor, A.B.; Demeler, B.; Pornillos, O.; Ganser-Pornillos, B.K.; Diaz-Griffero, F.; et al. Structural basis of HIV-1 capsid recognition by PF74 and CPSF6. Proc. Natl. Acad. Sci. USA 2014, 111, 18625–18630. [Google Scholar] [CrossRef]
- Achuthan, V.; Perreira, J.M.; Sowd, G.A.; Puray-Chavez, M.; McDougall, W.M.; Paulucci-Holthauzen, A.; Wu, X.; Fadel, H.J.; Poeschla, E.M.; Multani, A.S.; et al. Capsid-CPSF6 Interaction Licenses Nuclear HIV-1 Trafficking to Sites of Viral DNA Integration. Cell Host Microbe 2018, 24, 392–404.e8. [Google Scholar] [CrossRef]
- Sowd, G.A.; Serrao, E.; Wang, H.; Wang, W.; Fadel, H.J.; Poeschla, E.M.; Engelman, A.N. A critical role for alternative polyadenylation factor CPSF6 in targeting HIV-1 integration to transcriptionally active chromatin. Proc. Natl. Acad. Sci. USA 2016, 113, E1054–E1063. [Google Scholar] [CrossRef]
- Fernandez, J.; Machado, A.K.; Lyonnais, S.; Chamontin, C.; Gärtner, K.; Léger, T.; Henriquet, C.; Garcia, C.; Portilho, D.M.; Pugnière, M.; et al. Transportin-1 binds to the HIV-1 capsid via a nuclear localization signal and triggers uncoating. Nat. Microbiol. 2019, 4, 1840–1850. [Google Scholar] [CrossRef]
- Post, K.; Olson, E.D.; Naufer, M.N.; Gorelick, R.J.; Rouzina, I.; Williams, M.C.; Musier-Forsyth, K.; Levin, J.G. Mechanistic differences between HIV-1 and SIV nucleocapsid proteins and cross-species HIV-1 genomic RNA recognition. Retrovirology 2016, 13, 89. [Google Scholar] [CrossRef] [PubMed]
- Darlix, J.-L.; Godet, J.; Ivanyi-Nagy, R.; Fossé, P.; Mauffret, O.; Mély, Y. Flexible nature and specific functions of the HIV-1 nucleocapsid protein. J. Mol. Biol. 2011, 410, 565–581. [Google Scholar] [CrossRef] [PubMed]
- McLendon, G.; Hull, H.; Larkin, K.; Chang, W. Metal binding to the HIV nucleocapsid peptide. JBIC J. Biol. Inorg. Chem. 1999, 4, 171–174. [Google Scholar] [CrossRef]
- Mély, Y.; De Rocquigny, H.; Morellet, N.; Roques, B.P.; Gérard, D. Zinc Binding to the HIV-1 Nucleocapsid Protein: A Thermodynamic Investigation by Fluorescence Spectroscopy. Biochemistry 1996, 35, 5175–5182. [Google Scholar] [CrossRef] [PubMed]
- Mély, Y.; Jullian, N.; Morellet, N.; De Rocquigny, H.; Dong, C.Z.; Piémont, E.; Roques, B.P.; Gérard, D. Spatial proximity of the HIV-1 nucleocapsid protein zinc fingers investigated by time-resolved fluorescence and fluorescence resonance energy transfer. Biochemistry 1994, 33, 12085–12091. [Google Scholar] [CrossRef]
- Morellet, N.; de Rocquigny, H.; Mély, Y.; Jullian, N.; Déméné, H.; Ottmann, M.; Gérard, D.; Darlix, J.L.; Fournie-Zaluski, M.C.; Roques, B.P. Conformational behaviour of the active and inactive forms of the nucleocapsid NCp7 of HIV-1 studied by 1H NMR. J. Mol. Biol. 1994, 235, 287–301. [Google Scholar] [CrossRef]
- Morellet, N.; Jullian, N.; De Rocquigny, H.; Maigret, B.; Darlix, J.L.; Roques, B.P. Determination of the structure of the nucleocapsid protein NCp7 from the human immunodeficiency virus type 1 by 1H NMR. EMBO J. 1992, 11, 3059–3065. [Google Scholar] [CrossRef]
- Aldovini, A.; Young, R.A. Mutations of RNA and protein sequences involved in human immunodeficiency virus type 1 packaging result in production of noninfectious virus. J. Virol. 1990, 64, 1920–1926. [Google Scholar] [CrossRef]
- Comas-Garcia, M.; Datta, S.A.; Baker, L.; Varma, R.; Gudla, P.R.; Rein, A. Dissection of specific binding of HIV-1 Gag to the “packaging signal” in viral RNA. eLife 2017, 6, e27055. [Google Scholar] [CrossRef]
- Demene, H.; Dong, C.Z.; Ottmann, M.; Rouyez, M.C.; Jullian, N.; Morellet, N.; Mely, Y.; Darlix, J.L.; Fournie-Zaluski, M.C. 1H NMR structure and biological studies of the His23. fwdarw. Cys mutant nucleocapsid protein of HIV-1 indicate that the conformation of the first zinc finger is critical for virus infectivity. Biochemistry 1994, 33, 11707–11716. [Google Scholar] [CrossRef]
- Dorfman, T.; Luban, J.; Goff, S.P.; Haseltine, W.A.; Göttlinger, H.G. Mapping of functionally important residues of a cysteine-histidine box in the human immunodeficiency virus type 1 nucleocapsid protein. J. Virol. 1993, 67, 6159–6169. [Google Scholar] [CrossRef] [PubMed]
- Gorelick, R.J.; Nigida, S.M.; Bess, J.W.; Arthur, L.O.; Henderson, L.E.; Rein, A. Noninfectious human immunodeficiency virus type 1 mutants deficient in genomic RNA. J. Virol. 1990, 64, 3207–3211. [Google Scholar] [CrossRef] [PubMed]
- Amarasinghe, G.K.; De Guzman, R.N.; Turner, R.B.; Chancellor, K.J.; Wu, Z.R.; Summers, M.F. NMR structure of the HIV-1 nucleocapsid protein bound to stem-loop SL2 of the psi-RNA packaging signal. Implications for genome recognition. J. Mol. Biol. 2000, 301, 491–511. [Google Scholar] [CrossRef] [PubMed]
- Bourbigot, S.; Ramalanjaona, N.; Boudier, C.; Salgado, G.F.J.; Roques, B.P.; Mély, Y.; Bouaziz, S.; Morellet, N. How the HIV-1 Nucleocapsid Protein Binds and Destabilises the (−)Primer Binding Site During Reverse Transcription. J. Mol. Biol. 2008, 383, 1112–1128. [Google Scholar] [CrossRef] [PubMed]
- De Guzman, R.N. Structure of the HIV-1 Nucleocapsid Protein Bound to the SL3 -RNA Recognition Element. Science 1998, 279, 384–388. [Google Scholar] [CrossRef]
- Godet, J.; Kenfack, C.; Przybilla, F.; Richert, L.; Duportail, G.; Mély, Y. Site-selective probing of cTAR destabilization highlights the necessary plasticity of the HIV-1 nucleocapsid protein to chaperone the first strand transfer. Nucleic Acids Res. 2013, 41, 5036–5048. [Google Scholar] [CrossRef]
- Godet, J.; Boudier, C.; Humbert, N.; Ivanyi-Nagy, R.; Darlix, J.-L.; Mély, Y. Comparative nucleic acid chaperone properties of the nucleocapsid protein NCp7 and Tat protein of HIV-1. Virus Res. 2012, 169, 349–360. [Google Scholar] [CrossRef]
- Beltz, H.; Piémont, E.; Schaub, E.; Ficheux, D.; Roques, B.; Darlix, J.-L.; Mély, Y. Role of the Structure of the Top Half of HIV-1 cTAR DNA on the Nucleic Acid Destabilizing Activity of the Nucleocapsid Protein NCp7. J. Mol. Biol. 2004, 338, 711–723. [Google Scholar] [CrossRef]
- Beltz, H.; Azoulay, J.; Bernacchi, S.; Clamme, J.-P.; Ficheux, D.; Roques, B.; Darlix, J.-L.; Mély, Y. Impact of the Terminal Bulges of HIV-1 cTAR DNA on its Stability and the Destabilizing Activity of the Nucleocapsid Protein NCp7. J. Mol. Biol. 2003, 328, 95–108. [Google Scholar] [CrossRef]
- Bernacchi, S.; Stoylov, S.; Piémont, E.; Ficheux, D.; Roques, B.P.; Darlix, J.L.; Mély, Y. HIV-1 nucleocapsid protein activates transient melting of least stable parts of the secondary structure of TAR and its complementary sequence. J. Mol. Biol. 2002, 317, 385–399. [Google Scholar] [CrossRef]
- Comas-Garcia, M.; Davis, S.; Rein, A. On the Selective Packaging of Genomic RNA by HIV-1. Viruses 2016, 8, 246. [Google Scholar] [CrossRef] [PubMed]
- Cosa, G.; Zeng, Y.; Liu, H.-W.; Landes, C.F.; Makarov, D.E.; Musier-Forsyth, K.; Barbara, P.F. Evidence for Non-Two-State Kinetics in the Nucleocapsid Protein Chaperoned Opening of DNA Hairpins. J. Phys. Chem. B 2006, 110, 2419–2426. [Google Scholar] [CrossRef] [PubMed]
- Godet, J.; Mély, Y. Biophysical studies of the nucleic acid chaperone properties of the HIV-1 nucleocapsid protein. RNA Biol. 2010, 7, 687–699. [Google Scholar] [CrossRef] [PubMed]
- Hargittai, M.R.S.; Gorelick, R.J.; Rouzina, I.; Musier-Forsyth, K. Mechanistic Insights into the Kinetics of HIV-1 Nucleocapsid Protein-facilitated tRNA Annealing to the Primer Binding Site. J. Mol. Biol. 2004, 337, 951–968. [Google Scholar] [CrossRef] [PubMed]
- Rein, A.; Henderson, L.E.; Levin, J.G. Nucleic-acid-chaperone activity of retroviral nucleocapsid proteins: Significance for viral replication. Trends Biochem. Sci. 1998, 23, 297–301. [Google Scholar] [CrossRef]
- Vo, M.-N.; Barany, G.; Rouzina, I.; Musier-Forsyth, K. Mechanistic Studies of Mini-TAR RNA/DNA Annealing in the Absence and Presence of HIV-1 Nucleocapsid Protein. J. Mol. Biol. 2006, 363, 244–261. [Google Scholar] [CrossRef] [PubMed]
- Williams, M.C.; Rouzina, I.; Wenner, J.R.; Gorelick, R.J.; Musier-Forsyth, K.; Bloomfield, V.A. Mechanism for nucleic acid chaperone activity of HIV-1 nucleocapsid protein revealed by single molecule stretching. Proc. Natl. Acad. Sci. USA 2001, 98, 6121–6126. [Google Scholar] [CrossRef]
- Vo, M.-N.; Barany, G.; Rouzina, I.; Musier-Forsyth, K. HIV-1 Nucleocapsid Protein Switches the Pathway of Transactivation Response Element RNA/DNA Annealing from Loop–Loop “Kissing” to “Zipper”. J. Mol. Biol. 2009, 386, 789–801. [Google Scholar] [CrossRef]
- Levin, J.G.; Mitra, M.; Mascarenhas, A.; Musier-Forsyth, K. Role of HIV-1 nucleocapsid protein in HIV-1 reverse transcription. RNA Biol. 2010, 7, 754–774. [Google Scholar] [CrossRef]
- Levin, J.G.; Guo, J.; Rouzina, I.; Musier-Forsyth, K. Nucleic acid chaperone activity of HIV-1 nucleocapsid protein: Critical role in reverse transcription and molecular mechanism. Prog. Nucleic Acid Res. Mol. Biol. 2005, 80, 217–286. [Google Scholar] [CrossRef]
- Poljak, L.; Batson, S.M.; Ficheux, D.; Roques, B.P.; Darlix, J.-L.; Käs, E. Analysis of NCp7-dependent activation of HIV-1 cDNA integration and its conservation among retroviral nucleocapsid proteins. J. Mol. Biol. 2003, 329, 411–421. [Google Scholar] [CrossRef]
- Abd El-Wahab, E.W.; Smyth, R.P.; Mailler, E.; Bernacchi, S.; Vivet-Boudou, V.; Hijnen, M.; Jossinet, F.; Mak, J.; Paillart, J.-C.; Marquet, R. Specific recognition of the HIV-1 genomic RNA by the Gag precursor. Nat. Commun. 2014, 5, 4304. [Google Scholar] [CrossRef] [PubMed]
- Bernacchi, S.; Abd El-Wahab, E.W.; Dubois, N.; Hijnen, M.; Smyth, R.P.; Mak, J.; Marquet, R.; Paillart, J.-C. HIV-1 Pr55 Gag binds genomic and spliced RNAs with different affinity and stoichiometry. RNA Biol. 2017, 14, 90–103. [Google Scholar] [CrossRef] [PubMed]
- Dubois, N.; Khoo, K.K.; Ghossein, S.; Seissler, T.; Wolff, P.; McKinstry, W.J.; Mak, J.; Paillart, J.-C.; Marquet, R.; Bernacchi, S. The C-terminal p6 domain of the HIV-1 Pr55 Gag precursor is required for specific binding to the genomic RNA. RNA Biol. 2018, 15, 923–936. [Google Scholar] [CrossRef]
- Ferrer, M.; Clerté, C.; Chamontin, C.; Basyuk, E.; Lainé, S.; Hottin, J.; Bertrand, E.; Margeat, E.; Mougel, M. Imaging HIV-1 RNA dimerization in cells by multicolor super-resolution and fluctuation microscopies. Nucleic Acids Res. 2016, 44, 7922–7934. [Google Scholar] [CrossRef]
- Kutluay, S.B.; Bieniasz, P.D. Analysis of the initiating events in HIV-1 particle assembly and genome packaging. PLoS Pathog. 2010, 6, e1001200. [Google Scholar] [CrossRef]
- Kuzembayeva, M.; Dilley, K.; Sardo, L.; Hu, W.-S. Life of psi: How full-length HIV-1 RNAs become packaged genomes in the viral particles. Virology 2014, 454–455, 362–370. [Google Scholar] [CrossRef]
- Lu, K.; Heng, X.; Summers, M.F. Structural Determinants and Mechanism of HIV-1 Genome Packaging. J. Mol. Biol. 2011, 410, 609–633. [Google Scholar] [CrossRef]
