Modelling West Nile Virus and Usutu Virus Pathogenicity in Human Neural Stem Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Cells and Culture Protocols
2.1.1. Reprogramming of Erythroblasts into hiPSCs
2.1.2. Differentiation of hiPSCs into NSCs
2.2. Viral Strains
2.3. Infections with WNV, USUV and ZIKV
2.4. Analysis of Virus Replication Kinetics
2.4.1. Quantitative Real-Time RT-PCR Analysis of Virus RNA Load
2.4.2. Virus Titration by 50% Tissue Culture Infective Dose (TCID50) Assay
2.5. End-Point RT-PCR Analysis of Differentiation Marker Expression
2.6. qRT-PCR Analysis of mRNA Levels of Genes Involved in Innate Antiviral Response
2.7. Immunofluorescence Assays
2.8. Apoptosis Assay
2.9. Cell Viability Assay
2.10. Statistical Analysis
3. Results
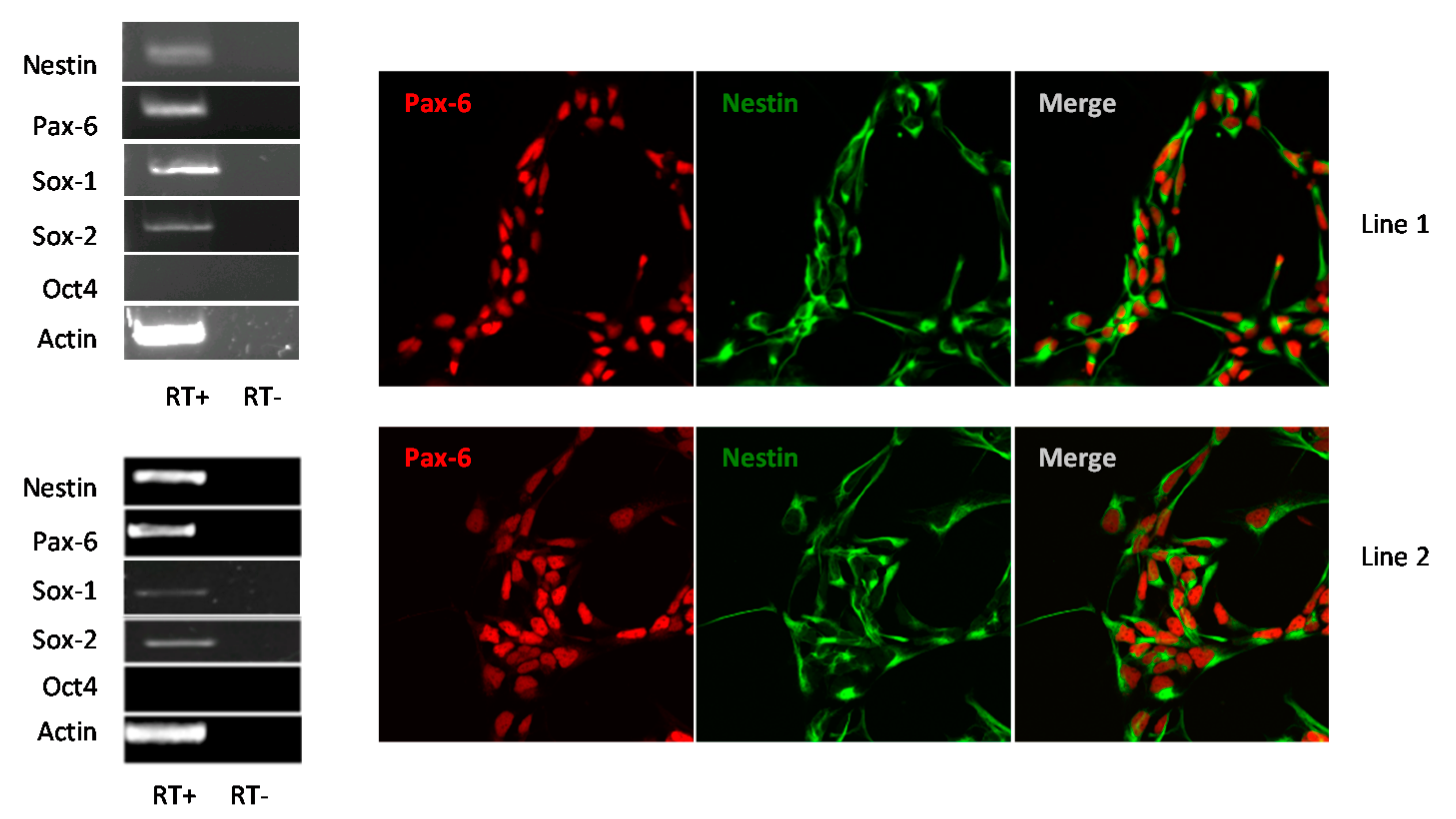
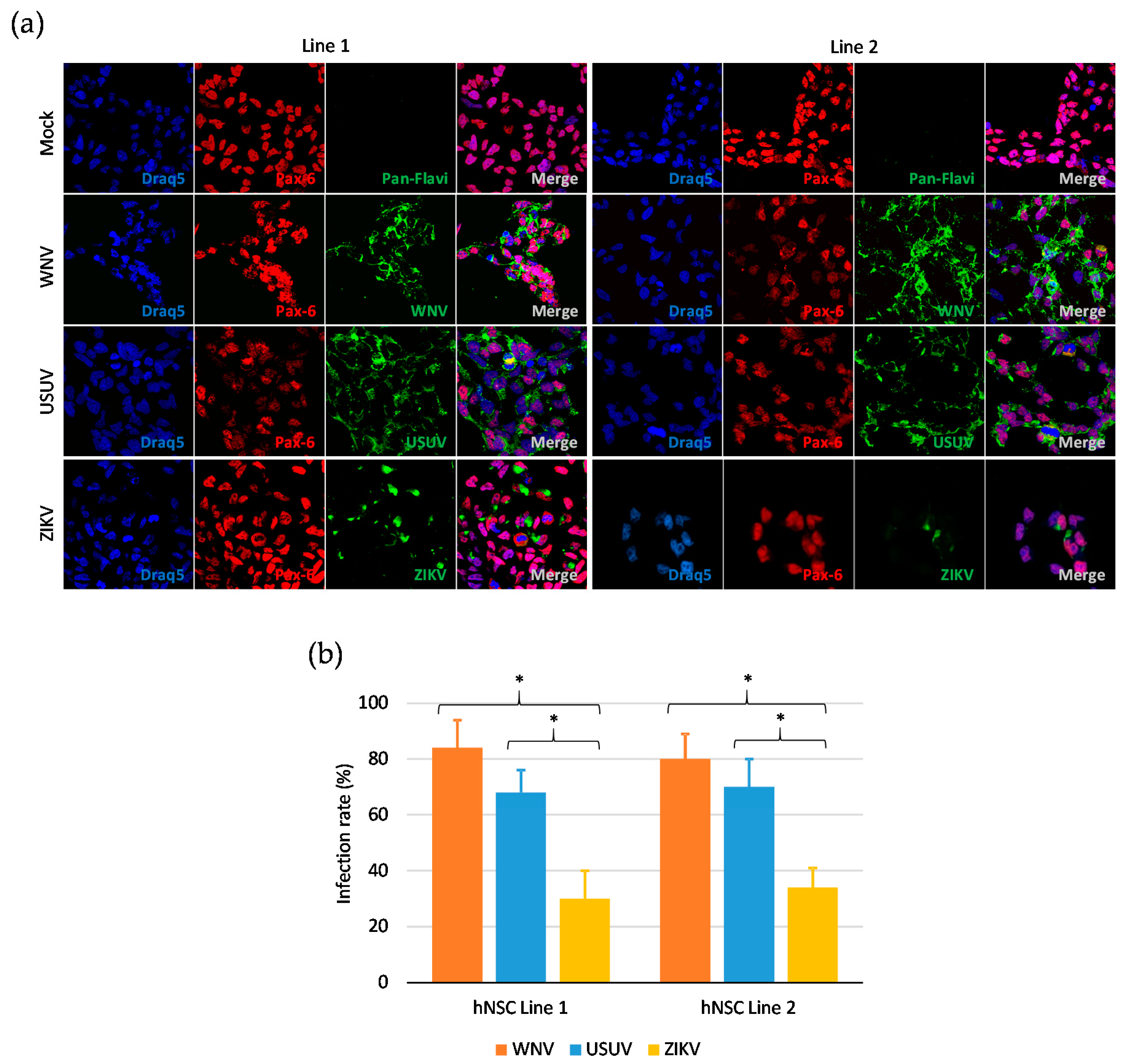
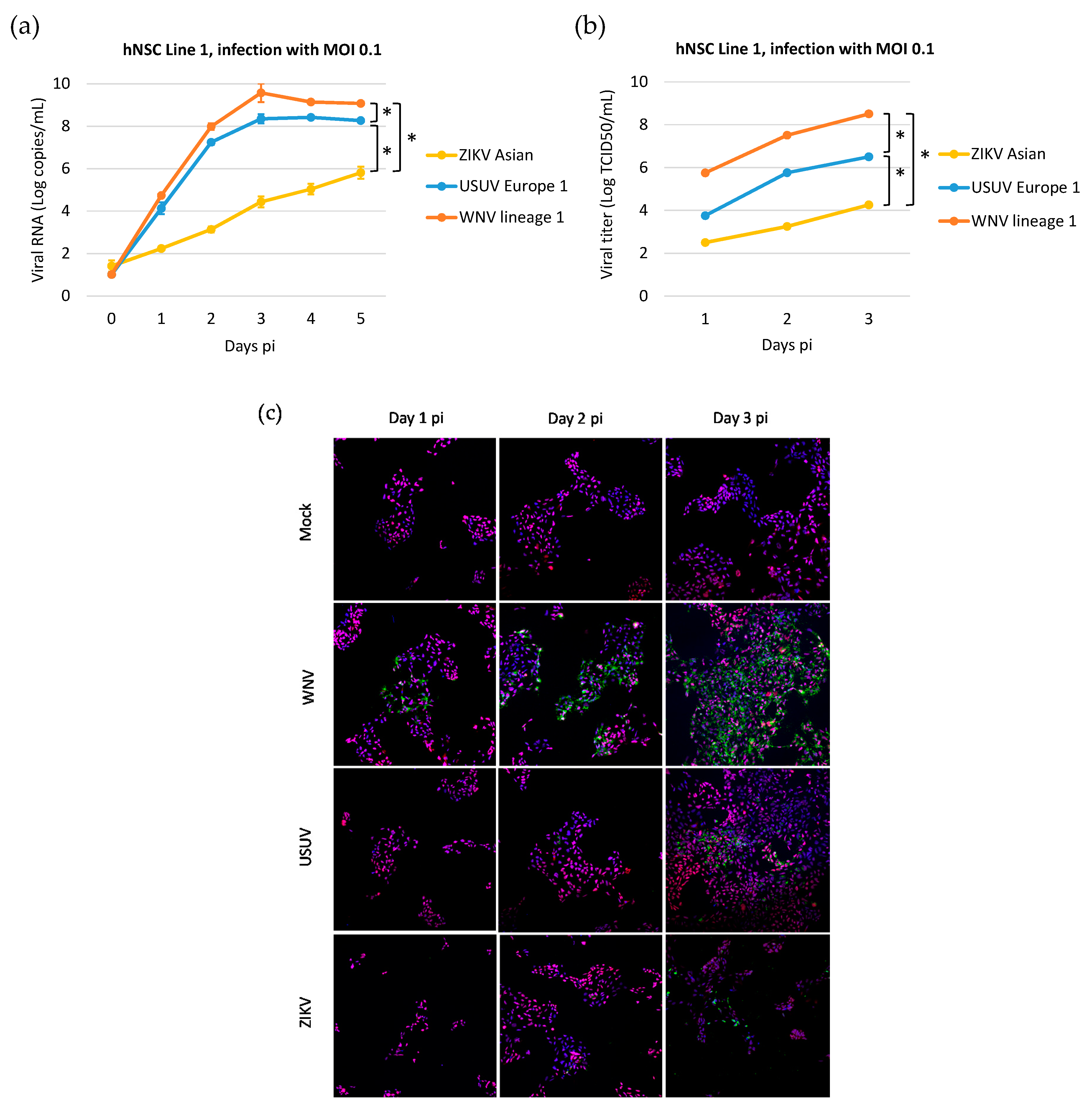
3.1. Human iPSC-Derived NSCs Are Permissive to WNV and USUV Infection
3.2. Kinetics of WNV, USUV, and ZIKV Production in hNSCs
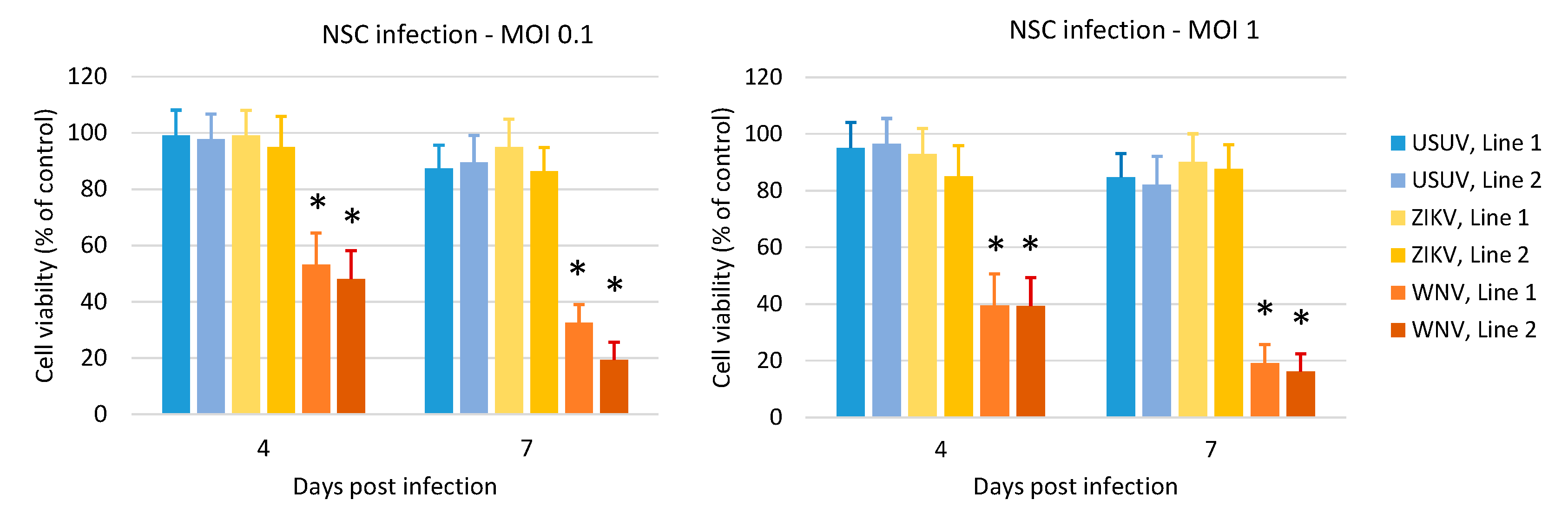
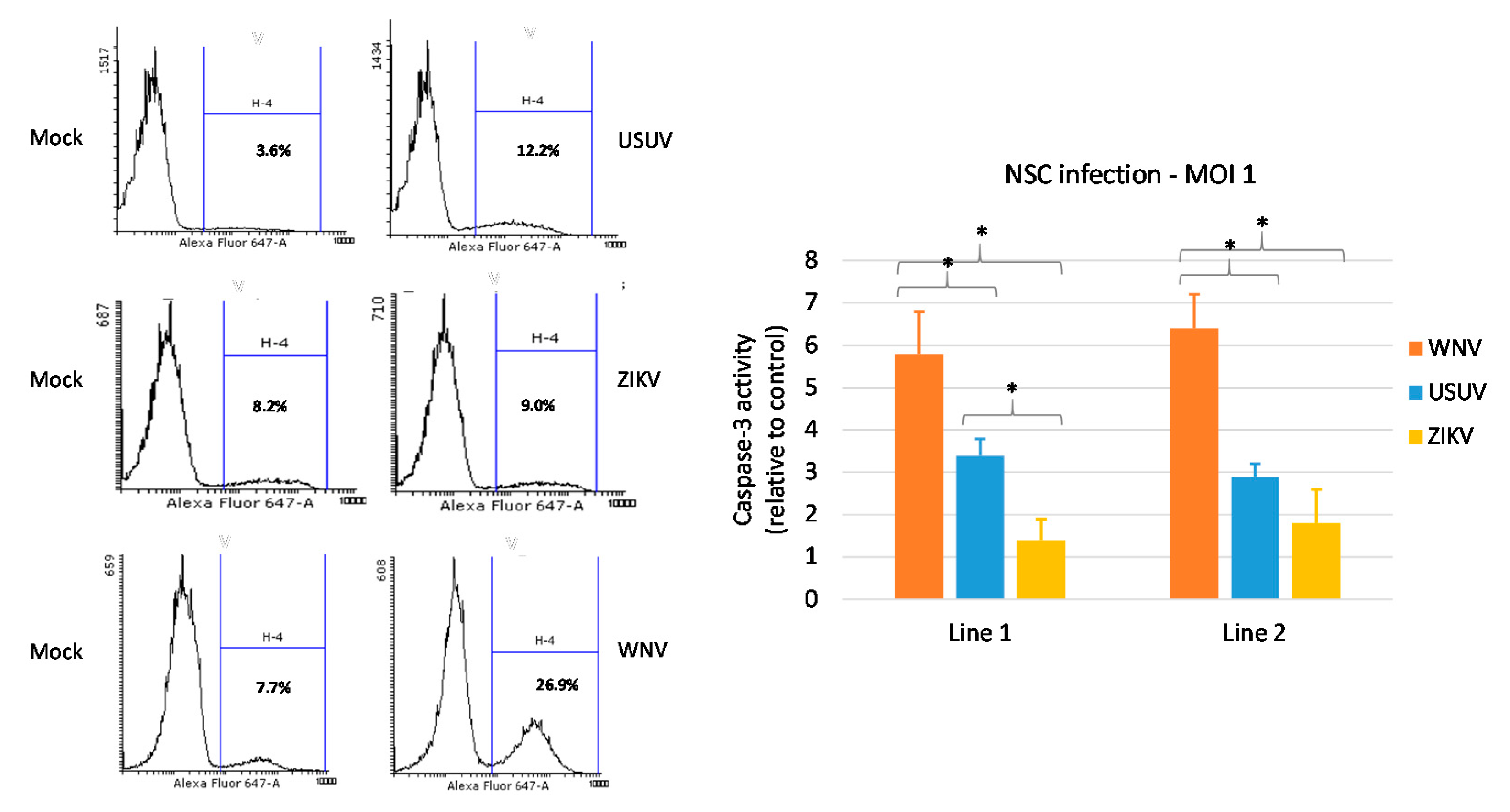
3.3. Effects of WNV, USUV and ZIKV Infection on hNSC Viability and Caspase-3 Activity in Infected Cells
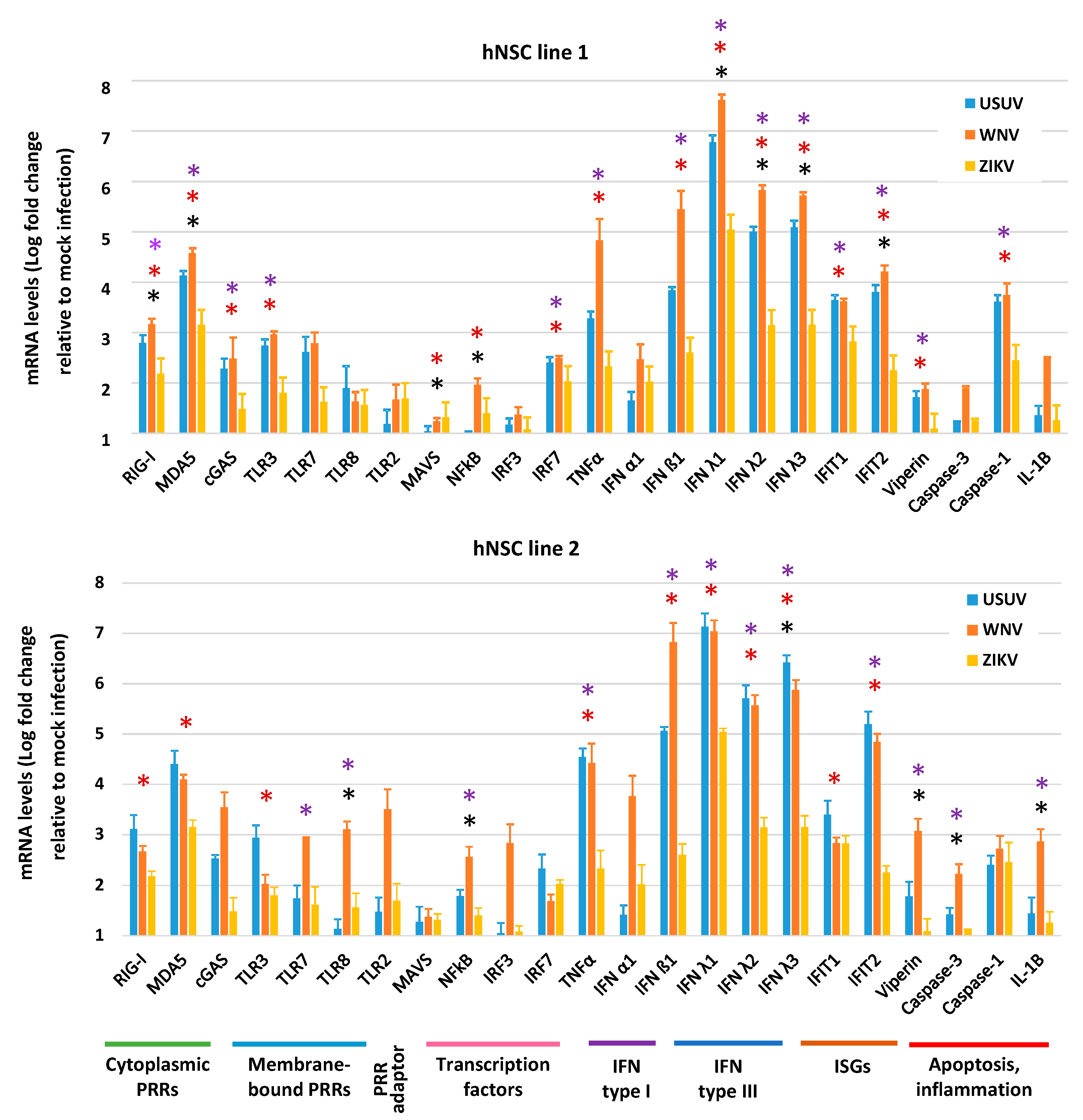
3.4. Expression of Innate Antiviral Immune Genes in hNSCs in Response to WNV, USUV and ZIKV Infection
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Barzon, L. Ongoing and emerging arbovirus threats in Europe. J. Clin. Virol. 2018, 107, 38–47. [Google Scholar] [CrossRef]
- Woodall, J.P. The viruses isolated from arthropods at the East African Virus Research Institute in the 26 years ending December 1963. Proc. E Afr. Acad. 1964, 2, 141–146. [Google Scholar]
- Weissenböck, H.; Bakonyi, T.; Rossi, G.; Mani, P.; Nowotny, N. Usutu virus, Italy, 1996. Emerg. Infect. Dis. 2013, 19, 274–277. [Google Scholar] [CrossRef]
- Weissenböck, H.; Kolodziejek, J.; Url, A.; Lussy, H.; Rebel-Bauder, B.; Nowotny, N. Emergence of Usutu virus, an African mosquito-borne flavivirus of the Japanese encephalitis virus group, central Europe. Emerg. Infect. Dis. 2002, 8, 652–656. [Google Scholar] [CrossRef]
- Zannoli, S.; Sambri, V. West Nile virus and Usutu virus co-circulation in Europe: Epidemiology and implications. Microorganisms 2019, 7, 184. [Google Scholar] [CrossRef]
- Sinigaglia, A.; Pacenti, M.; Martello, T.; Pagni, S.; Franchin, E.; Barzon, L. West Nile virus infection in individuals with pre-existing Usutu virus immunity, northern Italy, 2018. Eurosurveillance 2019, 24. [Google Scholar] [CrossRef]
- Pierro, A.; Gaibani, P.; Spadafora, C.; Ruggeri, D.; Randi, V.; Parenti, S.; Finarelli, A.C.; Rossini, G.; Landini, M.P.; Sambri, V. Detection of specific antibodies against West Nile and Usutu viruses in healthy blood donors in northern Italy, 2010-2011. Clin. Microbiol. Infect. 2013, 19, E451–E453. [Google Scholar] [CrossRef]
