Insights into Sensing of Murine Retroviruses
Abstract
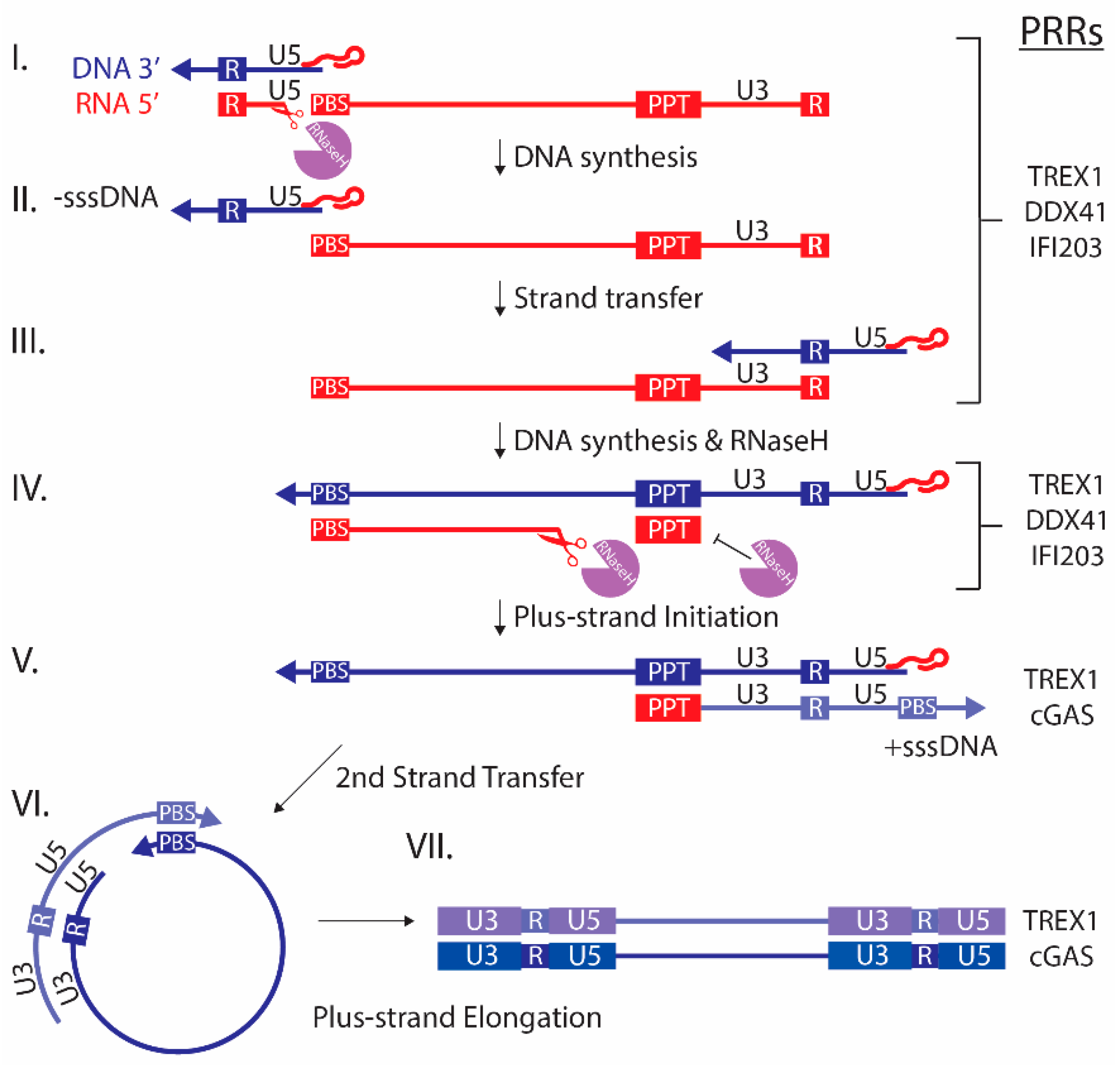
1. Introduction
2. Retroviruses Generate PAMPS during Replication
3. Murine Retroviruses and TLRs
4. ALRS, cGAS and Other Sensors
5. Viral Proteins that Block Host Sensors
6. Conclusions
Funding
Conflicts of Interest
References
- Akira, S.; Uematsu, S.; Takeuchi, O. Pathogen Recognition and Innate Immunity. Cell 2006, 124, 783–801. [Google Scholar] [CrossRef] [PubMed]
- Loo, Y.-M.; Gale, J.M. Immune Signaling by RIG-I-like Receptors. Immunity 2011, 34, 680–692. [Google Scholar] [CrossRef] [PubMed]
- Bhat, N.; Fitzgerald, K.A. Recognition of cytosolic DNA by cGAS and other STING-dependent sensors. Eur. J. Immunol. 2014, 44, 634–640. [Google Scholar] [CrossRef] [PubMed]
- Schattgen, S.A.; Fitzgerald, K.A. The PYHIN protein family as mediators of host defenses. Immunol. Rev. 2011, 243, 109–118. [Google Scholar] [CrossRef]
- Kawai, T.; Akira, S. The role of pattern-recognition receptors in innate immunity: Update on Toll-like receptors. Nat. Immunol. 2010, 11, 373–384. [Google Scholar] [CrossRef]
- Coffin, J.M.; Hughes, S.H.; Varmus, H.E. Retroviruses; CSHL Press: Cold Spring Harbor, NY, USA, 1997. [Google Scholar]
- Ganser-Pornillos, B.K.; Pornillos, O. Restriction of HIV-1 and other retroviruses by TRIM5. Nat. Rev. Genet. 2019, 17, 546–556. [Google Scholar] [CrossRef]
- Overbaugh, J.; Miller, A.; Eiden, M.V. Receptors and Entry Cofactors for Retroviruses Include Single and Multiple Transmembrane-Spanning Proteins as well as Newly Described Glycophosphatidylinositol-Anchored and Secreted Proteins. Microbiol. Mol. Boil. Rev. 2001, 65, 371–389. [Google Scholar] [CrossRef]
- McClure, M.O.; Sommerfelt, M.A.; Marsh, M.; Weiss, R.A. The pH independence of mammalian retrovirus infection. J. Gen. Virol. 1990, 71, 767–773. [Google Scholar] [CrossRef]
- Katen, L.J.; Januszeski, M.M.; Anderson, W.F.; Hasenkrug, K.J.; Evans, L.H. Infectious Entry by Amphotropic as well as Ecotropic Murine Leukemia Viruses Occurs through an Endocytic Pathway. J. Virol. 2001, 75, 5018–5026. [Google Scholar] [CrossRef] [PubMed]
- Kizhatil, K.; Albritton, L.M. Requirements for different components of the host cell cytoskeleton distinguish ecotropic murine leukemia virus entry via endocytosis from entry via surface fusion. J. Virol. 1997, 71, 7145–7156. [Google Scholar] [CrossRef]
- Ross, S.R.; Schofield, J.J.; Farr, C.J.; Bucan, M. Mouse transferrin receptor 1 is the cell entry receptor for mouse mammary tumor virus. Proc. Natl. Acad. Sci. USA 2002, 99, 12386–12390. [Google Scholar] [CrossRef]