- Nikolaitchik, O.A.; Dilley, K.A.; Fu, W.; Gorelick, R.J.; Tai, S.-H.S.; Soheilian, F.; Ptak, R.G.; Nagashima, K.; Pathak, V.K.; Hu, W.-S. Dimeric RNA Recognition Regulates HIV-1 Genome Packaging. PLoS Pathog. 2013, 9, e1003249. [Google Scholar] [CrossRef]
- Rein, A. RNA Packaging in HIV. Trends Microbiol. 2019, 27, 715–723. [Google Scholar] [CrossRef]
- Webb, J.A.; Jones, C.P.; Parent, L.J.; Rouzina, I.; Musier-Forsyth, K. Distinct binding interactions of HIV-1 Gag to Psi and non-Psi RNAs: Implications for viral genomic RNA packaging. RNA N. Y. N 2013, 19, 1078–1088. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Rahman, S.A.; Nikolaitchik, O.A.; Grunwald, D.; Sardo, L.; Burdick, R.C.; Plisov, S.; Liang, E.; Tai, S.; Pathak, V.K.; et al. HIV-1 RNA genome dimerizes on the plasma membrane in the presence of Gag protein. Proc. Natl. Acad. Sci. USA 2016, 113, E201–E208. [Google Scholar] [CrossRef] [PubMed]
- Dilley, K.A.; Nikolaitchik, O.A.; Galli, A.; Burdick, R.C.; Levine, L.; Li, K.; Rein, A.; Pathak, V.K.; Hu, W.-S. Interactions between HIV-1 Gag and Viral RNA Genome Enhance Virion Assembly. J. Virol. 2017, 91, e02319-16. [Google Scholar] [CrossRef] [PubMed]
- Datta, S.A.K.; Zhao, Z.; Clark, P.K.; Tarasov, S.; Alexandratos, J.N.; Campbell, S.J.; Kvaratskhelia, M.; Lebowitz, J.; Rein, A. Interactions between HIV-1 Gag molecules in solution: An inositol phosphate-mediated switch. J. Mol. Biol. 2007, 365, 799–811. [Google Scholar] [CrossRef]
- Datta, S.A.K.; Curtis, J.E.; Ratcliff, W.; Clark, P.K.; Crist, R.M.; Lebowitz, J.; Krueger, S.; Rein, A. Conformation of the HIV-1 Gag Protein in Solution. J. Mol. Biol. 2007, 365, 812–824. [Google Scholar] [CrossRef]
- Munro, J.B.; Nath, A.; Farber, M.; Datta, S.A.K.; Rein, A.; Rhoades, E.; Mothes, W. A Conformational Transition Observed in Single HIV-1 Gag Molecules during In Vitro Assembly of Virus-Like Particles. J. Virol. 2014, 88, 3577–3585. [Google Scholar] [CrossRef]
- Kempf, N.; Postupalenko, V.; Bora, S.; Didier, P.; Arntz, Y.; de Rocquigny, H.; Mély, Y. The HIV-1 Nucleocapsid Protein Recruits Negatively Charged Lipids To Ensure Its Optimal Binding to Lipid Membranes. J. Virol. 2015, 89, 1756–1767. [Google Scholar] [CrossRef]
- Sun, M.; Grigsby, I.F.; Gorelick, R.J.; Mansky, L.M.; Musier-Forsyth, K. Retrovirus-Specific Differences in Matrix and Nucleocapsid Protein-Nucleic Acid Interactions: Implications for Genomic RNA Packaging. J. Virol. 2014, 88, 1271–1280. [Google Scholar] [CrossRef]
- Tisné, C.; Roques, B.P.; Dardel, F. Heteronuclear NMR studies of the interaction of tRNA3Lys with HIV-1 nucleocapsid protein11Edited by M. F. Summers. J. Mol. Biol. 2001, 306, 443–454. [Google Scholar] [CrossRef]
- Liu, B.; Dai, R.; Tian, C.J.; Dawson, L.; Gorelick, R.; Yu, X.F. Interaction of the human immunodeficiency virus type 1 nucleocapsid with actin. J. Virol. 1999, 73, 2901–2908. [Google Scholar] [CrossRef]
- Alce, T.M.; Popik, W. APOBEC3G is incorporated into virus-like particles by a direct interaction with HIV-1 Gag nucleocapsid protein. J. Biol. Chem. 2004, 279, 34083–34086. [Google Scholar] [CrossRef]
- El Meshri, S.E.; Dujardin, D.; Godet, J.; Richert, L.; Boudier, C.; Darlix, J.L.; Didier, P.; Mély, Y.; de Rocquigny, H. Role of the Nucleocapsid Domain in HIV-1 Gag Oligomerization and Trafficking to the Plasma Membrane: A Fluorescence Lifetime Imaging Microscopy Investigation. J. Mol. Biol. 2015, 427, 1480–1494. [Google Scholar] [CrossRef] [PubMed]
- Friedrich, M.; Setz, C.; Hahn, F.; Matthaei, A.; Fraedrich, K.; Rauch, P.; Henklein, P.; Traxdorf, M.; Fossen, T.; Schubert, U. Glutamic Acid Residues in HIV-1 p6 Regulate Virus Budding and Membrane Association of Gag. Viruses 2016, 8, 117. [Google Scholar] [CrossRef]
- Garrus, J.E.; von Schwedler, U.K.; Pornillos, O.W.; Morham, S.G.; Zavitz, K.H.; Wang, H.E.; Wettstein, D.A.; Stray, K.M.; Côté, M.; Rich, R.L.; et al. Tsg101 and the vacuolar protein sorting pathway are essential for HIV-1 budding. Cell 2001, 107, 55–65. [Google Scholar] [CrossRef]
- Gurer, C.; Berthoux, L.; Luban, J. Covalent Modification of Human Immunodeficiency Virus Type 1 p6 by SUMO-1. J. Virol. 2005, 79, 910–917. [Google Scholar] [CrossRef] [PubMed]
- Hemonnot, B.; Cartier, C.; Gay, B.; Rebuffat, S.; Bardy, M.; Devaux, C.; Boyer, V.; Briant, L. The Host Cell MAP Kinase ERK-2 Regulates Viral Assembly and Release by Phosphorylating the p6gag Protein of HIV-1. J. Biol. Chem. 2004, 279, 32426–32434. [Google Scholar] [CrossRef]
- Müller, B.; Patschinsky, T.; Kräusslich, H.-G. The Late-Domain-Containing Protein p6 Is the Predominant Phosphoprotein of Human Immunodeficiency Virus Type 1 Particles. J. Virol. 2002, 76, 1015–1024. [Google Scholar] [CrossRef]
- Ott, D.E.; Coren, L.V.; Chertova, E.N.; Gagliardi, T.D.; Schubert, U. Ubiquitination of HIV-1 and MuLV Gag. Virology 2000, 278, 111–121. [Google Scholar] [CrossRef]
- Ott, D.E.; Coren, L.V.; Copeland, T.D.; Kane, B.P.; Johnson, D.G.; Sowder, R.C.; Yoshinaka, Y.; Oroszlan, S.; Arthur, L.O.; Henderson, L.E. Ubiquitin Is Covalently Attached to the p6GagProteins of Human Immunodeficiency Virus Type 1 and Simian Immunodeficiency Virus and to the p12Gag Protein of Moloney Murine Leukemia Virus. J. Virol. 1998, 72, 2962–2968. [Google Scholar] [CrossRef]
- Watanabe, S.M.; Chen, M.-H.; Khan, M.; Ehrlich, L.; Kemal, K.S.; Weiser, B.; Shi, B.; Chen, C.; Powell, M.; Anastos, K.; et al. The S40 residue in HIV-1 Gag p6 impacts local and distal budding determinants, revealing additional late domain activities. Retrovirology 2013, 10, 143. [Google Scholar] [CrossRef]
- Hurley, J.H.; Hanson, P.I. Membrane budding and scission by the ESCRT machinery: It’s all in the neck. Nat. Rev. Mol. Cell Biol. 2010, 11, 556–566. [Google Scholar] [CrossRef] [PubMed]
- Im, Y.J.; Hurley, J.H. Integrated structural model and membrane targeting mechanism of the human ESCRT-II complex. Dev. Cell 2008, 14, 902–913. [Google Scholar] [CrossRef] [PubMed]
- Odorizzi, G. Membrane manipulations by the ESCRT machinery. F1000Research 2015, 4, 516. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, O.; Teis, D. The ESCRT machinery. Curr. Biol. CB 2012, 22, R116–R120. [Google Scholar] [CrossRef] [PubMed]
- Göttlinger, H.G.; Dorfman, T.; Sodroski, J.G.; Haseltine, W.A. Effect of mutations affecting the p6 gag protein on human immunodeficiency virus particle release. Proc. Natl. Acad. Sci. USA 1991, 88, 3195–3199. [Google Scholar] [CrossRef]
- Huang, M.; Orenstein, J.M.; Martin, M.A.; Freed, E.O. p6Gag is required for particle production from full-length human immunodeficiency virus type 1 molecular clones expressing protease. J. Virol. 1995, 69, 6810–6818. [Google Scholar] [CrossRef]
- Demirov, D.G.; Ono, A.; Orenstein, J.M.; Freed, E.O. Overexpression of the N-terminal domain of TSG101 inhibits HIV-1 budding by blocking late domain function. Proc. Natl. Acad. Sci. USA 2002, 99, 955–960. [Google Scholar] [CrossRef]
- Goila-Gaur, R.; Demirov, D.G.; Orenstein, J.M.; Ono, A.; Freed, E.O. Defects in Human Immunodeficiency Virus Budding and Endosomal Sorting Induced by TSG101 Overexpression. J. Virol. 2003, 77, 6507–6519. [Google Scholar] [CrossRef]
- VerPlank, L.; Bouamr, F.; LaGrassa, T.J.; Agresta, B.; Kikonyogo, A.; Leis, J.; Carter, C.A. Tsg101, a homologue of ubiquitin-conjugating (E2) enzymes, binds the L domain in HIV type 1 Pr55Gag. Proc. Natl. Acad. Sci. USA 2001, 98, 7724–7729. [Google Scholar] [CrossRef]
- Morita, E.; Sandrin, V.; McCullough, J.; Katsuyama, A.; Baci Hamilton, I.; Sundquist, W.I. ESCRT-III protein requirements for HIV-1 budding. Cell Host Microbe 2011, 9, 235–242. [Google Scholar] [CrossRef]
- Peel, S.; Macheboeuf, P.; Martinelli, N.; Weissenhorn, W. Divergent pathways lead to ESCRT-III-catalyzed membrane fission. Trends Biochem. Sci. 2011, 36, 199–210. [Google Scholar] [CrossRef]
- Usami, Y.; Popov, S.; Popova, E.; Inoue, M.; Weissenhorn, W.; G Göttlinger, H. The ESCRT pathway and HIV-1 budding. Biochem. Soc. Trans. 2009, 37, 181–184. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, S.M.; Strickland, M.; Tjandra, N.; Carter, C.A. RNA Binding Suppresses Tsg101 Recognition of Ub-Modified Gag and Facilitates Recruitment to the Plasma Membrane. Viruses 2020, 12, 447. [Google Scholar] [CrossRef] [PubMed]
- Fisher, R.D.; Chung, H.-Y.; Zhai, Q.; Robinson, H.; Sundquist, W.I.; Hill, C.P. Structural and Biochemical Studies of ALIX/AIP1 and Its Role in Retrovirus Budding. Cell 2007, 128, 841–852. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Joshi, A.; Nagashima, K.; Freed, E.O.; Hurley, J.H. Structural basis for viral late-domain binding to Alix. Nat. Struct. Mol. Biol. 2007, 14, 194–199. [Google Scholar] [CrossRef] [PubMed]
- Martin-Serrano, J.; Yarovoy, A.; Perez-Caballero, D.; Bieniasz, P.D.; Yaravoy, A. Divergent retroviral late-budding domains recruit vacuolar protein sorting factors by using alternative adaptor proteins. Proc. Natl. Acad. Sci. USA 2003, 100, 12414–12419. [Google Scholar] [CrossRef]
- Von Schwedler, U.K.; Stuchell, M.; Müller, B.; Ward, D.M.; Chung, H.-Y.; Morita, E.; Wang, H.E.; Davis, T.; He, G.-P.; Cimbora, D.M.; et al. The Protein Network of HIV Budding. Cell 2003, 114, 701–713. [Google Scholar] [CrossRef]
- Zhai, Q.; Fisher, R.D.; Chung, H.-Y.; Myszka, D.G.; Sundquist, W.I.; Hill, C.P. Structural and functional studies of ALIX interactions with YPXnL late domains of HIV-1 and EIAV. Nat. Struct. Mol. Biol. 2008, 15, 43–49. [Google Scholar] [CrossRef]
- Martin-Serrano, J.; Bieniasz, P.D. A Bipartite Late-Budding Domain in Human Immunodeficiency Virus Type 1. J. Virol. 2003, 77, 12373–12377. [Google Scholar] [CrossRef]
- Strack, B.; Calistri, A.; Craig, S.; Popova, E.; Göttlinger, H.G. AIP1/ALIX is a binding partner for HIV-1 p6 and EIAV p9 functioning in virus budding. Cell 2003, 114, 689–699. [Google Scholar] [CrossRef]
- Usami, Y.; Popov, S.; Göttlinger, H.G. Potent rescue of human immunodeficiency virus type 1 late domain mutants by ALIX/AIP1 depends on its CHMP4 binding site. J. Virol. 2007, 81, 6614–6622. [Google Scholar] [CrossRef] [PubMed]
- Bendjennat, M.; Saffarian, S. The Race against Protease Activation Defines the Role of ESCRTs in HIV Budding. PLoS Pathog. 2016, 12, e1005657. [Google Scholar] [CrossRef]
- Kondo, E.; Göttlinger, H.G. A conserved LXXLF sequence is the major determinant in p6gag required for the incorporation of human immunodeficiency virus type 1 Vpr. J. Virol. 1996, 70, 159–164. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.L.; Bennett, R.P.; Wills, J.W.; Gorelick, R.; Ratner, L. A leucine triplet repeat sequence (LXX)4 in p6gag is important for Vpr incorporation into human immunodeficiency virus type 1 particles. J. Virol. 1995, 69, 6873–6879. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Jian, H.; Zhao, L.-J. Identification of the 15FRFG domain in HIV-1 Gag p6 essential for Vpr packaging into the virion. Retrovirology 2004, 1, 26. [Google Scholar] [CrossRef]