- Faggioni, G.; De Santis, R.; Pomponi, A.; Grottola, A.; Serpini, G.F.; Meacci, M.; Gennari, W.; Tagliazucchi, S.; Pecorari, M.; Monaco, F.; et al. Prevalence of Usutu and West Nile virus antibodies in human sera, Modena, Italy, 2012. J. Med. Virol. 2018, 90, 1666–1668. [Google Scholar] [CrossRef]
- Aberle, S.W.; Kolodziejek, J.; Jungbauer, C.; Stiasny, K.; Aberle, J.H.; Zoufaly, A.; Hourfar, M.K.; Weidner, L.; Nowotny, N. Increase in human West Nile and Usutu virus infections, Austria, 2018. Eurosurveillance 2018, 23. [Google Scholar] [CrossRef]
- Cadar, D.; Maier, P.; Müller, S.; Kress, J.; Chudy, M.; Bialonski, A.; Schlaphof, A.; Jansen, S.; Jöst, H.; Tannich, E.; et al. Blood donor screening for West Nile virus (WNV) revealed acute Usutu virus (USUV) infection, Germany, September 2016. Eurosurveillance 2017, 22, 30501. [Google Scholar] [CrossRef]
- Gaibani, P.; Pierro, A.M.; Cavrini, F.; Rossini, G.; Landini, M.P.; Sambri, V. False-positive transcription-mediated amplification assay detection of West Nile virus in blood from a patient with viremia caused by an Usutu virus infection. J. Clin. Microbiol. 2010, 48, 3338–3339. [Google Scholar] [CrossRef] [PubMed]
- Carletti, F.; Colavita, F.; Rovida, F.; Percivalle, E.; Baldanti, F.; Ricci, I.; De Liberato, C.; Rosone, F.; Messina, F.; Lalle, E.; et al. Expanding Usutu virus circulation in Italy: Detection in the Lazio region, central Italy, 2017 to 2018. Eurosurveillance 2019, 24. [Google Scholar] [CrossRef]
- Cavrini, F.; Gaibani, P.; Longo, G.; Pierro, A.M.; Rossini, G.; Bonilauri, P.; Gerunda, G.E.; Di Benedetto, F.; Pasetto, A.; Girardis, M.; et al. Usutu virus infection in a patient who underwent orthotropic liver transplantation, Italy, August-September 2009. Eurosurveillance 2009, 14, 19448. [Google Scholar]
- Pecorari, M.; Longo, G.; Gennari, W.; Grottola, A.; Sabbatini, A.; Tagliazucchi, S.; Savini, G.; Monaco, F.; Simone, M.; Lelli, R.; et al. First human case of Usutu virus neuroinvasive infection, Italy, August-September 2009. Eurosurveillance 2009, 14, 19446. [Google Scholar]
- Grottola, A.; Marcacci, M.; Tagliazucchi, S.; Gennari, W.; Di Gennaro, A.; Orsini, M.; Monaco, F.; Marchegiano, P.; Marini, V.; Meacci, M.; et al. Usutu virus infections in humans: A retrospective analysis in the municipality of Modena, Italy. Clin. Microbiol. Infect. 2017, 23, 33–37. [Google Scholar] [CrossRef]
- Pacenti, M.; Sinigaglia, A.; Martello, T.; De Rui, E.; Franchin, E.; Pagni, S.; Peta, E.; Riccetti, S.; Milani, A.; Montarsi, F.; et al. Clinical and virological findings in patients with Usutu virus infection, Northern Italy, 2018. Eurosurveillance 2019, 24, 1900180. [Google Scholar] [CrossRef]
- Nagy, A.; Mezei, E.; Nagy, O.; Bakonyi, T.; Csonka, N.; Kaposi, M.; Koroknai, A.; Szomor, K.; Rigó, Z.; Molnár, Z.; et al. Extraordinary increase in West Nile virus cases and first confirmed human Usutu virus infection in Hungary, 2018. Eurosurveillance 2019, 24, 1900038. [Google Scholar] [CrossRef]