- Kumar, P.; Nachagari, D.; Fields, C.; Franks, J.; Albritton, L.M. Host Cell Cathepsins Potentiate Moloney Murine Leukemia Virus Infection. J. Virol. 2007, 81, 10506–10514. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Stavrou, S.; Aguilera, A.N.; Blouch, K.; Ross, S.R. DDX41 Recognizes RNA/DNA Retroviral Reverse Transcripts and Is Critical for In Vivo Control of Murine Leukemia Virus Infection. mBio 2018, 9, e00923-18. [Google Scholar] [CrossRef] [PubMed]
- Blain, S.W.; Goff, S.P. Effects on DNA synthesis and translocation caused by mutations in the RNase H domain of Moloney murine leukemia virus reverse transcriptase. J. Virol. 1995, 69, 4440–4452. [Google Scholar] [CrossRef] [PubMed]
- Lim, D.; Orlova, M.; Goff, S.P. Mutations of the RNase H C Helix of the Moloney Murine Leukemia Virus Reverse Transcriptase Reveal Defects in Polypurine Tract Recognition. J. Virol. 2002, 76, 8360–8373. [Google Scholar] [CrossRef]
- Harel, J.; Rassart, E.; Jolicoeur, P. Cell cycle dependence of synthesis of unintegrated viral DNA in mouse cells newly infected with murine leukemia virus. Virology 1981, 110, 202–207. [Google Scholar] [CrossRef]
- Roe, T.; Reynolds, T.; Yu, G.; Brown, P. Integration of murine leukemia virus DNA depends on mitosis. EMBO J. 1993, 12, 2099–2108. [Google Scholar] [CrossRef]
- Wanaguru, M.; Barry, D.J.; Benton, D.J.; O’Reilly, N.; Bishop, K.N. Murine leukemia virus p12 tethers the capsid-containing pre-integration complex to chromatin by binding directly to host nucleosomes in mitosis. PLOS Pathog. 2018, 14, e1007117. [Google Scholar] [CrossRef]
- Guo, X.; Carroll, J.-W.N.; Macdonald, M.R.; Goff, S.P.; Gao, G. The Zinc Finger Antiviral Protein Directly Binds to Specific Viral mRNAs through the CCCH Zinc Finger Motifs. J. Virol. 2004, 78, 12781–12787. [Google Scholar] [CrossRef]
- Lee, H.; Komano, J.; Saitoh, Y.; Yamaoka, S.; Kozaki, T.; Misawa, T.; Takahama, M.; Satoh, T.; Takeuchi, O.; Yamamoto, N.; et al. Zinc-finger antiviral protein mediates retinoic acid inducible gene I–like receptor-independent antiviral response to murine leukemia virus. Proc. Natl. Acad. Sci. USA 2013, 110, 12379–12384. [Google Scholar] [CrossRef]
- Gao, G.; Guo, X.; Goff, S.P. Inhibition of Retroviral RNA Production by ZAP, a CCCH-Type Zinc Finger Protein. Science 2002, 297, 1703–1706. [Google Scholar] [CrossRef] [PubMed]
- Brubaker, S.W.; Bonham, K.S.; Zanoni, I.; Kagan, J.C. Innate immune pattern recognition: A cell biological perspective. Annu. Rev. Immunol. 2015, 33, 257–290. [Google Scholar] [CrossRef] [PubMed]
- Yarovinsky, F. TLR11 Activation of Dendritic Cells by a Protozoan Profilin-Like Protein. Science 2005, 308, 1626–1629. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Halle, A.; Kurt-Jones, E.A.; Cerny, A.M.; Porpiglia, E.; Rogers, M.; Golenbock, D.T.; Finberg, R.W. Lymphocytic choriomeningitis virus (LCMV) infection of CNS glial cells results in TLR2-MyD88/Mal-dependent inflammatory responses. J. Neuroimmunol. 2008, 194, 70–82. [Google Scholar] [CrossRef]