- Salgado, G.F.; Vogel, A.; Marquant, R.; Feller, S.E.; Bouaziz, S.; Alves, I.D. The Role of Membranes in the Organization of HIV-1 Gag p6 and Vpr: p6 Shows High Affinity for Membrane Bilayers Which Substantially Increases the Interaction between p6 and Vpr. J. Med. Chem. 2009, 52, 7157–7162. [Google Scholar] [CrossRef]
- Fritz, J.V.; Dujardin, D.; Godet, J.; Didier, P.; De Mey, J.; Darlix, J.-L.; Mély, Y.; de Rocquigny, H. HIV-1 Vpr oligomerization but not that of Gag directs the interaction between Vpr and Gag. J. Virol. 2010, 84, 1585–1596. [Google Scholar] [CrossRef]
- Dettenhofer, M.; Yu, X.-F. Proline Residues in Human Immunodeficiency Virus Type 1 p6Gag Exert a Cell Type-Dependent Effect on Viral Replication and Virion Incorporation of Pol Proteins. J. Virol. 1999, 73, 4696–4704. [Google Scholar] [CrossRef]
- Yu, X.-F.; Dawson, L.; Tian, C.-J.; Flexner, C.; Dettenhofer, M. Mutations of the Human Immunodeficiency Virus Type 1 p6Gag Domain Result in Reduced Retention of Pol Proteins during Virus Assembly. J. Virol. 1998, 72, 3412–3417. [Google Scholar] [CrossRef]
- Bleiber, G.; Peters, S.; Martinez, R.; Cmarko, D.; Meylan, P.; Telenti, A. The central region of human immunodeficiency virus type 1 p6 protein (Gag residues S14–I31) is dispensable for the virus in vitro. J. Gen. Virol. 2004, 85, 921–927. [Google Scholar] [CrossRef]
- Datta, S.A.K.; Clark, P.K.; Fan, L.; Ma, B.; Harvin, D.P.; Sowder, R.C.; Nussinov, R.; Wang, Y.-X.; Rein, A. Dimerization of the SP1 Region of HIV-1 Gag Induces a Helical Conformation and Association into Helical Bundles: Implications for Particle Assembly. J. Virol. 2016, 90, 1773–1787. [Google Scholar] [CrossRef] [PubMed]
- Datta, S.A.K.; Temeselew, L.G.; Crist, R.M.; Soheilian, F.; Kamata, A.; Mirro, J.; Harvin, D.; Nagashima, K.; Cachau, R.E.; Rein, A. On the Role of the SP1 Domain in HIV-1 Particle Assembly: A Molecular Switch? J. Virol. 2011, 85, 4111–4121. [Google Scholar] [CrossRef] [PubMed]
- Accola, M.A.; Höglund, S.; Göttlinger, H.G. A Putative α-Helical Structure Which Overlaps the Capsid-p2 Boundary in the Human Immunodeficiency Virus Type 1 Gag Precursor Is Crucial for Viral Particle Assembly. J. Virol. 1998, 72, 2072–2078. [Google Scholar] [CrossRef] [PubMed]
- Bell, N.M.; Lever, A.M.L. HIV Gag polyprotein: Processing and early viral particle assembly. Trends Microbiol. 2013, 21, 136–144. [Google Scholar] [CrossRef]
- Kräusslich, H.G.; Fäcke, M.; Heuser, A.M.; Konvalinka, J.; Zentgraf, H. The spacer peptide between human immunodeficiency virus capsid and nucleocapsid proteins is essential for ordered assembly and viral infectivity. J. Virol. 1995, 69, 3407–3419. [Google Scholar] [CrossRef]
- Morellet, N. Helical structure determined by NMR of the HIV-1 (345-392)Gag sequence, surrounding p2: Implications for particle assembly and RNA packaging. Protein Sci. 2005, 14, 375–386. [Google Scholar] [CrossRef]
- Guo, X.; Roldan, A.; Hu, J.; Wainberg, M.A.; Liang, C. Mutation of the SP1 Sequence Impairs both Multimerization and Membrane-Binding Activities of Human Immunodeficiency Virus Type 1 Gag. J. Virol. 2005, 79, 1803–1812. [Google Scholar] [CrossRef][Green Version]
- Dick, R.A.; Zadrozny, K.K.; Xu, C.; Schur, F.K.M.; Lyddon, T.D.; Ricana, C.L.; Wagner, J.M.; Perilla, J.R.; Ganser-Pornillos, B.K.; Johnson, M.C.; et al. Inositol phosphates are assembly co-factors for HIV-1. Nature 2018, 560, 509–512. [Google Scholar] [CrossRef]
- Kaye, J.F.; Lever, A.M.L. Nonreciprocal Packaging of Human Immunodeficiency Virus Type 1 and Type 2 RNA: A Possible Role for the p2 Domain of Gag in RNA Encapsidation. J. Virol. 1998, 72, 5877–5885. [Google Scholar] [CrossRef]
- Roldan, A.; Russell, R.S.; Marchand, B.; Götte, M.; Liang, C.; Wainberg, M.A. In Vitro Identification and Characterization of an Early Complex Linking HIV-1 Genomic RNA Recognition and Pr55 Gag Multimerization. J. Biol. Chem. 2004, 279, 39886–39894. [Google Scholar] [CrossRef]
- Russell, R.S.; Roldan, A.; Detorio, M.; Hu, J.; Wainberg, M.A.; Liang, C. Effects of a Single Amino Acid Substitution within thep2 Region of Human Immunodeficiency Virus Type 1 on Packagingof Spliced ViralRNA. J. Virol. 2003, 77, 12986–12995. [Google Scholar] [CrossRef] [PubMed]
- Ristic, N.; Chin, M.P. Mutations in matrix and SP1 repair the packaging specificity of a Human Immunodeficiency Virus Type 1 mutant by reducing the association of Gag with spliced viral RNA. Retrovirology 2010, 7, 73. [Google Scholar] [CrossRef] [PubMed]
- Hill, M.K.; Bellamy-McIntyre, A.; Vella, L.J.; Campbell, S.M.; Marshall, J.A.; Tachedjian, G.; Mak, J. Alteration of the proline at position 7 of the HIV-1 spacer peptide p1 suppresses viral infectivity in a strain dependent manner. Curr. HIV Res. 2007, 5, 69–78. [Google Scholar] [CrossRef] [PubMed]
- De Marco, A.; Heuser, A.-M.; Glass, B.; Krausslich, H.-G.; Muller, B.; Briggs, J.A.G. Role of the SP2 Domain and Its Proteolytic Cleavage in HIV-1 Structural Maturation and Infectivity. J. Virol. 2012, 86, 13708–13716. [Google Scholar] [CrossRef]
- Dooher, J.E.; Schneider, B.L.; Reed, J.C.; Lingappa, J.R. Host ABCE1 is at plasma membrane HIV assembly sites and its dissociation from Gag is linked to subsequent events of virus production. Traffic Cph. Den. 2007, 8, 195–211. [Google Scholar] [CrossRef]
- Dooher, J.E.; Lingappa, J.R. Conservation of a Stepwise, Energy-Sensitive Pathway Involving HP68 for Assembly of Primate Lentivirus Capsids in Cells. J. Virol. 2004, 78, 1645–1656. [Google Scholar] [CrossRef][Green Version]
- Lingappa, J.R. Basic Residues in the Nucleocapsid Domain of Gag Are Required for Interaction of HIV-1 Gag with ABCE1 (HP68), a Cellular Protein Important for HIV-1 Capsid Assembly. J. Biol. Chem. 2006, 281, 3773–3784. [Google Scholar] [CrossRef]
- Smirnova, E.V.; Collingwood, T.S.; Bisbal, C.; Tsygankova, O.M.; Bogush, M.; Meinkoth, J.L.; Henderson, E.E.; Annan, R.S.; Tsygankov, A.Y. TULA proteins bind to ABCE-1, a host factor of HIV-1 assembly, and inhibit HIV-1 biogenesis in a UBA-dependent fashion. Virology 2008, 372, 10–23. [Google Scholar] [CrossRef]
- Ibarrondo, F.J.; Choi, R.; Geng, Y.Z.; Canon, J.; Rey, O.; Baldwin, G.C.; Krogstad, P. HIV type 1 Gag and nucleocapsid proteins: Cytoskeletal localization and effects on cell motility. AIDS Res. Hum. Retroviruses 2001, 17, 1489–1500. [Google Scholar] [CrossRef]
- Wilk, T.; Gowen, B.; Fuller, S.D. Actin associates with the nucleocapsid domain of the human immunodeficiency virus Gag polyprotein. J. Virol. 1999, 73, 1931–1940. [Google Scholar] [CrossRef]
- Poole, E.; Strappe, P.; Mok, H.-P.; Hicks, R.; Lever, A.M.L. HIV-1 Gag-RNA Interaction Occurs at a Perinuclear/Centrosomal Site; Analysis by Confocal Microscopy and FRET: HIV-1 Gag-RNA Interaction Occurs in a Perinuclear Region. Traffic 2005, 6, 741–755. [Google Scholar] [CrossRef]
- Rey, O.; Canon, J.; Krogstad, P. HIV-1 Gag protein associates with F-actin present in microfilaments. Virology 1996, 220, 530–534. [Google Scholar] [CrossRef] [PubMed]
- Stauffer, S.; Rahman, S.A.; de Marco, A.; Carlson, L.-A.; Glass, B.; Oberwinkler, H.; Herold, N.; Briggs, J.A.G.; Müller, B.; Grünewald, K.; et al. The Nucleocapsid Domain of Gag Is Dispensable for Actin Incorporation into HIV-1 and for Association of Viral Budding Sites with Cortical F-Actin. J. Virol. 2014, 88, 7893–7903. [Google Scholar] [CrossRef] [PubMed]
- Orecchini, E.; Federico, M.; Doria, M.; Arenaccio, C.; Giuliani, E.; Ciafrè, S.A.; Michienzi, A. The ADAR1 editing enzyme is encapsidated into HIV-1 virions. Virology 2015, 485, 475–480. [Google Scholar] [CrossRef] [PubMed]
- Mercenne, G.; Alam, S.L.; Arii, J.; Lalonde, M.S.; Sundquist, W.I. Angiomotin functions in HIV-1 assembly and budding. eLife 2015, 4, e03778. [Google Scholar] [CrossRef]
- Bouttier, M.; Saumet, A.; Peter, M.; Courgnaud, V.; Schmidt, U.; Cazevieille, C.; Bertrand, E.; Lecellier, C.-H. Retroviral GAG proteins recruit AGO2 on viral RNAs without affecting RNA accumulation and translation. Nucleic Acids Res. 2012, 40, 775–786. [Google Scholar] [CrossRef]
- Dussupt, V.; Javid, M.P.; Abou-Jaoudé, G.; Jadwin, J.A.; de La Cruz, J.; Nagashima, K.; Bouamr, F. The nucleocapsid region of HIV-1 Gag cooperates with the PTAP and LYPXnL late domains to recruit the cellular machinery necessary for viral budding. PLoS Pathog. 2009, 5, e1000339. [Google Scholar] [CrossRef]
- Popov, S.; Popova, E.; Inoue, M.; Göttlinger, H.G. Divergent Bro1 domains share the capacity to bind human immunodeficiency virus type 1 nucleocapsid and to enhance virus-like particle production. J. Virol. 2009, 83, 7185–7193. [Google Scholar] [CrossRef]
- Popov, S.; Popova, E.; Inoue, M.; Göttlinger, H.G. Human immunodeficiency virus type 1 Gag engages the Bro1 domain of ALIX/AIP1 through the nucleocapsid. J. Virol. 2008, 82, 1389–1398. [Google Scholar] [CrossRef]
- Sette, P.; O’Connor, S.K.; Yerramilli, V.S.; Dussupt, V.; Nagashima, K.; Chutiraka, K.; Lingappa, J.; Scarlata, S.; Bouamr, F. HIV-1 Nucleocapsid Mimics the Membrane Adaptor Syntenin PDZ to Gain Access to ESCRTs and Promote Virus Budding. Cell Host Microbe 2016, 19, 336–348. [Google Scholar] [CrossRef]
- Sette, P.; Dussupt, V.; Bouamr, F. Identification of the HIV-1 NC Binding Interface in Alix Bro1 Reveals a Role for RNA. J. Virol. 2012, 86, 11608–11615. [Google Scholar] [CrossRef] [PubMed]
- Harrist, A.V.; Ryzhova, E.V.; Harvey, T.; González-Scarano, F. Anx2 interacts with HIV-1 Gag at phosphatidylinositol (4,5) bisphosphate-containing lipid rafts and increases viral production in 293T cells. PLoS ONE 2009, 4, e5020. [Google Scholar] [CrossRef] [PubMed]
- Ryzhova, E.V.; Vos, R.M.; Albright, A.V.; Harrist, A.V.; Harvey, T.; González-Scarano, F. Annexin 2: A Novel Human Immunodeficiency Virus Type 1 Gag Binding Protein Involved in Replication in Monocyte-Derived Macrophages. J. Virol. 2006, 80, 2694–2704. [Google Scholar] [CrossRef] [PubMed]
- Camus, G.; Segura-Morales, C.; Molle, D.; Lopez-Verges, S.; Begon-Pescia, C.; Cazevieille, C.; Schu, P.; Bertrand, E.; Berlioz-Torrent, C.; Basyuk, E. The Clathrin Adaptor Complex AP-1 Binds HIV-1 and MLV Gag and Facilitates Their Budding□D. Mol. Biol. Cell 2007, 18, 11. [Google Scholar] [CrossRef]
- Batonick, M.; Favre, M.; Boge, M.; Spearman, P.; Höning, S.; Thali, M. Interaction of HIV-1 Gag with the clathrin-associated adaptor AP-2. Virology 2005, 342, 190–200. [Google Scholar] [CrossRef]
- Dong, X.; Li, H.; Derdowski, A.; Ding, L.; Burnett, A.; Chen, X.; Peters, T.R.; Dermody, T.S.; Woodruff, E.; Wang, J.-J.; et al. AP-3 directs the intracellular trafficking of HIV-1 Gag and plays a key role in particle assembly. Cell 2005, 120, 663–674. [Google Scholar] [CrossRef]
- Miyakawa, K.; Nishi, M.; Matsunaga, S.; Okayama, A.; Anraku, M.; Kudoh, A.; Hirano, H.; Kimura, H.; Morikawa, Y.; Yamamoto, N.; et al. The tumour suppressor APC promotes HIV-1 assembly via interaction with Gag precursor protein. Nat. Commun. 2017, 8, 14259. [Google Scholar] [CrossRef]