- Vilibic-Cavlek, T.; Savic, V.; Sabadi, D.; Peric, L.; Barbic, L.; Klobucar, A.; Miklausic, B.; Tabain, I.; Santini, M.; Vucelja, M.; et al. Prevalence and molecular epidemiology of West Nile and Usutu virus infections in Croatia in the ‘One health’ context, 2018. Transbound. Emerg. Dis. 2019, 66, 1946–1957. [Google Scholar] [CrossRef]
- Weissenböck, H.; Bakonyi, T.; Chvala, S.; Nowotny, N. Experimental Usutu virus infection of suckling mice causes neuronal and glial cell apoptosis and demyelination. Acta Neuropathol. 2004, 108, 453–460. [Google Scholar] [CrossRef]
- Blázquez, A.B.; Escribano-Romero, E.; Martín-Acebes, M.A.; Petrovic, T.; Saiz, J.C. Limited susceptibility of mice to Usutu virus (USUV) infection and induction of flavivirus cross-protective immunity. Virology 2015, 482, 67–71. [Google Scholar] [CrossRef] [PubMed]
- Martín-Acebes, M.A.; Blázquez, A.B.; Cañas-Arranz, R.; Vázquez-Calvo, A.; Merino-Ramos, T.; Escribano-Romero, E.; Sobrino, F.; Saiz, J.C. A recombinant DNA vaccine protects mice deficient in the alpha/beta interferon receptor against lethal challenge with Usutu virus. Vaccine 2016, 34, 2066–2073. [Google Scholar] [CrossRef]
- Benzarti, E.; Sarlet, M.; Franssen, M.; Desmecht, D.; Schmidt-Chanasit, J.; Garigliany, M.M. New insights into the susceptibility of immunocompetent mice to Usutu virus. Viruses 2020, 12, 189. [Google Scholar] [CrossRef] [PubMed]
- Ndione, M.H.D.; Ndiaye, E.H.; Thiam, M.S.; Weidmann, M.; Faye, M.; Ba, Y.; Benkaroun, J.; Faye, O.; Loucoubar, C.; Mbacké Sembène, P.; et al. Impact of genetic diversity on biological characteristics of Usutu virus strains in Africa. Virus Res. 2019, 273, 197753. [Google Scholar] [CrossRef] [PubMed]
- Prow, N.A.; Edmonds, J.H.; Williams, D.T.; Setoh, Y.X.; Bielefeldt-Ohmann, H.; Suen, W.W.; Hobson-Peters, J.; van den Hurk, H.F.; Pyke, A.T.; Hall-Mendelin, S.; et al. Virulence and evolution of West Nile Virus, Australia, 1960–2012. Emerg. Infect. Dis. 2016, 22, 1353–1362. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Ramírez, E.; Llorente, F.; Del Amo, J.; Fall, G.; Sall, A.A.; Lubisi, A.; Lecollinet, S.; Vázquez, A.; Jiménez-Clavero, M.A. Pathogenicity evaluation of twelve West Nile virus strains belonging to four lineages from five continents in a mouse model: Discrimination between three pathogenicity categories. J. Gen. Virol. 2017, 98, 662–670. [Google Scholar] [CrossRef] [PubMed]
- Rizzoli, A.; Jimenez-Clavero, M.A.; Barzon, L.; Cordioli, P.; Figuerola, J.; Koraka, P.; Martina, B.; Moreno, A.; Nowotny, N.; Pardigon, N.; et al. The challenge of West Nile virus in Europe: Knowledge gaps and research priorities. Eurosurveillance 2015, 20, 21135. [Google Scholar] [CrossRef] [PubMed]
- Clé, M.; Barthelemy, J.; Desmetz, C.; Foulongne, V.; Lapeyre, L.; Bolloré, K.; Tuaillon, E.; Erkilic, N.; Kalatzis, V.; Lecollinet, S.; et al. Study of Usutu virus neuropathogenicity in mice and human cellular models. PLoS Negl. Trop. Dis. 2020, 14, e0008223. [Google Scholar] [CrossRef]