- Cuevas, C.D.; Ross, S.R. Toll-Like Receptor 2-Mediated Innate Immune Responses against Junín Virus in Mice Lead to Antiviral Adaptive Immune Responses during Systemic Infection and Do Not Affect Viral Replication in the Brain. J. Virol. 2014, 88, 7703–7714. [Google Scholar] [CrossRef]
- Bieback, K.; Lien, E.; Klagge, I.M.; Avota, E.; Schneider-Schaulies, J.; Duprex, W.P.; Wagner, H.; Kirschning, C.J.; Ter Meulen, V.; Schneider-Schaulies, S. Hemagglutinin Protein of Wild-Type Measles Virus Activates Toll-Like Receptor 2 Signaling. J. Virol. 2002, 76, 8729–8736. [Google Scholar] [CrossRef]
- Murawski, M.R.; Bowen, G.N.; Cerny, A.M.; Anderson, L.J.; Haynes, L.M.; Tripp, R.A.; Kurt-Jones, E.A.; Finberg, R.W. Respiratory Syncytial Virus Activates Innate Immunity through Toll-Like Receptor 2. J. Virol. 2008, 83, 1492–1500. [Google Scholar] [CrossRef]
- Aravalli, R.N.; Hu, S.; Rowen, T.N.; Palmquist, J.M.; Lokensgard, J.R. Cutting Edge: TLR2-Mediated Proinflammatory Cytokine and Chemokine Production by Microglial Cells in Response to Herpes Simplex Virus. J. Immunol. 2005, 175, 4189–4193. [Google Scholar] [CrossRef]
- Alexopoulou, L.; Holt, A.C.; Medzhitov, R.; Flavell, R.A. Recognition of double-stranded RNA and activation of NF-kappaB by Toll-like receptor 3. Nature 2001, 413, 732–738. [Google Scholar] [CrossRef]
- Tabeta, K.; Georgel, P.; Janssen, E.; Du, X.; Hoebe, K.; Crozat, K.; Mudd, S.; Shamel, L.; Sovath, S.; Goode, J.; et al. Toll-like receptors 9 and 3 as essential components of innate immune defense against mouse cytomegalovirus infection. Proc. Natl. Acad. Sci. USA 2004, 101, 3516–3521. [Google Scholar] [CrossRef]
- Wang, T.; Town, T.; Alexopoulou, L.; Anderson, J.F.; Fikrig, E.; Flavell, R.A. Toll-like receptor 3 mediates West Nile virus entry into the brain causing lethal encephalitis. Nat. Med. 2004, 10, 1366–1373. [Google Scholar] [CrossRef] [PubMed]
- Le Goffic, R.; Balloy, V.; Lagranderie, M.; Alexopoulou, L.; Escriou, N.; Flavell, R.; Chignard, M.; Si-Tahar, M. Detrimental Contribution of the Toll-Like Receptor (TLR)3 to Influenza A Virus–Induced Acute Pneumonia. PLOS Pathog. 2006, 2, e53. [Google Scholar] [CrossRef] [PubMed]
- Reinert, L.S.; Harder, L.; Holm, C.K.; Iversen, M.B.; Horan, K.A.; Dagnaes-Hansen, F.; Ulhøi, B.P.; Holm, T.H.; Mogensen, T.H.; Owens, T.; et al. TLR3 deficiency renders astrocytes permissive to herpes simplex virus infection and facilitates establishment of CNS infection in mice. J. Clin. Investig. 2012, 122, 1368–1376. [Google Scholar] [CrossRef] [PubMed]
- Diebold, S.S.; Kaisho, T.; Hemmi, H.; Akira, S.; Sousa, C.R. Innate Antiviral Responses by Means of TLR7-Mediated Recognition of Single-Stranded RNA. Science 2004, 303, 1529–1531. [Google Scholar] [CrossRef]