- Kudoh, A.; Takahama, S.; Sawasaki, T.; Ode, H.; Yokoyama, M.; Okayama, A.; Ishikawa, A.; Miyakawa, K.; Matsunaga, S.; Kimura, H.; et al. The phosphorylation of HIV-1 Gag by atypical protein kinase C facilitates viral infectivity by promoting Vpr incorporation into virions. Retrovirology 2014, 11, 9. [Google Scholar] [CrossRef]
- Cen, S.; Guo, F.; Niu, M.; Saadatmand, J.; Deflassieux, J.; Kleiman, L. The interaction between HIV-1 Gag and APOBEC3G. J. Biol. Chem. 2004, 279, 33177–33184. [Google Scholar] [CrossRef]
- Douaisi, M.; Dussart, S.; Courcoul, M.; Bessou, G.; Vigne, R.; Decroly, E. HIV-1 and MLV Gag proteins are sufficient to recruit APOBEC3G into virus-like particles. Biochem. Biophys. Res. Commun. 2004, 321, 566–573. [Google Scholar] [CrossRef]
- Schäfer, A.; Bogerd, H.P.; Cullen, B.R. Specific packaging of APOBEC3G into HIV-1 virions is mediated by the nucleocapsid domain of the gag polyprotein precursor. Virology 2004, 328, 163–168. [Google Scholar] [CrossRef] [PubMed]
- Stefas, E.; Rucheton, M.; Graafland, H.; Moynier, M.; Sompeyrac, C.; Bahraoui, E.M.; Veas, F. Human Plasmatic Apolipoprotein H Binds Human Immunodeficiency Virus Type 1 and Type 2 Proteins. AIDS Res. Hum. Retroviruses 1997, 13, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Mansharamani, M.; Graham, D.R.M.; Monie, D.; Lee, K.K.; Hildreth, J.E.K.; Siliciano, R.F.; Wilson, K.L. Barrier-to-Autointegration Factor BAF Binds p55 Gagand Matrix and Is a Host Component of Human ImmunodeficiencyVirus Type 1Virions. J. Virol. 2003, 77, 13084–13092. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ghanam, R.H.; Fernandez, T.F.; Fledderman, E.L.; Saad, J.S. Binding of calmodulin to the HIV-1 matrix protein triggers myristate exposure. J. Biol. Chem. 2010, 285, 41911–41920. [Google Scholar] [CrossRef] [PubMed]
- Samal, A.B.; Ghanam, R.H.; Fernandez, T.F.; Monroe, E.B.; Saad, J.S. NMR, biophysical, and biochemical studies reveal the minimal Calmodulin binding domain of the HIV-1 matrix protein. J. Biol. Chem. 2011, 286, 33533–33543. [Google Scholar] [CrossRef] [PubMed]
- Taylor, J.E.; Chow, J.Y.H.; Jeffries, C.M.; Kwan, A.H.; Duff, A.P.; Hamilton, W.A.; Trewhella, J. Calmodulin binds a highly extended HIV-1 MA protein that refolds upon its release. Biophys. J. 2012, 103, 541–549. [Google Scholar] [CrossRef]
- Grigorov, B.; Attuil-Audenis, V.; Perugi, F.; Nedelec, M.; Watson, S.; Pique, C.; Darlix, J.-L.; Conjeaud, H.; Muriaux, D. A role for CD81 on the late steps of HIV-1 replication in a chronically infected T cell line. Retrovirology 2009, 6, 28. [Google Scholar] [CrossRef]
- Ding, J.; Zhao, J.; Sun, L.; Mi, Z.; Cen, S. Citron kinase enhances ubiquitination of HIV-1 Gag protein and intracellular HIV-1 budding. Arch. Virol. 2016, 161, 2441–2448. [Google Scholar] [CrossRef]
- Wilson, S.J.; Schoggins, J.W.; Zang, T.; Kutluay, S.B.; Jouvenet, N.; Alim, M.A.; Bitzegeio, J.; Rice, C.M.; Bieniasz, P.D. Inhibition of HIV-1 Particle Assembly by 2′,3′-Cyclic-Nucleotide 3′-Phosphodiesterase. Cell Host Microbe 2012, 12, 585–597. [Google Scholar] [CrossRef]
- Braaten, D.; Luban, J. Cyclophilin A regulates HIV-1 infectivity, as demonstrated by gene targeting in human T cells. EMBO J. 2001, 20, 1300–1309. [Google Scholar] [CrossRef]
- DeBoer, J.; Madson, C.J.; Belshan, M. Cyclophilin B enhances HIV-1 infection. Virology 2016, 489, 282–291. [Google Scholar] [CrossRef] [PubMed]
- Franke, E.K.; Yuan, H.E.; Luban, J. Specific incorporation of cyclophilin A into HIV-1 virions. Nature 1994, 372, 359–362. [Google Scholar] [CrossRef] [PubMed]
- Thali, M.; Bukovsky, A.; Kondo, E.; Rosenwirth, B.; Walsh, C.T.; Sodroski, J.; Göttlinger, H.G. Functional association of cyclophilin A with HIV-1 virions. Nature 1994, 372, 363–365. [Google Scholar] [CrossRef] [PubMed]
- Reed, J.C.; Molter, B.; Geary, C.D.; McNevin, J.; McElrath, J.; Giri, S.; Klein, K.C.; Lingappa, J.R. HIV-1 Gag co-opts a cellular complex containing DDX6, a helicase that facilitates capsid assembly. J. Cell Biol. 2012, 198, 439–456. [Google Scholar] [CrossRef] [PubMed]
- Lorgeoux, R.-P.; Pan, Q.; Le Duff, Y.; Liang, C. DDX17 promotes the production of infectious HIV-1 particles through modulating viral RNA packaging and translation frameshift. Virology 2013, 443, 384–392. [Google Scholar] [CrossRef] [PubMed]
- Perugi, F.; Muriaux, D.; Ramirez, B.C.; Chabani, S.; Decroly, E.; Darlix, J.-L.; Blot, V.; Pique, C. Human Discs Large is a new negative regulator of human immunodeficiency virus-1 infectivity. Mol. Biol. Cell 2009, 20, 498–508. [Google Scholar] [CrossRef] [PubMed]
- Ghoujal, B.; Milev, M.P.; Ajamian, L.; Abel, K.; Mouland, A.J. ESCRT-II’s involvement in HIV-1 genomic RNA trafficking and assembly. Biol. Cell 2012, 104, 706–721. [Google Scholar] [CrossRef]
- Cimarelli, A.; Luban, J. Translation elongation factor 1-alpha interacts specifically with the human immunodeficiency virus type 1 Gag polyprotein. J. Virol. 1999, 73, 5388–5401. [Google Scholar] [CrossRef]
- Li, D.; Wei, T.; Rawle, D.J.; Qin, F.; Wang, R.; Soares, D.C.; Jin, H.; Sivakumaran, H.; Lin, M.-H.; Spann, K.; et al. Specific Interaction between eEF1A and HIV RT Is Critical for HIV-1 Reverse Transcription and a Potential Anti-HIV Target. PLoS Pathog. 2015, 11, e1005289. [Google Scholar] [CrossRef]
- Valiente-Echeverría, F.; Melnychuk, L.; Vyboh, K.; Ajamian, L.; Gallouzi, I.-E.; Bernard, N.; Mouland, A.J. eEF2 and Ras-GAP SH3 domain-binding protein (G3BP1) modulate stress granule assembly during HIV-1 infection. Nat. Commun. 2014, 5, 4819. [Google Scholar] [CrossRef]
- Cooper, J.; Liu, L.; Woodruff, E.A.; Taylor, H.E.; Goodwin, J.S.; D’Aquila, R.T.; Spearman, P.; Hildreth, J.E.K.; Dong, X. Filamin A protein interacts with human immunodeficiency virus type 1 Gag protein and contributes to productive particle assembly. J. Biol. Chem. 2011, 286, 28498–28510. [Google Scholar] [CrossRef] [PubMed]
- Pan, Q.; Rong, L.; Zhao, X.; Liang, C. Fragile X mental retardation protein restricts replication of human immunodeficiency virus type 1. Virology 2009, 387, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Kishimoto, N.; Onitsuka, A.; Kido, K.; Takamune, N.; Shoji, S.; Misumi, S. Glyceraldehyde 3-phosphate dehydrogenase negatively regulates human immunodeficiency virus type 1 infection. Retrovirology 2012, 9, 107. [Google Scholar] [CrossRef] [PubMed]
- Peytavi, R.; Hong, S.S.; Gay, B.; d’Angeac, A.D.; Selig, L.; Bénichou, S.; Benarous, R.; Boulanger, P. HEED, the product of the human homolog of the murine eed gene, binds to the matrix protein of HIV-1. J. Biol. Chem. 1999, 274, 1635–1645. [Google Scholar] [CrossRef] [PubMed]
- Wilson, S.A.; Sieiro-Vazquez, C.; Edwards, N.J.; Iourin, O.; Byles, E.D.; Kotsopoulou, E.; Adamson, C.S.; Kingsman, S.M.; Kingsman, A.J.; Martin-Rendon, E. Cloning and characterization of hIF2, a human homologue of bacterial translation initiation factor 2, and its interaction with HIV-1 matrix. Biochem. J. 1999, 342 Pt 1, 97–103. [Google Scholar] [CrossRef]
- Lama, J.; Trono, D. Human immunodeficiency virus type 1 matrix protein interacts with cellular protein HO3. J. Virol. 1998, 72, 1671–1676. [Google Scholar] [CrossRef]
- Beauséjour, Y.; Tremblay, M.J. Interaction between the cytoplasmic domain of ICAM-1 and Pr55Gag leads to acquisition of host ICAM-1 by human immunodeficiency virus type 1. J. Virol. 2004, 78, 11916–11925. [Google Scholar] [CrossRef]
- Jalaguier, P.; Cantin, R.; Maaroufi, H.; Tremblay, M.J. Selective acquisition of host-derived ICAM-1 by HIV-1 is a matrix-dependent process. J. Virol. 2015, 89, 323–336. [Google Scholar] [CrossRef]
- Mark-Danieli, M.; Laham, N.; Kenan-Eichler, M.; Castiel, A.; Melamed, D.; Landau, M.; Bouvier, N.M.; Evans, M.J.; Bacharach, E. Single Point Mutations in the Zinc Finger Motifs of the Human Immunodeficiency Virus Type 1 Nucleocapsid Alter RNA Binding Specificities of the Gag Protein and Enhance Packaging and Infectivity. J. Virol. 2005, 79, 7756–7767. [Google Scholar] [CrossRef][Green Version]
- Zhou, Y.; Rong, L.; Lu, J.; Pan, Q.; Liang, C. Insulin-like growth factor II mRNA binding protein 1 associates with Gag protein of human immunodeficiency virus type 1, and its overexpression affects virus assembly. J. Virol. 2008, 82, 5683–5692. [Google Scholar] [CrossRef]
- Ehrlich, L.S.; Medina, G.N.; Photiadis, S.; Whittredge, P.B.; Watanabe, S.; Taraska, J.W.; Carter, C.A. Tsg101 regulates PI(4,5)P2/Ca2+ signaling for HIV-1 Gag assembly. Front. Microbiol. 2014, 5. [Google Scholar] [CrossRef] [PubMed]
- Sabo, Y.; de los Santos, K.; Goff, S.P. IQGAP1 Negatively Regulates HIV-1 Gag Trafficking and Virion Production. Cell Rep. 2020, 30, 4065–4081.e4. [Google Scholar] [CrossRef] [PubMed]
- Martinez, N.W.; Xue, X.; Berro, R.G.; Kreitzer, G.; Resh, M.D. Kinesin KIF4 regulates intracellular trafficking and stability of the human immunodeficiency virus type 1 Gag polyprotein. J. Virol. 2008, 82, 9937–9950. [Google Scholar] [CrossRef]
- Sabo, Y.; Walsh, D.; Barry, D.S.; Tinaztepe, S.; de los Santos, K.; Goff, S.P.; Gundersen, G.G.; Naghavi, M.H. HIV-1 Induces the Formation of Stable Microtubules to Enhance Early Infection. Cell Host Microbe 2013, 14, 535–546. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Winkler, U.; Freed, E.O.; Torrey, T.A.; Kim, W.; Li, H.; Goff, S.P.; Morse, H.C. Cellular motor protein KIF-4 associates with retroviral Gag. J. Virol. 1999, 73, 10508–10513. [Google Scholar] [CrossRef]
- Kyei, G.B.; Dinkins, C.; Davis, A.S.; Roberts, E.; Singh, S.B.; Dong, C.; Wu, L.; Kominami, E.; Ueno, T.; Yamamoto, A.; et al. Autophagy pathway intersects with HIV-1 biosynthesis and regulates viral yields in macrophages. J. Cell Biol. 2009, 186, 255–268. [Google Scholar] [CrossRef] [PubMed]
- Engeland, C.E.; Oberwinkler, H.; Schümann, M.; Krause, E.; Müller, G.A.; Kräusslich, H.-G. The cellular protein lyric interacts with HIV-1 Gag. J. Virol. 2011, 85, 13322–13332. [Google Scholar] [CrossRef]
- Halwani, R.; Cen, S.; Javanbakht, H.; Saadatmand, J.; Kim, S.; Shiba, K.; Kleiman, L. Cellular Distribution of Lysyl-tRNA Synthetase and Its Interaction with Gag during Human Immunodeficiency Virus Type 1 Assembly. J. Virol. 2004, 78, 7553–7564. [Google Scholar] [CrossRef]
- Javanbakht, H.; Halwani, R.; Cen, S.; Saadatmand, J.; Musier-Forsyth, K.; Gottlinger, H.; Kleiman, L. The Interaction between HIV-1 Gag and Human Lysyl-tRNA Synthetase during Viral Assembly. J. Biol. Chem. 2003, 278, 27644–27651. [Google Scholar] [CrossRef]
- Kovaleski, B.J.; Kennedy, R.; Khorchid, A.; Kleiman, L.; Matsuo, H.; Musier-Forsyth, K. Critical Role of Helix 4 of HIV-1 Capsid C-terminal Domain in Interactions with Human Lysyl-tRNA Synthetase. J. Biol. Chem. 2007, 282, 32274–32279. [Google Scholar] [CrossRef]
- Kovaleski, B.J.; Kennedy, R.; Hong, M.K.; Datta, S.A.; Kleiman, L.; Rein, A.; Musier-Forsyth, K. In vitro characterization of the interaction between HIV-1 Gag and human lysyl-tRNA synthetase. J. Biol. Chem. 2006, 281, 19449–19456. [Google Scholar] [CrossRef]