- Scagnolari, C.; Caputo, B.; Trombetti, S.; Cacciotti, G.; Soldà, A.; Spano, L.; Villari, P.; della Torre, A.; Nowotny, N.; Antonelli, G. Usutu virus growth in human cell lines: Induction of and sensitivity to type I and III interferons. J. Gen. Virol. 2013, 94, 789–795. [Google Scholar] [CrossRef]
- Salinas, S.; Constant, O.; Desmetz, C.; Barthelemy, J.; Lemaitre, J.-M.; Milhavet, O.; Nagot, N.; Foulongne, V.; Perrin, F.E.; Saiz, J.C.; et al. Deleterious effect of Usutu virus on human neural cells. PLoS Negl. Trop. Dis. 2017, 11, e0005913. [Google Scholar] [CrossRef]
- Desole, G.; Sinigaglia, A.; Riccetti, S.; Masi, G.; Pacenti, M.; Trevisan, M.; Barzon, L. Modelling neurotropic flavivirus infection in human induced pluripotent stem cell-derived systems. Int. J. Mol. Sci. 2019, 20, 5404. [Google Scholar] [CrossRef]
- Barzon, L.; Trevisan, M.; Sinigaglia, A.; Lavezzo, E.; Palù, G. Zika virus: From pathogenesis to disease control. FEMS Microbiol. Lett. 2016, 363, 202. [Google Scholar] [CrossRef] [PubMed]
- Cacciotti, G.; Caputo, B.; Selvaggi, C.; la Sala, A.; Vitiello, L.; Diallo, D.; Ceianu, C.; Antonelli, G.; Nowotny, N. Variation in interferon sensitivity and induction between Usutu and West Nile (lineages 1 and 2) viruses. Virology 2015, 485, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Trevisan, M.; Desole, G.; Costanzi, G.; Lavezzo, E.; Palù, G.; Barzon, L. Reprogramming methods do not affect gene expression profile of human induced pluripotent stem cells. Int. J. Mol. Sci. 2017, 18, 206. [Google Scholar] [CrossRef]
- Brien, J.D.; Lazear, H.M.; Diamond, M.S. Propagation, quantification, detection, and storage of West Nile virus. Curr. Protoc. Microbiol. 2013, 31, 15. [Google Scholar] [CrossRef]
- Cavrini, F.; Della Pepa, M.E.; Gaibani, P.; Pierro, A.M.; Rossini, G.; Landini, M.P.; Sambri, V. A rapid and specific real-time RT-PCR assay to identify Usutu virus in human plasma, serum, and cerebrospinal fluid. J. Clin. Virol. 2011, 50, 221–223. [Google Scholar] [CrossRef] [PubMed]
- Linke, S.; Ellerbrok, H.; Niedrig, M.; Nitsche, A.; Pauli, G. Detection of West Nile virus lineages 1 and 2 by real-time PCR. J. Virol. Methods 2007, 146, 355–358. [Google Scholar] [CrossRef]
- Lanciotti, R.S.; Kosoy, O.L.; Laven, J.J.; Velez, J.O.; Lambert, A.J.; Johnson, A.J.; Stanfield, S.M.; Duffy, M.R. Genetic and serologic properties of Zika virus associated with an epidemic, Yap State, Micronesia, 2007. Emerg. Infect. Dis. 2008, 14, 1232–1239. [Google Scholar] [CrossRef] [PubMed]
- Barzon, L.; Pacenti, M.; Franchin, E.; Pagni, S.; Martello, T.; Cattai, M.; Cusinato, R.; Palù, G. Excretion of West Nile virus in urine during acute infection. J. Infect. Dis. 2013, 208, 1086–1092. [Google Scholar] [CrossRef]
- Barzon, L.; Percivalle, E.; Pacenti, M.; Rovida, F.; Zavattoni, M.; Del Bravo, P.; Cattelan, A.M.; Palù, G.; Baldanti, F. Virus and antibody dynamics in travelers with acute Zika virus infection. Clin. Infect. Dis. 2018, 66, 1173–1180. [Google Scholar] [CrossRef]