- Lund, J.M.; Alexopoulou, L.; Sato, A.; Karow, M.; Adams, N.C.; Gale, N.W.; Iwasaki, A.; Flavell, R.A. Recognition of single-stranded RNA viruses by Toll-like receptor 7. Proc. Natl. Acad. Sci. USA 2004, 101, 5598–5603. [Google Scholar] [CrossRef]
- Town, T.; Bai, F.; Wang, T.; Kaplan, A.; Qian, F.; Montgomery, R.R.; Anderson, J.F.; Flavell, R.A.; Fikrig, E. Toll-like Receptor 7 Mitigates Lethal West Nile Encephalitis via Interleukin 23-Dependent Immune Cell Infiltration and Homing. Immunity 2009, 30, 242–253. [Google Scholar] [CrossRef]
- Heil, F.; Hemmi, H.; Hochrein, H.; Ampenberger, F.; Kirschning, C.; Akira, S.; Lipford, G.; Wagner, H.; Bauer, S. Species-Specific Recognition of Single-Stranded RNA via Toll-like Receptor 7 and 8. Science 2004, 303, 1526–1529. [Google Scholar] [CrossRef]
- Krug, A.; Luker, G.D.; Barchet, W.; Leib, D.A.; Akira, S.; Colonna, M. Herpes simplex virus type 1 activates murine natural interferon-producing cells through toll-like receptor 9. Blood 2004, 103, 1433–1437. [Google Scholar] [CrossRef]
- Samuelsson, C.; Hausmann, J.; Lauterbach, H.; Schmidt, M.; Akira, S.; Wagner, H.; Chaplin, P.; Suter, M.; O’Keeffe, M.; Hochrein, H. Survival of lethal poxvirus infection in mice depends on TLR9, and therapeutic vaccination provides protection. J. Clin. Investig. 2008, 118, 1776–1784. [Google Scholar] [CrossRef]
- Zhu, J.; Huang, X.; Yang, Y. Innate Immune Response to Adenoviral Vectors Is Mediated by both Toll-Like Receptor-Dependent and -Independent Pathways. J. Virol. 2007, 81, 3170–3180. [Google Scholar] [CrossRef]
- Browne, E.P. Toll-Like Receptor 7 Inhibits Early Acute Retroviral Infection through Rapid Lymphocyte Responses. J. Virol. 2013, 87, 7357–7366. [Google Scholar] [CrossRef] [PubMed]
- Browne, E.P.; Littman, D.R. Myd88 Is Required for an Antibody Response to Retroviral Infection. PLoS Pathog. 2009, 5, e1000298. [Google Scholar] [CrossRef] [PubMed]
- Browne, E.P. Toll-like Receptor 7 Controls the Anti-Retroviral Germinal Center Response. PLoS Pathog. 2011, 7, e1002293. [Google Scholar] [CrossRef]
- Kane, M.; Case, L.K.; Wang, C.; Yurkovetskiy, L.A.; Dikiy, S.; Golovkina, T.V. Innate Immune Sensing of Retroviral Infection via Toll-like Receptor 7 Occurs upon Viral Entry. Immunity 2011, 35, 135–145. [Google Scholar] [CrossRef] [PubMed]
- Pi, R.; Iwasaki, A.; Sewald, X.; Mothes, W.; Uchil, P.D. Murine Leukemia Virus Exploits Innate Sensing by Toll-Like Receptor 7 in B-1 Cells To Establish Infection and Locally Spread in Mice. J. Virol. 2019, 93, 21. [Google Scholar] [CrossRef]
- Gibbert, K.; Francois, S.; Sigmund, A.M.; Harper, M.S.; Barrett, B.S.; Kirschning, C.J.; Lu, M.; Santiago, M.L.; Dittmer, U. Friend retrovirus drives cytotoxic effectors through Toll-like receptor 3. Retrovirology 2014, 11, 126. [Google Scholar] [CrossRef]