- Na Nakorn, P.; Treesuwan, W.; Choowongkomon, K.; Hannongbua, S.; Boonyalai, N. In vitro and in silico binding study of the peptide derived from HIV-1 CA-CTD and LysRS as a potential HIV-1 blocking site. J. Theor. Biol. 2011, 270, 88–97. [Google Scholar] [CrossRef]
- Gupta, P.; Singhal, P.K.; Rajendrakumar, P.; Padwad, Y.; Tendulkar, A.V.; Kalyanaraman, V.S.; Schmidt, R.E.; Srinivasan, A.; Mahalingam, S. Mechanism of Host Cell MAPK/ERK-2 Incorporation into Lentivirus Particles: Characterization of the Interaction between MAPK/ERK-2 and Proline-Rich-Domain Containing Capsid Region of Structural Protein Gag. J. Mol. Biol. 2011, 410, 681–697. [Google Scholar] [CrossRef]
- Abudu, A.; Wang, X.; Dang, Y.; Zhou, T.; Xiang, S.-H.; Zheng, Y.-H. Identification of Molecular Determinants from Moloney Leukemia Virus 10 Homolog (MOV10) Protein for Virion Packaging and Anti-HIV-1 Activity. J. Biol. Chem. 2012, 287, 1220–1228. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Han, Y.; Dang, Y.; Fu, W.; Zhou, T.; Ptak, R.G.; Zheng, Y.-H. Moloney Leukemia Virus 10 (MOV10) Protein Inhibits Retrovirus Replication. J. Biol. Chem. 2010, 285, 14346–14355. [Google Scholar] [CrossRef] [PubMed]
- Sette, P.; Jadwin, J.A.; Dussupt, V.; Bello, N.F.; Bouamr, F. The ESCRT-associated protein Alix recruits the ubiquitin ligase Nedd4-1 to facilitate HIV-1 release through the LYPXnL L domain motif. J. Virol. 2010, 84, 8181–8192. [Google Scholar] [CrossRef] [PubMed]
- Usami, Y.; Popov, S.; Popova, E.; Göttlinger, H.G. Efficient and specific rescue of human immunodeficiency virus type 1 budding defects by a Nedd4-like ubiquitin ligase. J. Virol. 2008, 82, 4898–4907. [Google Scholar] [CrossRef]
- Weiss, E.R.; Popova, E.; Yamanaka, H.; Kim, H.C.; Huibregtse, J.M.; Göttlinger, H. Rescue of HIV-1 Release by Targeting Widely Divergent NEDD4-Type Ubiquitin Ligases and Isolated Catalytic HECT Domains to Gag. PLoS Pathog. 2010, 6, e1001107. [Google Scholar] [CrossRef]
- Bacharach, E.; Gonsky, J.; Alin, K.; Orlova, M.; Goff, S.P. The Carboxy-Terminal Fragment of Nucleolin Interacts with the Nucleocapsid Domain of Retroviral Gag Proteins and Inhibits Virion Assembly. J. Virol. 2000, 74, 11027–11039. [Google Scholar] [CrossRef][Green Version]
- Gao, W.; Li, M.; Zhang, J. Tandem immunoprecipitation approach to identify HIV-1 Gag associated host factors. J. Virol. Methods 2014, 203, 116–119. [Google Scholar] [CrossRef]
- Ueno, T.; Tokunaga, K.; Sawa, H.; Maeda, M.; Chiba, J.; Kojima, A.; Hasegawa, H.; Shoya, Y.; Sata, T.; Kurata, T.; et al. Nucleolin and the Packaging Signal, ψ, Promote the Budding of Human Immunodeficiency Virus Type-1 (HIV-1). Microbiol. Immunol. 2004, 48, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Popov, S.; Popova, E.; Inoue, M.; Wu, Y.; Göttlinger, H. HIV-1 gag recruits PACSIN2 to promote virus spreading. Proc. Natl. Acad. Sci. USA 2018, 115, 7093–7098. [Google Scholar] [CrossRef] [PubMed]
- Guth, C.A.; Sodroski, J. Contribution of PDZD8 to Stabilization of the Human Immunodeficiency Virus Type 1 Capsid. J. Virol. 2014, 88, 4612–4623. [Google Scholar] [CrossRef] [PubMed]
- Henning, M.S.; Morham, S.G.; Goff, S.P.; Naghavi, M.H. PDZD8 is a novel Gag-interacting factor that promotes retroviral infection. J. Virol. 2010, 84, 8990–8995. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Sodroski, J. Efficient human immunodeficiency virus (HIV-1) infection of cells lacking PDZD8. Virology 2015, 481, 73–78. [Google Scholar] [CrossRef]
- Mekdad, H.E.; Boutant, E.; Karnib, H.; Biedma, M.E.; Sharma, K.K.; Malytska, I.; Laumond, G.; Roy, M.; Réal, E.; Paillart, J.-C.; et al. Characterization of the interaction between the HIV-1 Gag structural polyprotein and the cellular ribosomal protein L7 and its implication in viral nucleic acid remodeling. Retrovirology 2016, 13, 54. [Google Scholar] [CrossRef]
- Le Sage, V.; Cinti, A.; Mouland, A.J. No-Go’ing Back: Co-opting RVB-2 to Control HIV-1 Gene Expression and Immune Response. Trends Microbiol. 2015, 23, 593–595. [Google Scholar] [CrossRef]
- Mu, X.; Fu, Y.; Zhu, Y.; Wang, X.; Xuan, Y.; Shang, H.; Goff, S.P.; Gao, G. HIV-1 Exploits the Host Factor RuvB-like 2 to Balance Viral Protein Expression. Cell Host Microbe 2015, 18, 233–242. [Google Scholar] [CrossRef]
- Joshi, A.; Garg, H.; Ablan, S.D.; Freed, E.O. Evidence of a role for soluble N-ethylmaleimide-sensitive factor attachment protein receptor (SNARE) machinery in HIV-1 assembly and release. J. Biol. Chem. 2011, 286, 29861–29871. [Google Scholar] [CrossRef]
- Nishi, M.; Ryo, A.; Tsurutani, N.; Ohba, K.; Sawasaki, T.; Morishita, R.; Perrem, K.; Aoki, I.; Morikawa, Y.; Yamamoto, N. Requirement for microtubule integrity in the SOCS1-mediated intracellular dynamics of HIV-1 Gag. FEBS Lett. 2009, 583, 1243–1250. [Google Scholar] [CrossRef]
- Ryo, A.; Tsurutani, N.; Ohba, K.; Kimura, R.; Komano, J.; Nishi, M.; Soeda, H.; Hattori, S.; Perrem, K.; Yamamoto, M.; et al. SOCS1 is an inducible host factor during HIV-1 infection and regulates the intracellular trafficking and stability of HIV-1 Gag. Proc. Natl. Acad. Sci. USA 2008, 105, 294–299. [Google Scholar] [CrossRef] [PubMed]
- Abrahamyan, L.G.; Chatel-Chaix, L.; Ajamian, L.; Milev, M.P.; Monette, A.; Clement, J.-F.; Song, R.; Lehmann, M.; DesGroseillers, L.; Laughrea, M.; et al. Novel Staufen1 ribonucleoproteins prevent formation of stress granules but favour encapsidation of HIV-1 genomic RNA. J. Cell Sci. 2010, 123, 369–383. [Google Scholar] [CrossRef] [PubMed]
- Chatel-Chaix, L.; Clement, J.-F.; Martel, C.; Beriault, V.; Gatignol, A.; DesGroseillers, L.; Mouland, A.J. Identification of Staufen in the Human Immunodeficiency Virus Type 1 Gag Ribonucleoprotein Complex and a Role in Generating Infectious Viral Particles. Mol. Cell. Biol. 2004, 24, 2637–2648. [Google Scholar] [CrossRef] [PubMed]
- Chatel-Chaix, L.; Boulay, K.; Mouland, A.J.; Desgroseillers, L. The host protein Staufen1 interacts with the Pr55Gag zinc fingers and regulates HIV-1 assembly via its N-terminus. Retrovirology 2008, 5, 41. [Google Scholar] [CrossRef]
- Milev, M.P.; Brown, C.M.; Mouland, A.J. Live cell visualization of the interactions between HIV-1 Gag and the cellular RNA-binding protein Staufen1. Retrovirology 2010, 7, 41. [Google Scholar] [CrossRef]
- Rao, S.; Cinti, A.; Temzi, A.; Amorim, R.; You, J.C.; Mouland, A.J. HIV-1 NC-induced stress granule assembly and translation arrest are inhibited by the dsRNA binding protein Staufen1. RNA 2018, 24, 219–236. [Google Scholar] [CrossRef]
- Chatel-Chaix, L.; Abrahamyan, L.; Fréchina, C.; Mouland, A.J.; DesGroseillers, L. The Host Protein Staufen1 Participates in Human Immunodeficiency Virus Type 1 Assembly in Live Cells by Influencing pr55Gag Multimerization. J. Virol. 2007, 81, 6216–6230. [Google Scholar] [CrossRef]
- Milev, M.P.; Ravichandran, M.; Khan, M.F.; Schriemer, D.C.; Mouland, A.J. Characterization of Staufen1 Ribonucleoproteins by Mass Spectrometry and Biochemical Analyses Reveal the Presence of Diverse Host Proteins Associated with Human Immunodeficiency Virus Type 1. Front. Microbiol. 2012, 3. [Google Scholar] [CrossRef]
- Bauby, H.; Lopez-Vergès, S.; Hoeffel, G.; Delcroix-Genête, D.; Janvier, K.; Mammano, F.; Hosmalin, A.; Berlioz-Torrent, C. TIP47 is Required for the Production of Infectious HIV-1 Particles from Primary Macrophages. Traffic 2010, 11, 455–467. [Google Scholar] [CrossRef]
- Checkley, M.A.; Luttge, B.G.; Mercredi, P.Y.; Kyere, S.K.; Donlan, J.; Murakami, T.; Summers, M.F.; Cocklin, S.; Freed, E.O. Reevaluation of the Requirement for TIP47 in Human Immunodeficiency Virus Type 1 Envelope Glycoprotein Incorporation. J. Virol. 2013, 87, 3561–3570. [Google Scholar] [CrossRef]
- Lopez-Verges, S.; Camus, G.; Blot, G.; Beauvoir, R.; Benarous, R.; Berlioz-Torrent, C. Tail-interacting protein TIP47 is a connector between Gag and Env and is required for Env incorporation into HIV-1 virions. Proc. Natl. Acad. Sci. USA 2006, 103, 14947–14952. [Google Scholar] [CrossRef] [PubMed]
- Luban, J. TRIM5 and the Regulation of HIV-1 Infectivity. Mol. Biol. Int. 2012, 2012, 426840. [Google Scholar] [CrossRef] [PubMed]
- Sakuma, R.; Ohmine, S.; Ikeda, Y. Determinants for the rhesus monkey TRIM5alpha-mediated block of the late phase of HIV-1 replication. J. Biol. Chem. 2010, 285, 3784–3793. [Google Scholar] [CrossRef] [PubMed]
- Sakuma, R.; Noser, J.A.; Ohmine, S.; Ikeda, Y. Rhesus monkey TRIM5alpha restricts HIV-1 production through rapid degradation of viral Gag polyproteins. Nat. Med. 2007, 13, 631–635. [Google Scholar] [CrossRef]
- Stremlau, M.; Owens, C.M.; Perron, M.J.; Kiessling, M.; Autissier, P.; Sodroski, J. The cytoplasmic body component TRIM5alpha restricts HIV-1 infection in Old World monkeys. Nature 2004, 427, 848–853. [Google Scholar] [CrossRef]
- Dussupt, V.; Sette, P.; Bello, N.F.; Javid, M.P.; Nagashima, K.; Bouamr, F. Basic residues in the nucleocapsid domain of Gag are critical for late events of HIV-1 budding. J. Virol. 2011, 85, 2304–2315. [Google Scholar] [CrossRef]
- El Meshri, S.E.; Boutant, E.; Mouhand, A.; Thomas, A.; Larue, V.; Richert, L.; Vivet-Boudou, V.; Mély, Y.; Tisné, C.; Muriaux, D.; et al. The NC domain of HIV-1 Gag contributes to the interaction of Gag with TSG101. Biochim. Biophys. Acta BBA-Gen. Subj. 2018, 1862, 1421–1431. [Google Scholar] [CrossRef]
- Bohl, C.R.; Abrahamyan, L.G.; Wood, C. Human Ubc9 Is Involved in Intracellular HIV-1 Env Stability after Trafficking out of the Trans-Golgi Network in a Gag Dependent Manner. PLoS ONE 2013, 8, e69359. [Google Scholar] [CrossRef]
- Jaber, T.; Bohl, C.R.; Lewis, G.L.; Wood, C.; West, J.T.; Weldon, R.A. Human Ubc9 Contributes to Production of Fully Infectious Human Immunodeficiency Virus Type 1 Virions. J. Virol. 2009, 83, 10448–10459. [Google Scholar] [CrossRef]
- Callahan, M.A.; Handley, M.A.; Lee, Y.H.; Talbot, K.J.; Harper, J.W.; Panganiban, A.T. Functional interaction of human immunodeficiency virus type 1 Vpu and Gag with a novel member of the tetratricopeptide repeat protein family. J. Virol. 1998, 72, 8461. [Google Scholar] [CrossRef]
- Ajamian, L.; Abel, K.; Rao, S.; Vyboh, K.; García-de-Gracia, F.; Soto-Rifo, R.; Kulozik, A.; Gehring, N.; Mouland, A. HIV-1 Recruits UPF1 but Excludes UPF2 to Promote Nucleocytoplasmic Export of the Genomic RNA. Biomolecules 2015, 5, 2808–2839. [Google Scholar] [CrossRef] [PubMed]
- Ajamian, L.; Abrahamyan, L.; Milev, M.; Ivanov, P.V.; Kulozik, A.E.; Gehring, N.H.; Mouland, A.J. Unexpected roles for UPF1 in HIV-1 RNA metabolism and translation. RNA 2008, 14, 914–927. [Google Scholar] [CrossRef] [PubMed]
- Gupta, K.; Ott, D.; Hope, T.J.; Siliciano, R.F.; Boeke, J.D. A Human Nuclear Shuttling Protein That Interacts with Human Immunodeficiency Virus Type 1 Matrix Is Packaged into Virions. J. Virol. 2000, 74, 11811–11824. [Google Scholar] [CrossRef] [PubMed]