- Farahzadi, R.; Fathi, E.; Mesbah-Namin, S.A.; Zarghami, N. Zinc sulfate contributes to promote telomere length extension via increasing telomerase gene expression, telomerase activity and change in the TERT gene promoter CpG island methylation status of human adipose-derived mesenchymal stem cells. PLoS ONE 2017, 12, e0188052. [Google Scholar] [CrossRef]
- Trevisan, M.; Barbaro, V.; Riccetti, S.; Masi, G.; Barzon, L.; Nespeca, P.; Alvisi, G.; Di Iorio, E.; Palù, G. Generation of a transgene-free induced pluripotent stem cells line (UNIPDi002-A) from oral mucosa epithelial stem cells carrying the R304Q mutation in TP63 gene. Stem Cell Res. 2018, 28, 149–152. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, H.; Liao, H.; Minami, K.; Toyoda, M.; Akutsu, H.; Miyagawa, Y.; Okita, H.; Kiyokawa, N.; Umezawa, A.; Imadome, K.; et al. Human cytomegalovirus induces apoptosis in neural stem/progenitor cells derived from induced pluripotent stem cells by generating mitochondrial dysfunction and endoplasmic reticulum stress. Herpesviridae 2013, 4, 2. [Google Scholar] [CrossRef]
- Xu, C.; Tabebordbar, M.; Iovino, S.; Ciarlo, C.; Liu, J.; Castiglioni, A.; Price, E.; Liu, M.; Barton, E.R.; Kahn, C.R.; et al. A zebrafish embryo culture system defines factors that promote vertebrate myogenesis across species. Cell 2013, 155, 909–921. [Google Scholar] [CrossRef] [PubMed]
- Manel, N.; Unutmaz, D.; Littman, D.R. The differentiation of human TH-17 cells requires transforming growth factor-beta and induction of the nuclear receptor RORγt. Nat. Immunol. 2008, 9, 641–649. [Google Scholar] [CrossRef] [PubMed]
- Webster Marketon, J.I.; Corry, J.; Teng, M.N. The respiratory syncytial virus (RSV) nonstructural proteins mediate RSV suppression of glucocorticoid receptor transactivation. Virology 2014, 449, 62–69. [Google Scholar] [CrossRef] [PubMed]
- Prescott, J.B.; Hall, P.R.; Bondu-Hawkins, V.S.; Ye, C.; Hjelle, B. Early innate immune responses to Sin Nombre Hantavirus occur independently of IFN regulatory factor 3, characterized pattern recognition receptors, and viral entry. J. Immunol. 2007, 179, 1796–1802. [Google Scholar] [CrossRef]
- Zhang, Y.; Yeruva, L.; Marinov, A.; Prantner, D.; Wyrick, P.B.; Lupashin, V.; Nagarajan, U.M. The DNA Sensor, Cyclic GMP–AMP Synthase, Is Essential for Induction of IFN-β during Chlamydia trachomatis Infection. J. Immunol. 2014, 193, 2394–2404. [Google Scholar] [CrossRef]
- Abrahams, V.M.; Potter, J.A.; Bhat, G.; Peltier, M.R.; Saade, G.; Menon, R. Bacterial modulation of human fetal membrane Toll-like receptor expression. Am. J. Reprod. Immunol. 2013, 69, 33–40. [Google Scholar] [CrossRef]
- Hsu, Y.-L.; Wang, M.-Y.; Ho, L.-J.; Laia, J.-H. Dengue virus infection induces interferon-lambda1 to facilitate cell migration. Sci. Rep. 2016, 6, 24530. [Google Scholar] [CrossRef]
- Sha, Q.; Truong-Tran, A.Q.; Plitt, J.R.; Beck, L.A.; Schleimer, R.P. Activation of airway epithelial cells by toll-like receptor agonists. Am. J. Respir. Cell Mol. Biol. 2004, 31, 358–364. [Google Scholar] [CrossRef]