- Gibbert, K.; Dietze, K.K.; Zelinskyy, G.; Lang, K.S.; Barchet, W.; Kirschning, C.J.; Dittmer, U. Polyinosinic-Polycytidylic Acid Treatment of Friend Retrovirus-Infected Mice Improves Functional Properties of Virus-Specific T Cells and Prevents Virus-Induced Disease. J. Immunol. 2010, 185, 6179–6189. [Google Scholar] [CrossRef]
- Rigby, R.E.; Webb, L.M.; MacKenzie, K.J.; Li, Y.; Leitch, A.; Reijns, M.; Lundie, R.J.; Revuelta, A.; Davidson, N.J.; Diebold, S.; et al. RNA:DNA hybrids are a novel molecular pattern sensed by TLR9. EMBO J. 2014, 33, 542–558. [Google Scholar] [CrossRef]
- Beignon, A.-S.; McKenna, K.; Škoberne, M.; Manches, O.; DaSilva, I.; Kavanagh, D.G.; Larsson, M.; Gorelick, R.J.; Lifson, J.D.; Bhardwaj, N. Endocytosis of HIV-1 activates plasmacytoid dendritic cells via Toll-like receptor– viral RNA interactions. J. Clin. Investig. 2005, 115, 3265–3275. [Google Scholar] [CrossRef]
- Young, G.; Eksmond, U.; Salcedo, R.; Alexopoulou, L.; Stoye, J.P.; Kassiotis, G. Resurrection of endogenous retroviruses in antibody-deficient mice. Nature 2012, 491, 774–778. [Google Scholar] [CrossRef]
- Yu, P.; Lübben, W.; Slomka, H.; Gebler, J.; Konert, M.; Cai, C.; Neubrandt, L.; Da Costa, O.P.; Paul, S.; Dehnert, S.; et al. Nucleic Acid-Sensing Toll-like Receptors Are Essential for the Control of Endogenous Retrovirus Viremia and ERV-Induced Tumors. Immunity 2012, 37, 867–879. [Google Scholar] [CrossRef] [PubMed]
- Kane, M.; Case, L.K.; Kopaskie, K.; Kozlova, A.; MacDearmid, C.; Chervonsky, A.V.; Golovkina, T.V. Successful Transmission of a Retrovirus Depends on the Commensal Microbiota. Science 2011, 334, 245–249. [Google Scholar] [CrossRef] [PubMed]
- Wilks, J.; Lien, E.; Jacobson, A.N.; Fischbach, M.A.; Qureshi, N.; Chervonsky, A.; Golovkina, T. Mammalian Lipopolysaccharide Receptors Incorporated into the Retroviral Envelope Augment Virus Transmission. Cell Host Microbe 2015, 18, 456–462. [Google Scholar] [CrossRef] [PubMed]
- Rassa, J.C.; Meyers, J.L.; Zhang, Y.; Kudaravalli, R.; Ross, S.R. Murine retroviruses activate B cells via interaction with toll-like receptor 4. Proc. Natl. Acad. Sci. USA 2002, 99, 2281–2286. [Google Scholar] [CrossRef] [PubMed]
- Lau, C.M.; Broughton, C.; Tabor, A.S.; Akira, S.; Flavell, R.A.; Mamula, M.J.; Christensen, S.R.; Shlomchik, M.J.; Viglianti, G.A.; Rifkin, I.R.; et al. RNA-associated autoantigens activate B cells by combined B cell antigen receptor/Toll-like receptor 7 engagement. J. Exp. Med. 2005, 202, 1171–1177. [Google Scholar] [CrossRef]
- Savarese, E.; Chae, O.-W.; Trowitzsch, S.; Weber, G.; Kastner, B.; Akira, S.; Wagner, H.; Schmid, R.M.; Bauer, S.; Krug, A. U1 small nuclear ribonucleoprotein immune complexes induce type I interferon in plasmacytoid dendritic cells through TLR7. Blood 2006, 107, 3229–3234. [Google Scholar] [CrossRef]