- Luo, K.; Wang, T.; Liu, B.; Tian, C.; Xiao, Z.; Kappes, J.; Yu, X.-F. Cytidine Deaminases APOBEC3G and APOBEC3F Interact with Human Immunodeficiency Virus Type 1 Integrase and Inhibit Proviral DNA Formation. J. Virol. 2007, 81, 7238–7248. [Google Scholar] [CrossRef]
- Zennou, V.; Perez-Caballero, D.; Göttlinger, H.; Bieniasz, P.D. APOBEC3G Incorporation into Human Immunodeficiency Virus Type 1 Particles. J. Virol. 2004, 78, 12058–12061. [Google Scholar] [CrossRef]
- Sheehy, A.M.; Gaddis, N.C.; Choi, J.D.; Malim, M.H. Isolation of a human gene that inhibits HIV-1 infection and is suppressed by the viral Vif protein. Nature 2002, 418, 646–650. [Google Scholar] [CrossRef]
- Goila-Gaur, R.; Strebel, K. HIV-1 Vif, APOBEC, and Intrinsic Immunity. Retrovirology 2008, 5, 51. [Google Scholar] [CrossRef]
- Harris, R.S.; Bishop, K.N.; Sheehy, A.M.; Craig, H.M.; Petersen-Mahrt, S.K.; Watt, I.N.; Neuberger, M.S.; Malim, M.H. DNA deamination mediates innate immunity to retroviral infection. Cell 2003, 113, 803–809. [Google Scholar] [CrossRef]
- Mbisa, J.L.; Barr, R.; Thomas, J.A.; Vandegraaff, N.; Dorweiler, I.J.; Svarovskaia, E.S.; Brown, W.L.; Mansky, L.M.; Gorelick, R.J.; Harris, R.S.; et al. Human Immunodeficiency Virus Type 1 cDNAs Produced in the Presence of APOBEC3G Exhibit Defects in Plus-Strand DNA Transfer and Integration. J. Virol. 2007, 81, 7099–7110. [Google Scholar] [CrossRef]
- Guo, F.; Saadatmand, J.; Niu, M.; Kleiman, L. Roles of Gag and NCp7 in Facilitating tRNA(Lys)(3) Annealing to Viral RNA in Human Immunodeficiency Virus Type 1. J. Virol. 2009, 83, 8099–8107. [Google Scholar] [CrossRef]
- Guo, F.; Cen, S.; Niu, M.; Yang, Y.; Gorelick, R.J.; Kleiman, L. The Interaction of APOBEC3G with Human Immunodeficiency Virus Type 1 Nucleocapsid Inhibits tRNA3Lys Annealing to Viral RNA. J. Virol. 2007, 81, 11322–11331. [Google Scholar] [CrossRef] [PubMed]
- Bishop, K.N.; Verma, M.; Kim, E.-Y.; Wolinsky, S.M.; Malim, M.H. APOBEC3G Inhibits Elongation of HIV-1 Reverse Transcripts. PLoS Pathog. 2008, 4, e1000231. [Google Scholar] [CrossRef] [PubMed]
- Iwatani, Y.; Takeuchi, H.; Strebel, K.; Levin, J.G. Biochemical Activities of Highly Purified, Catalytically Active Human APOBEC3G: Correlation with Antiviral Effect. J. Virol. 2006, 80, 5992–6002. [Google Scholar] [CrossRef] [PubMed]
- Stavrou, S.; Crawford, D.; Blouch, K.; Browne, E.P.; Kohli, R.M.; Ross, S.R. Different Modes of Retrovirus Restriction by Human APOBEC3A and APOBEC3G In Vivo. PLoS Pathog. 2014, 10, e1004145. [Google Scholar] [CrossRef]
- Burdick, R.; Smith, J.L.; Chaipan, C.; Friew, Y.; Chen, J.; Venkatachari, N.J.; Delviks-Frankenberry, K.A.; Hu, W.-S.; Pathak, V.K. P Body-Associated Protein Mov10 Inhibits HIV-1 Replication at Multiple Stages. J. Virol. 2010, 84, 10241–10253. [Google Scholar] [CrossRef]
- Furtak, V.; Mulky, A.; Rawlings, S.A.; Kozhaya, L.; Lee, K.; KewalRamani, V.N.; Unutmaz, D. Perturbation of the P-Body Component Mov10 Inhibits HIV-1 Infectivity. PLoS ONE 2010, 5, e9081. [Google Scholar] [CrossRef]
- Chen, C.; Ma, X.; Hu, Q.; Li, X.; Huang, F.; Zhang, J.; Pan, T.; Xia, J.; Liu, C.; Zhang, H. Moloney leukemia virus 10 (MOV10) inhibits the degradation of APOBEC3G through interference with the Vif-mediated ubiquitin–proteasome pathway. Retrovirology 2017, 14, 56. [Google Scholar] [CrossRef]
- Merrick, W.C. Eukaryotic Protein Synthesis: Still a Mystery. J. Biol. Chem. 2010, 285, 21197–21201. [Google Scholar] [CrossRef]
- Khan, R.; Giedroc, D.P. Recombinant human immunodeficiency virus type 1 nucleocapsid (NCp7) protein unwinds tRNA. J. Biol. Chem. 1992, 267, 6689–6695. [Google Scholar]
- Li, D.; Wei, T.; Abbott, C.M.; Harrich, D. The Unexpected Roles of Eukaryotic Translation Elongation Factors in RNA Virus Replication and Pathogenesis. Microbiol. Mol. Biol. Rev. 2013, 77, 253–266. [Google Scholar] [CrossRef]
- Rao, S.; Hassine, S.; Monette, A.; Amorim, R.; DesGroseillers, L.; Mouland, A.J. HIV-1 requires Staufen1 to dissociate stress granules and to produce infectious viral particles. RNA 2019, 25, 727–736. [Google Scholar] [CrossRef] [PubMed]
- Valiente-Echeverría, F.; Melnychuk, L.; Mouland, A.J. Viral modulation of stress granules. Virus Res. 2012, 169, 430–437. [Google Scholar] [CrossRef]
- Ingelfinger, D.; Arndt-Jovin, D.J.; Lührmann, R.; Achsel, T. The human LSm1-7 proteins colocalize with the mRNA-degrading enzymes Dcp1/2 and Xrnl in distinct cytoplasmic foci. RNA N. Y. N 2002, 8, 1489–1501. [Google Scholar]
- Cougot, N.; Babajko, S.; Séraphin, B. Cytoplasmic foci are sites of mRNA decay in human cells. J. Cell Biol. 2004, 165, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Bish, R.; Cuevas-Polo, N.; Cheng, Z.; Hambardzumyan, D.; Munschauer, M.; Landthaler, M.; Vogel, C. Comprehensive Protein Interactome Analysis of a Key RNA Helicase: Detection of Novel Stress Granule Proteins. Biomolecules 2015, 5, 1441–1466. [Google Scholar] [CrossRef] [PubMed]
- Mazroui, R. Trapping of messenger RNA by Fragile X Mental Retardation protein into cytoplasmic granules induces translation repression. Hum. Mol. Genet. 2002, 11, 3007–3017. [Google Scholar] [CrossRef]
- Kedersha, N.; Chen, S.; Gilks, N.; Li, W.; Miller, I.J.; Stahl, J.; Anderson, P. Evidence That Ternary Complex (eIF2-GTP-tRNA i Met)–Deficient Preinitiation Complexes Are Core Constituents of Mammalian Stress Granules. Mol. Biol. Cell 2002, 13, 195–210. [Google Scholar] [CrossRef]
- Kang, D.; Su, Z.; Sarkar, D.; Emdad, L.; Volsky, D.J.; Fisher, P.B. Cloning and characterization of HIV-1-inducible astrocyte elevated gene-1, AEG-1. Gene 2005, 353, 8–15. [Google Scholar] [CrossRef]
- Su, Z.; Chen, Y.; Kang, D.; Chao, W.; Simm, M.; Volsky, D.J.; Fisher, P.B. Customized rapid subtraction hybridization (RaSH) gene microarrays identify overlapping expression changes in human fetal astrocytes resulting from human immunodeficiency virus-1 infection or tumor necrosis factor-α treatment. Gene 2003, 306, 67–78. [Google Scholar] [CrossRef]
- Vartak-Sharma, N.; Nooka, S.; Ghorpade, A. Astrocyte elevated gene-1 (AEG-1) and the A(E)Ging HIV/AIDS-HAND. Prog. Neurobiol. 2017, 157, 133–157. [Google Scholar] [CrossRef]
- Vartak-Sharma, N.; Gelman, B.B.; Joshi, C.; Borgamann, K.; Ghorpade, A. Astrocyte Elevated Gene-1 Is a Novel Modulator of HIV-1-associated Neuroinflammation via Regulation of Nuclear Factor-κB Signaling and Excitatory Amino Acid Transporter-2 Repression. J. Biol. Chem. 2014, 289, 19599–19612. [Google Scholar] [CrossRef] [PubMed]
- Zimmerman, C.; Klein, K.C.; Kiser, P.K.; Singh, A.R.; Firestein, B.L.; Riba, S.C.; Lingappa, J.R. Identification of a host protein essential for assembly of immature HIV-1 capsids. Nature 2002, 415, 88–92. [Google Scholar] [CrossRef] [PubMed]
- Mouland, A.J.; Mercier, J.; Luo, M.; Bernier, L.; DesGroseillers, L.; Cohen, E.A. The Double-Stranded RNA-Binding Protein Staufen Is Incorporated in Human Immunodeficiency Virus Type 1: Evidence for a Role in Genomic RNA Encapsidation. J. Virol. 2000, 74, 5441–5451. [Google Scholar] [CrossRef] [PubMed]
- Weldon, R.A.; Sarkar, P.; Brown, S.M.; Weldon, S.K. Mason–Pfizer monkey virus Gag proteins interact with the human sumo conjugating enzyme, hUbc9. Virology 2003, 314, 62–73. [Google Scholar] [CrossRef]
- Ott, D.E.; Coren, L.V.; Johnson, D.G.; Kane, B.P.; Sowder, R.C.; Kim, Y.D.; Fisher, R.J.; Zhou, X.Z.; Lu, K.P.; Henderson, L.E. Actin-binding cellular proteins inside human immunodeficiency virus type 1. Virology 2000, 266, 42–51. [Google Scholar] [CrossRef] [PubMed]
- Perrin-Tricaud, C.; Davoust, J.; Jones, I.M. Tagging the Human Immunodeficiency Virus Gag Protein with Green Fluorescent Protein. Virology 1999, 255, 20–25. [Google Scholar] [CrossRef][Green Version]
- Sasaki, H.; Nakamura, M.; Ohno, T.; Matsuda, Y.; Yuda, Y.; Nonomura, Y. Myosin-actin interaction plays an important role in human immunodeficiency virus type 1 release from host cells. Proc. Natl. Acad. Sci. USA 1995, 92, 2026–2030. [Google Scholar] [CrossRef]
- Gladnikoff, M.; Shimoni, E.; Gov, N.S.; Rousso, I. Retroviral Assembly and Budding Occur through an Actin-Driven Mechanism. Biophys. J. 2009, 97, 2419–2428. [Google Scholar] [CrossRef]
- Carlson, L.-A.; de Marco, A.; Oberwinkler, H.; Habermann, A.; Briggs, J.A.G. Cryo Electron Tomography of Native HIV-1 Budding Sites. PLoS Pathog. 2010, 6, 11. [Google Scholar] [CrossRef]
- Leung, J.; Yueh, A.; Appah, F.S.K.; Yuan, B.; de los Santos, K.; Goff, S.P. Interaction of Moloney murine leukemia virus matrix protein with IQGAP. EMBO J. 2006, 25, 2155–2166. [Google Scholar] [CrossRef]
- Zhou, Y.; Rong, L.; Zhang, J.; Aloysius, C.; Pan, Q.; Liang, C. Insulin-like growth factor II mRNA binding protein 1 modulates Rev-dependent human immunodeficiency virus type 1 RNA expression. Virology 2009, 393, 210–220. [Google Scholar] [CrossRef] [PubMed]
- Popova, E.; Popov, S.; Göttlinger, H.G. Human Immunodeficiency Virus Type 1 Nucleocapsid p1 Confers ESCRT Pathway Dependence. J. Virol. 2010, 84, 6590–6597. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Parent, L.J.; Bennett, R.P.; Craven, R.C.; Nelle, T.D.; Krishna, N.K.; Bowzard, J.B.; Wilson, C.B.; Puffer, B.A.; Montelaro, R.C.; Wills, J.W. Positionally independent and exchangeable late budding functions of the Rous sarcoma virus and human immunodeficiency virus Gag proteins. J. Virol. 1995, 69, 5455–5460. [Google Scholar] [CrossRef] [PubMed]
- Shehu-Xhilaga, M.; Ablan, S.; Demirov, D.G.; Chen, C.; Montelaro, R.C.; Freed, E.O. Late Domain-Dependent Inhibition of Equine Infectious Anemia Virus Budding. J. Virol. 2004, 78, 724–732. [Google Scholar] [CrossRef]
- Chamontin, C.; Rassam, P.; Ferrer, M.; Racine, P.-J.; Neyret, A.; Lainé, S.; Milhiet, P.-E.; Mougel, M. HIV-1 nucleocapsid and ESCRT-component Tsg101 interplay prevents HIV from turning into a DNA-containing virus. Nucleic Acids Res. 2015, 43, 336–347. [Google Scholar] [CrossRef]
- Bello, N.F.; Dussupt, V.; Sette, P.; Rudd, V.; Nagashima, K.; Bibollet-Ruche, F.; Chen, C.; Montelaro, R.C.; Hahn, B.H.; Bouamr, F. Budding of Retroviruses Utilizing Divergent L Domains Requires Nucleocapsid. J. Virol. 2012, 86, 4182–4193. [Google Scholar] [CrossRef]
- Le Blanc, I.; Prévost, M.-C.; Dokhélar, M.-C.; Rosenberg, A.R. The PPPY Motif of Human T-Cell Leukemia Virus Type 1 Gag Protein Is Required Early in the Budding Process. J. Virol. 2002, 76, 10024–10029. [Google Scholar] [CrossRef]
- Bouamr, F.; Melillo, J.A.; Wang, M.Q.; Nagashima, K.; de Los Santos, M.; Rein, A.; Goff, S.P. PPPYEPTAP Motif Is the Late Domain of Human T-Cell Leukemia Virus Type 1 Gag and Mediates Its Functional Interaction with Cellular Proteins Nedd4 and Tsg101. J. Virol. 2003, 77, 11882–11895. [Google Scholar] [CrossRef]
- Chung, H.-Y.; Morita, E.; von Schwedler, U.; Muller, B.; Krausslich, H.-G.; Sundquist, W.I. NEDD4L Overexpression Rescues the Release and Infectivity of Human Immunodeficiency Virus Type 1 Constructs Lacking PTAP and YPXL Late Domains. J. Virol. 2008, 82, 4884–4897. [Google Scholar] [CrossRef]