- Zong, C.; Kimura, Y.; Kinoshita, K.; Takasu, S.; Zhang, X.; Sakurai, T.; Sekido, Y.; Ichihara, S.; Endo, G.; Ichihara, G. Exposure to 1,2-dichloropropane upregulates the expression of activation-induced cytidine deaminase (AID) in human cholangiocytes co-cultured with macrophages. Toxicol. Sci. 2019, 168, 137–148. [Google Scholar] [CrossRef]
- Reimer, T.; Schweizer, M.; Jungi, T.W. Type I IFN induction in response to Listeria monocytogenes in human macrophages: Evidence for a differential activation of IFN regulatory factor 3 (IRF3). J. Immunol. 2007, 179, 1166–1177. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Li, Q.; Tian, S.; Cui, W.X.J.; Wang, R.-F. TRIM9 short isoform preferentially promotes DNA and RNA virus-induced production of type I interferon by recruiting GSK3β to TBK1. Cell Res. 2016, 26, 613–628. [Google Scholar] [CrossRef] [PubMed]
- Teng, T.S.; Foo, S.S.; Simamarta, D.; Lum, F.M.; Teo, T.H.; Lulla, A.; Yeo, N.K.; Koh, E.G.; Chow, A.; Leo, Y.S.; et al. Viperin restricts chikungunya virus replication and pathology. J. Clin. Investig. 2012, 122, 4447–4460. [Google Scholar] [CrossRef] [PubMed]
- Dill, M.T.; Duong, F.H.; Vogt, J.E.; Bibert, S.; Bochud, P.Y.; Terracciano, L.; Papassotiropoulos, A.; Roth, V.; Heim, M.H. Interferon-induced gene expression is a stronger predictor of treatment response than IL28B genotype in patients with hepatitis C. Gastroenterology 2011, 140, 1021–1031. [Google Scholar] [CrossRef]
- Niu, L.; Zhang, S.; Wu, J.; Chen, L.; Wang, Y. Upregulation of NLRP3 inflammasome in the tears and ocular surface of dry eye patients. PLoS ONE 2015, 10, e0126277. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, R.; Nakamura, E.; Harigaya, T. Vasoinhibin, an N-terminal prolactin fragment, directly inhibits cardiac angiogenesis in three-dimensional heart culture. Front. Endocrinol. (Lausanne) 2017, 8, 4. [Google Scholar] [CrossRef]
- Riedmaier, I.; Tichopad, A.; Reiter, M.; Pfaffl, M.W.; Meyer, H.H. Influence of testosterone and a novel SARM on gene expression in whole blood of Macaca fascicularis. J. Steroid Biochem. Mol. Biol. 2009, 114, 167–173. [Google Scholar] [CrossRef]
- Gordon, F.E.; Nutt, C.L.; Cheunsuchon, P.; Nakayama, Y.; Provencher, K.A.; Rice, K.A.; Zhou, Y.; Zhang, X.; Klibanski, A. Increased expression of angiogenic genes in the brains of mouse meg3-null embryos. Endocrinology 2010, 151, 2443–2452. [Google Scholar] [CrossRef]
- Graham, J.B.; Thomas, S.; Swarts, J.; McMillan, A.A.; Ferris, M.T.; Suthar, M.S.; Treuting, P.M.; Ireton, R.; Gale, M., Jr.; Lund, J.M. Genetic diversity in the collaborative cross model recapitulates human West Nile virus disease outcomes. MBio 2015, 6, e00493-15. [Google Scholar] [CrossRef]
- Huang, B.; West, N.; Vider, J.; Zhang, P.; Griffiths, R.E.; Wolvetang, E.; Burtonclay, P.; Warrilow, D. Inflammatory responses to a pathogenic West Nile virus strain. BMC Infect. Dis. 2019, 19, 912. [Google Scholar] [CrossRef] [PubMed]