- Brunette, R.L.; Young, J.M.; Whitley, D.G.; Brodsky, I.E.; Malik, H.S.; Stetson, D.B. Extensive evolutionary and functional diversity among mammalian AIM2-like receptors. J. Exp. Med. 2012, 209, 1969–1983. [Google Scholar] [CrossRef]
- Sun, L.; Wu, J.; Du, F.; Chen, X.; Chen, Z.J. Cyclic GMP-AMP Synthase Is a Cytosolic DNA Sensor That Activates the Type I Interferon Pathway. Science 2012, 339, 786–791. [Google Scholar] [CrossRef]
- Tan, X.; Sun, L.; Chen, J.; Chen, Z.J. Detection of Microbial Infections Through Innate Immune Sensing of Nucleic Acids. Annu. Rev. Microbiol. 2018, 72, 447–478. [Google Scholar] [CrossRef]
- Nakaya, Y.; Lilue, J.; Stavrou, S.; Moran, E.A.; Ross, S.R. AIM2-Like Receptors Positively and Negatively Regulate the Interferon Response Induced by Cytosolic DNA. mBio 2017, 8, e00944-17. [Google Scholar] [CrossRef]
- Cridland, J.A.; Curley, E.Z.; Wykes, M.; Schroder, K.; Sweet, M.J.; Roberts, T.L.; Ragan, M.A.; Kassahn, K.; Stacey, K.J. The mammalian PYHIN gene family: Phylogeny, evolution and expression. BMC Evol. Boil. 2012, 12, 140. [Google Scholar] [CrossRef] [PubMed]
- Stavrou, S.; Blouch, K.; Kotla, S.; Bass, A.; Ross, S.R. Nucleic acid recognition orchestrates the anti-viral response to retroviruses. Cell Host Microbe 2015, 17, 478–488. [Google Scholar] [CrossRef] [PubMed]
- Unterholzner, L.; Keating, S.E.; Baran, M.; Horan, K.A.; Jensen, S.B.; Sharma, S.; Sirois, C.M.; Jin, T.; Latz, E.; Xiao, T.S.; et al. IFI16 is an innate immune sensor for intracellular DNA. Nat. Immunol. 2010, 11, 997–1004. [Google Scholar] [CrossRef] [PubMed]
- Barber, G.N. STING: Infection, inflammation and cancer. Nat. Rev. Immunol. 2015, 15, 760–770. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Lieberman, J. A Mechanistic Understanding of Pyroptosis: The Fiery Death Triggered by Invasive Infection. Adv. Immunol. 2017, 135, 81–117. [Google Scholar] [CrossRef]
- Monroe, K.M.; Yang, Z.; Johnson, J.R.; Geng, X.; Doitsh, G.; Krogan, N.J.; Greene, W.C. IFI16 DNA sensor is required for death of lymphoid CD4 T cells abortively infected with HIV. Science 2014, 343, 428–432. [Google Scholar] [CrossRef]
- Storek, K.M.; Gertsvolf, N.A.; Ohlson, M.B.; Monack, D.M. cGAS and Ifi204 Cooperate to Produce Type I IFNs in Response to Francisella Infection. J. Immunol. 2015, 194, 3236–3245. [Google Scholar] [CrossRef]
- Li, X.-D.; Wu, J.; Gao, D.; Wang, H.; Sun, L.; Chen, Z.J. Pivotal Roles of cGAS-cGAMP Signaling in Antiviral Defense and Immune Adjuvant Effects. Science 2013, 341, 1390–1394. [Google Scholar] [CrossRef]
- Chunfa, L.; Xin, S.; Qiang, L.; Sreevatsan, S.; Yang, L.; Zhao, D.; Zhou, X. The Central Role of IFI204 in IFN-beta Release and Autophagy Activation during Mycobacterium bovis Infection. Front. Cell Infect. Microbiol. 2017, 7, 169. [Google Scholar] [CrossRef]