- Garg, H.; Joshi, A. SNAREs in HIV-1 assembly. Commun. Integr. Biol. 2012, 5, 172–174. [Google Scholar] [CrossRef]
- Ginisty, H.; Sicard, H.; Roger, B.; Bouvet, P. Structure and functions of nucleolin. J. Cell Sci. 1999, 112 Pt 6, 761–772. [Google Scholar]
- Darlix, J.-L.; Garrido, J.L.; Morellet, N.; Mély, Y.; de Rocquigny, H. Properties, functions, and drug targeting of the multifunctional nucleocapsid protein of the human immunodeficiency virus. Adv. Pharmacol. San Diego Calif. 2007, 55, 299–346. [Google Scholar] [CrossRef]
- Iraci, N.; Tabarrini, O.; Santi, C.; Sancineto, L. NCp7: Targeting a multitask protein for next-generation anti-HIV drug development part 2. Noncovalent inhibitors and nucleic acid binders. Drug Discov. Today 2018, 23, 687–695. [Google Scholar] [CrossRef] [PubMed]
- Sancineto, L.; Iraci, N.; Tabarrini, O.; Santi, C. NCp7: Targeting a multitasking protein for next-generation anti-HIV drug development part 1: Covalent inhibitors. Drug Discov. Today 2018, 23, 260–271. [Google Scholar] [CrossRef] [PubMed]
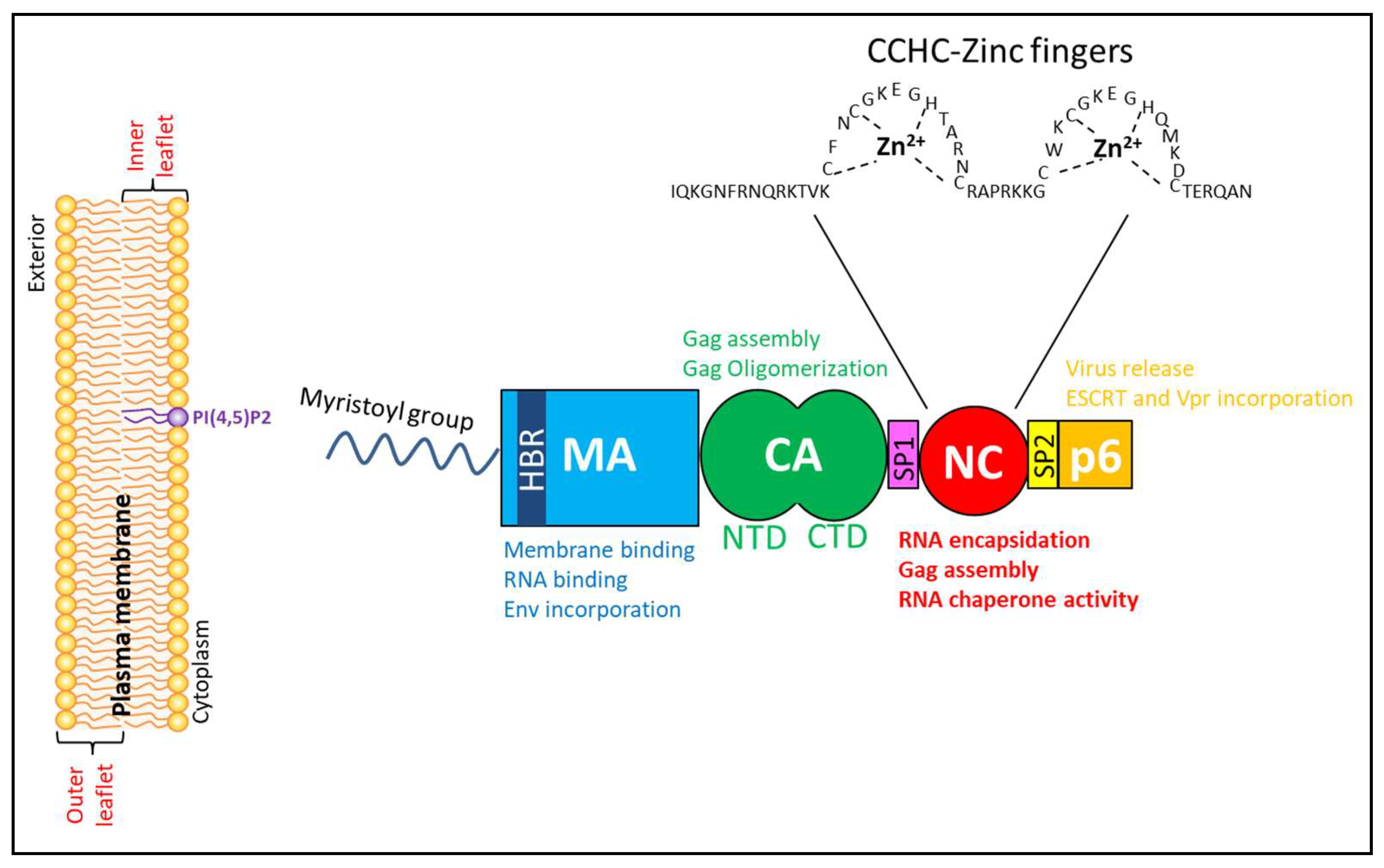
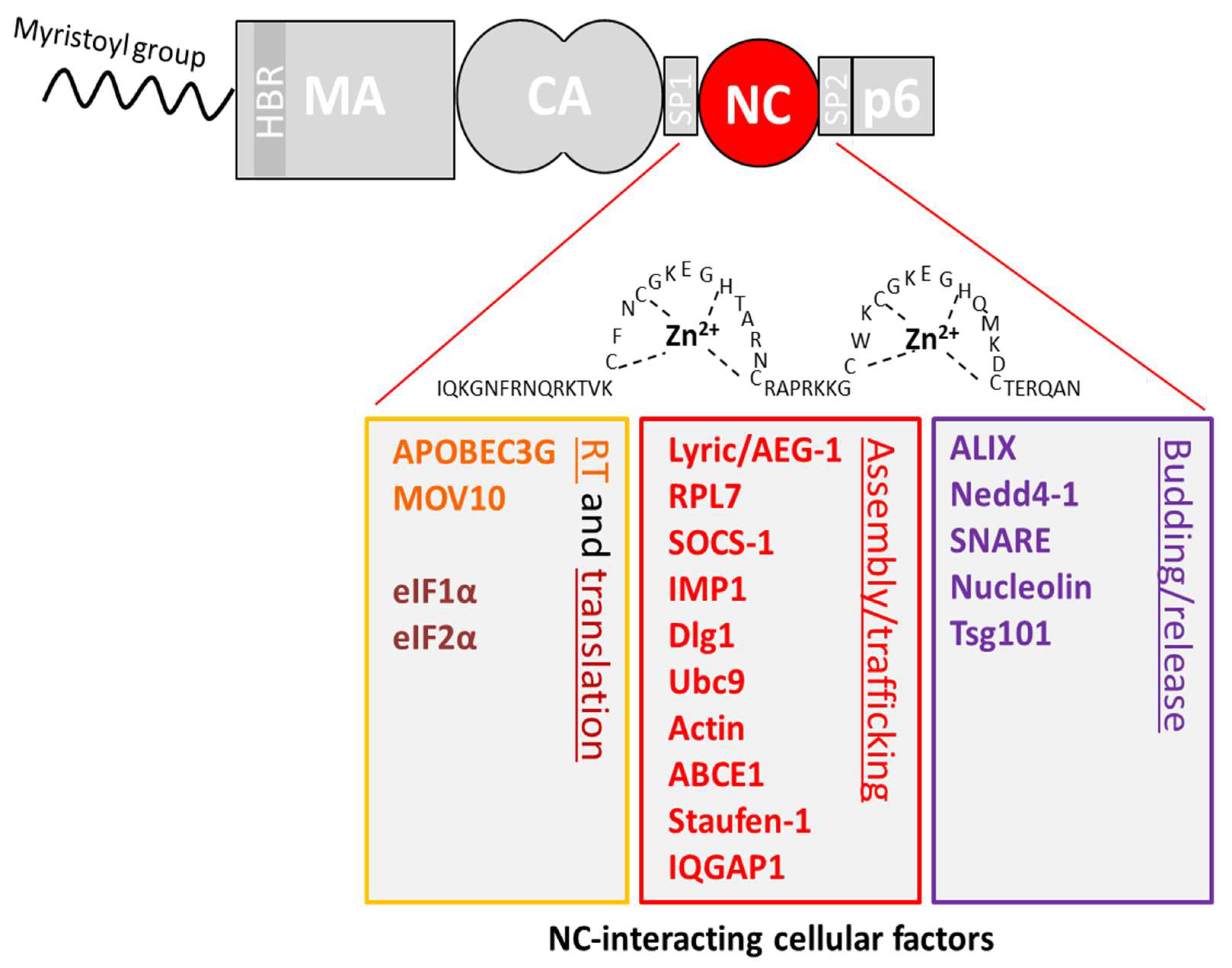
| Partner of Interaction | Classification of the Protein | Role of the Protein | Gag Interaction Domain(s) | Role of the Protein or the Interaction in the Replication Cycle | Methods Used to Demonstrate the Interaction | References |
|---|---|---|---|---|---|---|
| ABCE1 (ATP-binding cassette sub-family E member 1) = HP68 | ATPase, member of the ATP-binding cassette (ABC) transporters superfamily and ATP-binding cassette, sub-family E (OABP) | Inhibits the action of ribonuclease L (which presents antiviral activities) and has an effect on tumor cell proliferation and anti-apoptosis | NC | Essential for post-translational events in immature capsid assembly, Recruits a protein interfering with the late steps of the replication cycle | Radiolabeling Co-IP | [215,216,217,218] |
| Actin | Family of globular multi-functional proteins, forming microfilaments Component of the cytoskeleton and of the contractile apparatus in muscle cells | Essential for muscle contraction, cell mobility and remodeling, cell division and cytokinesis, intracellular trafficking and cell division | NC | Could play a role in assembly and/or other steps of the replication cycle | Co-IP in vitro protein binding assay Co-Fractionation | [160,219,220] |
| Gag | Viral budding | Intensity-based FRET Co-Fractionation in vitro protein binding assay | [221,222,223] | |||
| MA | Involved in proper localization and activation of the Reverse Transcription complex | Co-IP | [41] | |||
| ADAR1 (adenosine deaminase acting on RNA1) | Adenosine deaminase | Catalyzes the conversion of adenosine to inosine within a dsRNA | nd | Incorporated into particles, role needs to be elucidated | Dual tag affinity purification IP | [224] |
| Angiomotin AMOT (also AMOTL1, AMOTL2) | Motin family member (also angiomotin-like protein) | Functions related to endothelial cell migration, angiogenesis, embryonic cell movements, and maintenance of cell polarity | nd | Links Gag and NEDD4L, promotes HIV release from infected cells. Helps to complete immature virion assembly prior to budding. | Pull down experiment | [225] |
| AGO2 (Argonaute-2) | Active part of RNA-induced silencing complex (RISC) | Necessary for RNA-mediated gene silencing (RNAi) by the RISC | nd | Probably involved in capsid assembly (this function is independent of miRNA and translation regulation) | Co-IP | [226] |
| ALIX (apoptosis-linked gene 2-interacting protein X) = AIP1 | Adaptor protein | Involved in endocytosis, multivesicular body biogenesis, membrane repair, cytokinesis, apoptosis and maintenance of tight junction integrity | NC and p6 | Recruits ESCRT proteins for the release of newly formed viral particles | GST pull-down | [190,191,227,228,229,230,231] |
| Anx2 (annexin II) | Calcium-dependent phospholipid-binding protein | Involved in exocytosis, endocytosis, membrane organization, linkage of the F-actin cytoskeleton to the PM fibrinolysis… | nd | Increases Gag processing and virus production | Co-IP BiFC In vitro protein binding assay, Co-IP | [232,233] |
| AP-1 (clathrin-associated adaptor protein 1) | Clathrin adaptor proteins | Involved in intracellular transport of lysosomal hydrolases between the trans-Golgi network, and endosomes | MA | Transports Gag to the sites of active budding, facilitates Gag interactions with other cellular proteins | Yeast two-hybrid GST pull down Co-IP | [234] |
| AP-2 (clathrin-associated adaptor protein 2) | Works on the cell membrane to internalize cargos in clathrin-mediated endocytosis | MA-CA junction | AP-2 complex plays a role in the regulation of the virus assembly/release | GST pull-down In-vitro SPR measurements | [235] | |
| AP-3 (clathrin-associated adaptor protein 3) | Responsible for the transport of proteins to lysosomes and other organelles | MA | Directs Gag trafficking to MVB and participates in virus assembly | Yeast two-hybrid GST pull-down Co-IP | [236] | |
| APC (adenomatous polyposis coli protein) | Tumor suppressor | Suppresses Wnt signaling Implicated in cell adhesion and migration | MA HBR domain | Promotes Gag multimerization at the PM, vRNA incorporation and targeting at virological synapses | TAP- tag/MS screen GST pull-down Co-IP with endogenous protein | [237] |
| aPKC (atypical protein kinase C) | Serine/Threonine kinase | Kinase implicated in cell polarity and migration | nd | aPKC regulates HIV-1 infection via the phosphorylation of Gag-p6 which enhances the incorporation of Vpr into virions | Luminescent proximity assay Co-IP | [238] |
| APOBEC3G (A3G) APOBEC3F (A3F) | RNA/DNA cytidine deaminase-editing enzyme | Innate antiviral immunity | NC | Cellular HIV restriction factor Once incorporated in virions causes deamination of nascent DNA during RT | BiFC GST pull-down Co-IP | [161,239,240,241] |
| ApoH (apolipoprotein H) | Apolipoprotein component of circulating plasma lipoproteins | Beta-2-glycoprotein I Component of circulating plasma lipoproteins | MA | nd | Capture of sera virus Immunoblot with MA | [242] |
| BAF (barrier-to-autointegration factor) | Chromatin protein | Potential roles in cell division, but no precise function defined | MA | Potential role in PIC assembly and structure | Co-IP | [243] |
| CaM (calmodulin) | Ubiquitous multifunctional calcium-binding messenger | Implicated in numerous pathways, such as inflammation, metabolism, apopotosis, immune response | MA | Binding leads to MA myristate exposure Binding may induce MA transient unfolding Binds also Gp41 | Isothermal titration calorimetry Mass spectrometry Gel filtration | [244,245,246] |
| CD81, CD82 | Tetraspanins | Membrane glycoproteins involved in cell-cell adhesion, fusion, signal transduction, proliferation and differentiation | nd | Facilitates the viral egress | Co-IP | [247] |
| Citron kinase | Ser/Thr protein kinase | Effector of RhoA GTPase Implicated in mitosis and DNA damage control | nd | Enhances virus production by promoting Gag ubiquitination Induces viral release via the MVB pathway | Co-IP | [248] |
| CNP (2′,3′-cyclic-nucleotide 3′-phosphodiesterase) | Membrane-associated enzyme | 2H phosphoesterase superfamily | MA | Inhibits particle formation at the PM | GST pull-down with crosslinking Co-IP | [249] |
| CypA and CypB (cyclophilin A and B) | Peptidyl-prolyl cis-trans isomerase | Facilitates protein folding and trafficking CypA is involved in T-cell activation | CA | Incorporated into virions, Protects HIV-1 from restriction factor TRIM5α | Yeast two-hybrid GST pull-down Co-IP | [96,250,251,252,253] |
| DCP1 and DCP2 (decapping protein) | mRNA decapping enzymes | Component of mRNA decapping complex with DDX6 Role in 5′-to-3′ RNA degradation pathway | nd | May be a component of HIV-1 assembly intermediates | Co-IP with overexpression | [226,254] |
| DDX6 (DEAD-box RNA helicase 6) | RNA helicase | Role in mRNA decapping and degradation | nd | Facilitates capsid assembly | Co-IP | [254] |
| DDX17 (DEAD-box RNA helicase17) | RNA helicase | Role in RNA metabolism | nd | Modulates HIV RNA metabolism May promote infectious particle production | Proximity biotinylation assay Co-IP | [36,255] |
| DLG1 (discs large homolog 1) = SAP97) | Membrane-associated guanylate kinase (MAGUK) family | Scaffold, anchor and adaptor protein at the PM | NC | RNA independent interaction Restricts HIV infectivity Modulates Gag cellular distribution | Co-IP with endogenous DLG1 GST-pull down | [256] |
| EAP30 (ELL associated protein of 30 kDa) | ESCRT-II | Roles of ESCRT-II in mRNA trafficking and in promotion of the inward budding of vesicles from the membranes of late endosomes | nd | EAP30-Gag-Staufen1 are part of the HIV-1 RNP, promoting the trafficking of HIV-1 Gag and the vRNA to directly influence viral production | Co-IP TriFC | [257] |
| eEF1α (eukaryotic elongation factor 1-alpha) | Translation elongation factor | Responsible for the enzymatic delivery of aminoacyl tRNA to the ribosome | MA(HBR) and NC | May contribute to tRNA incorporation into virions and plays a role in viral uncoating Interacts with RT | Yeast two-hybrid GST pull-down Co-IP | [258,259] |
| eEF2 (eukaryotic elongation factor 2) | Translation elongation factor | Promotes GTP-dependent translocation of the ribosome | CA | Required in assembly blockade of Gag-mediated stress granules | Mass spectrometry Co-IP with endogenous protein | [260] |
| Filamin A | Filamin | Promotes orthogonal branching of actin filament and links them to membrane glycoproteins | CA | Involved in Gag trafficking to the PM | Yeast two-hybrid GST pull-down Co-IP | [261] |
| FMRP1 (fragile X mental retardation protein) | mRNA binding protein | Part of neuronal granules May participate to RNA transport and expression regulation | NC | RNA independent interaction Incorporated in virions May regulate HIV infectivity and modulate RNP packaging into virions | Co-IP with endogenous protein | [262] |
| GAPDH (glyceraldehyde 3-phosphate dehydrogenase) | Oxidoreductase | Enzyme implicated in glycolysis | MA and CA | Incorporated in virions Negatively regulates infection | Co-IP Yeast two-hybrid | [263] |
| HEED (human polycomb protein EED) | WD-40 repeat and Polycomb-group protein families | Involved in maintaining the transcriptional repressive state of genes | MA | Negative effect at early phase of infection Binds also IN and Nef | Yeast two-hybrid GST pull-down | [264] |
| eIF5B (eukaryotic initiation factor 5 B) = eIF2 | Translation initiation factor | Mediates the joining of ribosome 60S and 40S subunits | MA | MA inhibits eIF5B mediated translation | Yeast two-hybrid GST pull-down Co-IP | [265] |