- Errett, J.S.; Suthar, M.S.; McMillan, A.; Diamond, M.S.; Gale, M., Jr. The essential, nonredundant roles of RIG-I and MDA5 in detecting and controlling West Nile virus infection. J. Virol. 2013, 87, 11416–11425. [Google Scholar] [CrossRef] [PubMed]
- Lazear, H.M.; Daniels, B.P.; Pinto, A.K.; Huang, A.C.; Vick, S.C.; Doyle, S.E.; Gale, M., Jr.; Klein, R.S.; Diamond, M.S. Interferon-λ restricts West Nile virus neuroinvasion by tightening the blood-brain barrier. Sci. Transl. Med. 2015, 7, 284ra59. [Google Scholar] [CrossRef] [PubMed]
- Samuel, M.A.; Morrey, J.D.; Diamond, M.S. Caspase 3-dependent cell death of neurons contributes to the pathogenesis of West Nile virus encephalitis. J. Virol. 2007, 81, 2614–2623. [Google Scholar] [CrossRef]
- Ramos, H.J.; Lanteri, M.C.; Blahnik, G.; Negash, A.; Suthar, M.S.; Brassil, M.M.; Sodhi, K.; Treuting, P.M.; Busch, M.P.; Norris, P.J.; et al. IL-1β signaling promotes CNS-intrinsic immune control of West Nile virus infection. PLoS Pathog. 2012, 8, e1003039. [Google Scholar] [CrossRef]
- Gack, M.U.; Diamond, M.S. Innate immune escape by dengue and West Nile viruses. Curr. Opin. Virol. 2016, 20, 119–128. [Google Scholar] [CrossRef]
- Liu, W.J.; Wang, X.J.; Clark, D.C.; Lobigs, M.; Hall, R.A.; Khromykh, A.A. A single amino acid substitution in the West Nile virus nonstructural protein NS2A disables its ability to inhibit alpha/beta interferon induction and attenuates virus virulence in mice. J. Virol. 2006, 80, 2396–2404. [Google Scholar] [CrossRef]
- Setoh, Y.X.; Prow, N.A.; Rawle, D.J.; Tan, C.S.; Edmonds, J.H.; Hall, R.A.; Khromykh, A.A. Systematic analysis of viral genes responsible for differential virulence between American and Australian West Nile virus strains. J. Gen. Virol. 2015, 96, 1297–1308. [Google Scholar] [CrossRef]
- Xia, H.; Luo, H.; Shan, C.; Muruato, A.E.; Nunes, B.T.D.; Medeiros, D.B.A.; Zou, J.; Xie, X.; Giraldo, M.I.; Vasconcelos, P.F.C.; et al. An evolutionary NS1 mutation enhances Zika virus evasion of host interferon induction. Nat. Commun. 2018, 9, 414. [Google Scholar] [CrossRef]
- Delgado-Enciso, I.; López-Lemus, U.A.; Valcarcel-Gamiño, J.A.; Rodriguez-Sanchez, I.P.; Valle-Reyes, S.; Martinez-Fierro, M.L.; Melnikov, V.; Guzmán-Esquivel, J.; Vaca-Paniagua, F.; Valdez-Velazquez, L.; et al. Dengue virus-1 NS5 genetic variant associated with a severe clinical infection: Possible reduction of the innate immune response by inhibition of interferon type 1 and the Janus kinase-signal transducer and activator of transcription signaling pathway. Int. J. Mol. Med. 2018, 41, 2263–2269. [Google Scholar] [CrossRef]


| Target Gene | Forward/Reverse Primers (5′–3′) | References | |
|---|---|---|---|
| Neural stem cells markers | NESTIN | GAAGGTGAAGGGCAAATCTG CCTCTTCTTCCCATATTTCCTG | [40] |
| PAX6 | TCTAATCGAAGGGCCAAATG TGTGAGGGCTGTGTCTGTTC | [41] | |