- Aguirre, S.; Luthra, P.; Sánchez-Aparicio, M.T.; Maestre, A.M.; Patel, J.R.; Lamothe, F.; Fredericks, A.C.; Tripathi, S.; Zhu, T.; Pintado-Silva, J.; et al. Dengue virus NS2B protein targets cGAS for degradation and prevents mitochondrial DNA sensing during infection. Nat. Microbiol. 2017, 2, 17037. [Google Scholar] [CrossRef]
- Yan, N.; Regalado-Magdos, A.D.; Stiggelbout, B.; Lee-Kirsch, M.A.; Lieberman, J. The cytosolic exonuclease TREX1 inhibits the innate immune response to human immunodeficiency virus type 1. Nat. Immunol. 2010, 11, 1005–1013. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Morrison, J.H.; Dingli, D.; Poeschla, E.M. HIV-1 Activation of Innate Immunity Depends Strongly on the Intracellular Level of TREX1 and Sensing of Incomplete Reverse Transcription Products. J. Virol. 2018, 92, e00001-18. [Google Scholar] [CrossRef] [PubMed]
- Gao, D.; Wu, J.; Wu, Y.-T.; Du, F.; Aroh, C.; Yan, N.; Sun, L.; Chen, Z.J. Cyclic GMP-AMP Synthase Is an Innate Immune Sensor of HIV and Other Retroviruses. Science 2013, 341, 903–906. [Google Scholar] [CrossRef] [PubMed]
- Merkl, P.E.; Knipe, D.M.; Damania, B.; Lieberman, P. Role for a Filamentous Nuclear Assembly of IFI16, DNA, and Host Factors in Restriction of Herpesviral Infection. mBio 2019, 10, e02621-18. [Google Scholar] [CrossRef] [PubMed]
- Hotter, D.; Bosso, M.; Jønsson, K.L.; Krapp, C.; Stürzel, C.M.; Das, A.; Littwitz-Salomon, E.; Berkhout, B.; Russ, A.; Wittmann, S.; et al. IFI16 Targets the Transcription Factor Sp1 to Suppress HIV-1 Transcription and Latency Reactivation. Cell Host Microbe 2019, 25, 858–872.e13. [Google Scholar] [CrossRef] [PubMed]
- Gray, E.E.; Winship, D.; Snyder, J.M.; Child, S.J.; Geballe, A.P.; Stetson, D.B. The AIM2-like Receptors Are Dispensable for the Interferon Response to Intracellular DNA. Immunity 2016, 45, 255–266. [Google Scholar] [CrossRef]
- Gao, D.; Li, T.; Li, X.-D.; Chen, X.; Li, Q.-Z.; Wight-Carter, M.; Chen, Z.J. Activation of cyclic GMP-AMP synthase by self-DNA causes autoimmune diseases. Proc. Natl. Acad. Sci. USA 2015, 112, E5699–E5705. [Google Scholar] [CrossRef]
- Morita, M.; Stamp, G.; Robins, P.; Dulic, A.; Rosewell, I.; Hrivnak, G.; Daly, G.; Lindahl, T.; Barnes, D.E. Gene-Targeted Mice Lacking the Trex1 (DNase III) 3′-5′ DNA Exonuclease Develop Inflammatory Myocarditis. Mol. Cell. Boil. 2004, 24, 6719–6727. [Google Scholar] [CrossRef]
- Crow, Y.; Hayward, B.E.; Parmar, R.; Robins, P.; Leitch, A.; Ali, M.; Black, D.N.; van Bokhoven, H.; Brunner, H.G.; Hamel, B.C.; et al. Mutations in the gene encoding the 3′-5′ DNA exonuclease TREX1 cause Aicardi-Goutières syndrome at the AGS1 locus. Nat. Genet. 2006, 38, 917–920. [Google Scholar] [CrossRef]
- Ghosh, S.; Wallerath, C.; Covarrubias, S.; Hornung, V.; Carpenter, S.B.; Fitzgerald, K.A.; Wallareth, C. The PYHIN Protein p205 Regulates the Inflammasome by Controlling Asc Expression. J. Immunol. 2017, 199, 3249–3260. [Google Scholar] [CrossRef]