| HARS2 (histidyl-tRNA synthetase homolog) = HO3) | Aminoacyl-tRNA synthetase | Catalyzes the ATP-dependent ligation of histidine to the 3′-end of its cognate tRNA | MA | Enhances infectivity Found in virions | Yeast two-hybrid Co-IP | [266] |
| ICAM-1 (intercellular adhesion molecule A) = CD54 | Immunoglobulin superfamily | Endothelial- and leucocyte-associated protein Mediates cell -cell adhesion | MA | Promotes HIV-mediated syncitia formation Important for binding of HIV-infected dendritic cells to CD4+ T cells ICAM-1 promotes HIV entry into cells | Virus immunocapture assay | [267,268] |
| IMP1 (insulin-like growth factor II mRNA binding protein 1) | RNA binding factor | Recruits target transcripts to cytoplasmic protein-RNA complexes (mRNPs) | NC (2nd zinc finger) | Blocks the formation of infectious particles | Co-IP | [269,270] |
| IP3R (1,4,5-inositol trisphosphate receptor) | Ca2+ signaling protein | Functions as Ca2+ ion-specific channel on the membrane of the endoplasmic reticulum (ER) | nd, potentially p6 | Gag modulates both ER Ca2+ release and refilling via its PTAP domain | Co-IP close proximity experiments: IEM (immunoelectron microscopy) TIRF | [271] |
| IQGAP1 (IQ motif-containing GTPase activating protein) | Scaffold protein | Regulator of many cellular processes (vesicle trafficking, endocytosis…), - cytoskeleton regulator affecting both microtubules and actin | NC and p6 | Negative regulator: factor inhibiting efficient budding by preventing the accumulation of Gag at the cellular PM | Co-IP | [272] |
| KIF4 (kinesin superfamily protein) | microtubule (MT)-stimulated ATPase in the kinesin motor family | KIF4 regulates the movement of multiple intracellular components, implicated in chromosome segregation during mitosis, and cytokinesis as well as in the regulation of programmed cell death in juvenile neurons. | MA | Regulates Gag trafficking and stability | Yeast two-hybrid Co-IP | [273,274,275] |
| LC3 = Atg8 (autophagy-related protein 8) | Autophagy factor | Facilitates autophagosome biogenesis and wrapping around autophagic targets | nd | Increases Gag processing and HIV yields | Co-IP | [276] |
| Lyric (lysine-rich carcinoembryonic antigen-related cell adhesion molecule coisolated) = astrocyte-elevated gene 1EG-1 or metadherin | Adhesion molecule | Implicated in various signaling pathways, suggested to have anti-apoptotic effects and to be involved in tumorigenesis | MA and NC | Potential role in regulating infectivity Implicated in HIV-1 associated neuropathy and potentially promoting HIV replication | Affinity purification Co-IP | [37,277] |
| LysRS (lysyl-tRNA synthetase) | Lysyl-tRNA synthetase | Catalyzes the formation of Lys-tRNA | CA (Helix 4 of the C-terminal domain) | Packaging of tRNALys into the virions | GST pull-down Fluorescence anisotropy FPLC Circular dichroism molecular dynamics | [278,279,280,281,282] |
| MAP1A and MAP1S (microtubule-associated proteins) | Microtubule-associated protein family | Regulate the stability and the dynamics of microtubules, guide the microtubules to a specific location, mediate interactions with cellular proteins | CA | Promote HIV trafficking to the nucleus, help tether viral capsids to microtubules | Yeast two-hybrid Capsid-binding assay Proximity ligation assay | [97] |
| MAPK/ERK-2 (mitogen-activated protein kinase/extracellular signal-regulated kinase 2) | Serine–threonine kinases | Involved in regulation of meiosis, mitosis, and post-mitotic functions in differentiated cells | CA (Proline residues) | Interaction results in Gag incorporation into virus particles and may be essential for retroviral replication | Co-IP GST pull-down | [283] |
| MOV10 (Moloney leukemia virus 10 homolog) | Super family-1 RNA helicase | Type I interferon stimulating gene Plays a role in miRNA-mediated regulation Broad antiviral activity | NC | Incorporated into virions Inhibitory effect on HIV infection | GST pull-down Co-IP | [284,285] |
| Nedd4-1 (neural precursor cell expressed developmentally down-regulated protein 4) and Nedd4-2 | E3 ubiquitin-protein ligase | Ubiquitinate and target cargo proteins into the MVB sorting pathway | p6 and NC | Stimulate viral release by ubiquitination of Gag leading to the recruitment of ESCRT complexes | Co-IP Yeast two-hybrid Incorporation of Nedd4-2 into VLPs | [286,287,288] |
| Nucleolin | RNA binding protein | Major non-ribosomal nucleolar proteins nucleolus, Role in ribosome biogenesis | NC | Enhances virion assembly and release | Yeast two-hybrid Co-IP Tandem affinity purification | [289,290,291] |
| PACSIN2 (protein kinase C and casein kinase substrate in neurons 2) | F-BAR domain family | Implicated in remodeling membrane and actin cytoskeleton | p6 | Promotes cell-to-cell transmission, enhancing HIV-1 spreading (connecting Gag to actin?) | Co-IP | [292] |
| PDZD8 (PDZ domain-containing protein 8) | Cytoskeletal regulatory protein, ER membrane protein | Plays a role in the regulation of cell morphology, cytoskeletal organization and endosomal maturation | CA | Positive mediator of retroviral infection, promoting early stage of infection by stabilizing CA to support HIV-1 infection | Yeast two-hybrid Co-IP | [293,294,295] |
| RPL7 (ribosomal protein large 7) | Ribosomal protein | Involved in ribosome biogenesis and regulation of mRNA translation | NC (zinc fingers) | Promotes Gag chaperone activity | Yeast two-hybrid Co-IP | [296] |
| RVB-2 (RuvB-like 2) | AAA+ superfamily member | Multifunctional protein involved in DNA repair, nonsense-mediated mRNA decay, humoral immunity regulator … | MA | Role in controlling viral protein expression (Env and Gag) | Tandem affinity purification Co-IP | [297,298] |
| RPS6 (ribosomal protein small 6) | Ribosomal protein | Ribosome biogenesis | nd | nd | Proximity-dependent biotin identification (BioID) Co-IP | [36] |
| SNARE (soluble N-ethylmaleimide-sensitive factor attachment protein receptor) | Soluble NSF Attachment protein receptor family | Mediates vesicle fusion | NC | Role in assembly and release, likely by affecting cellular trafficking pathways required for Gag transport and association with the PM | In vitro protein binding | [299] |
| SOCS1 (suppressor of cytokine signaling protein 1) | Suppressor of cytokine signaling (SOCS) family | Takes part in a negative feedback loop to attenuate cytokine signaling | MA and NC | Regulates positively late stages of HIV-1 infection by facilitating Gag intracellular trafficking to the PM and its stability via the microtubule network which may as well enhance Gag ubiquitination | GST-pull down Co-IP | [300,301] |
| Staufen-1 (dsRNA-binding Staufen homolog 1) | dsRNA-binding proteins family | Involved in the transport and/or localization of mRNAs to different subcellular compartments and/or organelles | NC | Part of RNP complex (Gag+vRNA+Staufen-1 +other proteins). Participates in HIV-1 assembly by influencing Gag multimerization, and in the intracellular trafficking of Gag during viral egress. Staufen1 also plays important rescue roles (vRNA translation…) during cellular stress. | Co-IP BRET BiFC Tandem affinity purification TriFC | [257,302,303,304,305,306,307,308] |
| TIP47 (tail-interacting protein of 47bkDa) = M6PRBP1 (mannose-6-phosphate binding protein) | Peripilin protein family | Involved in the endosome-to-TGN retrograde transport of mannose-6 phosphate receptors | MA | Involved in the incorporation of HIV-1 Env into HIV-1 Gag particle during viral assembly (T-cell and macrophage) | Yeast two-hybrid GST-pull down Co-IP NMR Surface plasmon resonance (SPR) binding assay | [309,310,311] |
| TRIM5α (tripartite motif-containing protein 5) | TRIM (tripartite motif) protein family | Retrovirus restriction factor, mediates species-specific, early block to retrovirus infection | CA | Mediator of innate cellular resistance to infection acting on the capsid; cellular factor blocking virus production by actively degrading viral Gag polyproteins | Trim5α restriction assay | [312,313,314,315] |
| Tsg101 (tumor susceptibility gene 101) | VPS (vacuolar protein sorting) family, component of ESCRT I complex | Regulates the vesicular trafficking. Involved in sorting of cargos into MVBs. Required for cytokinesis, plays a role in cell growth and differentiation and acts as a negative growth regulator | p6 and NC | The binding to p6 leads to the recruitment of ESCRT proteins and the following viral release | GST pull-down, Yeast two-hybrid Co-IPFRET-FLIM NMR chemical shift mapping | [164,179,316,317] |
| Ubc9 (ubiquitin carrier protein 9) | E2 SUMO-1 conjugating enzyme | Post-translationally modifies target proteins and alters their function by SUMOylation | NC-p6 | Plays a role in the production of infectious HIV-1 virions, influencing the stability and trafficking of Env proteins to the site of assembly | GST-pull down Yeast two-hybrid Co-IP | [165,318,319] |
| UBP (U-binding protein) | TPR (tetratricopeptide repeat) family of proteins | TPR family: organelle-targeting proteins, proteins involved in mitosis, immunophilins and nuclear phosphatases | nd | Intermediary between Vpu and Gag and likely plays a role in virus assembly or release | Yeast two-hybrid in vitro protein binding assay | [320] |
| UPF1 (upframeshift protein 1) = UPF3B | ATP-dependent RNA helicase of the SFI superfamily | Required for nonsense-mediated mRNA decay in eukaryotes (RNA stability). Also involved in DNA repair, cell cycle progression, DNA replication, telomere metabolism | nd | Part of the Staufen-1 RNP complex in the cytoplasm. Role in the maintenance of HIV-1 RNA stability and protein synthesis | Co-IP Tandem affinity purification assay | [308,321,322] |
| VAN (virion-associated nuclear shuttling protein) | Nuclear/cytoplasm shuttling protein | unknown | MA | Role during early phase of replication. Potentially facilitating nuclear import and retention of the PIC. | Yeast two-hybrid GST pull down | [323] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Klingler, J.; Anton, H.; Réal, E.; Zeiger, M.; Moog, C.; Mély, Y.; Boutant, E. How HIV-1 Gag Manipulates Its Host Cell Proteins: A Focus on Interactors of the Nucleocapsid Domain. Viruses 2020, 12, 888. https://doi.org/10.3390/v12080888
Klingler J, Anton H, Réal E, Zeiger M, Moog C, Mély Y, Boutant E. How HIV-1 Gag Manipulates Its Host Cell Proteins: A Focus on Interactors of the Nucleocapsid Domain. Viruses. 2020; 12(8):888. https://doi.org/10.3390/v12080888
Chicago/Turabian StyleKlingler, Jéromine, Halina Anton, Eléonore Réal, Manon Zeiger, Christiane Moog, Yves Mély, and Emmanuel Boutant. 2020. "How HIV-1 Gag Manipulates Its Host Cell Proteins: A Focus on Interactors of the Nucleocapsid Domain" Viruses 12, no. 8: 888. https://doi.org/10.3390/v12080888
APA StyleKlingler, J., Anton, H., Réal, E., Zeiger, M., Moog, C., Mély, Y., & Boutant, E. (2020). How HIV-1 Gag Manipulates Its Host Cell Proteins: A Focus on Interactors of the Nucleocapsid Domain. Viruses, 12(8), 888. https://doi.org/10.3390/v12080888