| SOX1 | GCGGAAAGCGTTTTCTTTG TAATCTGACTTCTCCTCCC | [42] | |
| SOX2 | TTGTCGGAGACGGAGAAGCG TGACCACCGAACCCATGGAG | [42] | |
| Pluripotency marker | OCT4 | GTGGAGGAAGCTGACAACAA CAGGTTTTCTTTCCCTAGCT | [43] |
| Housekeeping gene | ACTIN | GGACTTCGAGCAAGAGATGG AGCACTGTGTTGGCGTACAG | [44] |
| Target Gene | Forward/Reverse Primers/TaqMan Probe (5′–3′) | Reference |
|---|---|---|
| RIG1 | ACCAGAGCACTTGTGGACGCT TGCCGGGAGGGTCATTCCTGT | [45] |
| MDA5 | CAGAAGGAAGTGTCAGCTGCTTAG TGCTGCCACATTCTCTTCATCT | [46] |
| CGAS | CCTGCTGTAACACTTCTTAT TTAGTCGTAGTTGCTTCCT | [47] |
| TLR3 | GAAAGGCTAGCAGTCATCCA CATCGGGTACCTGAGTCAAC | [48] |
| TLR7 | CTTGGCACCTCTCATGCTCT GTCTGTGCAGTCCACGATCA | [49] |
| TLR8 | AGTTTCTCTTCTCGGCCACC GGAACATGTTTTCCATGTTTCTGT | [49] |
| TLR2 | GGCCAGCAAATTACCTGTGTG AGGCGGACATCCTGAACCT FAM-CCATCCCATGTGCGTGG-MGB | [50] |
| MAVS | AGCAAGAGACCAGGATCGACTG CGCAATGAAGTACTCCACCCA | [46] |
| NFKB | GCCAACAGATGGCCCATACC TGCTGGTCCCACATAGTTGC | [51] |
| IRF3 | AGCAGAGGACCGGAGCAA AGAGGTGTCTGGCTGGGAAA FAM-ACCCTCACGACCCACATAAAATCTACGAGTTTG-TAMRA | [52] |
| IRF7 | TACCATCTACCTGGGCTTCG AGGGTTCCAGCTTCACCA | [46] |
| TNFA | CCAGACCAAGGTCAACCTCC CCCTCCCAGATAGATGGGCT | [53] |
| IFNA1 | AGAATCTCTCCTTTCTCCTG TCTGACAACCTCCCAGGCAC | [54] |
| IFNB1 | GAGCTACAACTTGCTTGGATTCC CAAGCCTCCCATTCAATTGC FAM-ACAAAGAAGCAGCAATTTTCAGTGTCAGAAGCT-TAMRA | [52] |
| IFNL1 | GAGGCCCCCAAAAAGGAGTC AGGTTCCCATCGGCCACATA | [49] |
| IFNL2 | AATTGTGTTGCCAGTGGGGA GCGACTGGGTGGCAATAAAT | [49] |
| IFNL3 | AGGGCCAAAGATGCCTTAG CAGCTCAGCCTCCAAAGC | [49] |
| IFIT1 | TCTCAGAGGAGCCTGGCTAA TGACATCTCAATTGCTCCAG | [49] |
| IFIT2 | AAGAGTGCAGCTGCCTGAA GGCATTTTAGTTGCCGTAGG | [49] |
| RSAD2 | CTTTGTGCTGCCCCTTGAG TCCATACCAGCTTCCTTAAGCAA | [55] |
| CASP1 | AAGACCCGAGCTTTGATTGACTC AAATCTCTGCCGACTTTTGTTTCC | [56] |
| CASP3 | TGCATACTCCACAGCACCTG TTCTGTTGCCACCTTTCGGT | [57] |
| IL1B | GAGCAACAAGTGGTGTTCTCC AACACGCAGGACAGGTACAG | [58] |
| GAPDH | GAAGGTGAAGGTCGGAGTC GAAGATGGTGATGGGATTTC FAM-CAAGCTTCCCGTTCTCAGCC-TAMRA | [59] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Riccetti, S.; Sinigaglia, A.; Desole, G.; Nowotny, N.; Trevisan, M.; Barzon, L. Modelling West Nile Virus and Usutu Virus Pathogenicity in Human Neural Stem Cells. Viruses 2020, 12, 882. https://doi.org/10.3390/v12080882
Riccetti S, Sinigaglia A, Desole G, Nowotny N, Trevisan M, Barzon L. Modelling West Nile Virus and Usutu Virus Pathogenicity in Human Neural Stem Cells. Viruses. 2020; 12(8):882. https://doi.org/10.3390/v12080882
Chicago/Turabian StyleRiccetti, Silvia, Alessandro Sinigaglia, Giovanna Desole, Norbert Nowotny, Marta Trevisan, and Luisa Barzon. 2020. "Modelling West Nile Virus and Usutu Virus Pathogenicity in Human Neural Stem Cells" Viruses 12, no. 8: 882. https://doi.org/10.3390/v12080882
APA StyleRiccetti, S., Sinigaglia, A., Desole, G., Nowotny, N., Trevisan, M., & Barzon, L. (2020). Modelling West Nile Virus and Usutu Virus Pathogenicity in Human Neural Stem Cells. Viruses, 12(8), 882. https://doi.org/10.3390/v12080882