- Choubey, D.; Panchanathan, R. Interferon-inducible Ifi200-family genes in systemic lupus erythematosus. Immunol. Lett. 2008, 119, 32–41. [Google Scholar] [CrossRef] [PubMed]
- Conticello, S.; Thomas, C.J.F.; Petersen-Mahrt, S.K.; Neuberger, M.S. Evolution of the AID/APOBEC Family of Polynucleotide (Deoxy)cytidine Deaminases. Mol. Boil. Evol. 2004, 22, 367–377. [Google Scholar] [CrossRef] [PubMed]
- Harris, R.; Dudley, J.P. APOBECs and virus restriction. Virology 2015, 479, 131–145. [Google Scholar] [CrossRef]
- Pollpeter, D.; Parsons, M.; Sobala, A.E.; Coxhead, S.; Lang, R.D.; Bruns, A.M.; Papaioannou, S.; McDonnell, J.M.; Apolonia, L.; Chowdhury, J.A.; et al. Deep sequencing of HIV-1 reverse transcripts reveals the multifaceted antiviral functions of APOBEC3G. Nat. Microbiol. 2017, 3, 220–233. [Google Scholar] [CrossRef] [PubMed]
- Macmillan, A.L.; Kohli, R.M.; Ross, S.R. APOBEC3 Inhibition of Mouse Mammary Tumor Virus Infection: The Role of Cytidine Deamination versus Inhibition of Reverse Transcription. J. Virol. 2013, 87, 4808–4817. [Google Scholar] [CrossRef] [PubMed]
- Stavrou, S.; Nitta, T.; Kotla, S.; Ha, D.; Nagashima, K.; Rein, A.R.; Fan, H.; Ross, S.R. Murine leukemia virus glycosylated Gag blocks apolipoprotein B editing complex 3 and cytosolic sensor access to the reverse transcription complex. Proc. Natl. Acad. Sci. USA 2013, 110, 9078–9083. [Google Scholar] [CrossRef]
- Guo, X.; Ma, J.; Sun, J.; Gao, G. The zinc-finger antiviral protein recruits the RNA processing exosome to degrade the target mRNA. Proc. Natl. Acad. Sci. USA 2006, 104, 151–156. [Google Scholar] [CrossRef]
- Law, L.M.J.; Razooky, B.S.; Li, M.M.H.; You, S.; Jurado, A.; Rice, C.M.; Macdonald, M.R. ZAP’s stress granule localization is correlated with its antiviral activity and induced by virus replication. PLOS Pathog. 2019, 15, e1007798. [Google Scholar] [CrossRef]
- Gerpe, M.C.R.; Renner, T.M.; Bélanger, K.; Lam, C.; Aydin, H.; Langlois, M.-A. N-Linked Glycosylation Protects Gammaretroviruses against Deamination by APOBEC3 Proteins. J. Virol. 2014, 89, 2342–2357. [Google Scholar] [CrossRef][Green Version]
- Hsu, H.W.; Schwartzberg, P.; Goff, S.P. Point mutations in the P30 domain of the gag gene of Moloney murine leukemia virus. Virology 1985, 142, 211–214. [Google Scholar] [CrossRef]
- Hagen, B.; Kraase, M.; Indikova, I.; Indik, S. A high rate of polymerization during synthesis of mouse mammary tumor virus DNA alleviates hypermutation by APOBEC3 proteins. PLoS Pathog. 2019, 15, e1007533. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Akkawi, C.; Mougel, M.; Ross, S.R. Murine leukemia virus P50 protein counteracts APOBEC3 by blocking its packaging. J. Virol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, E.E.; Shioda, T. Role of Human TRIM5α in Intrinsic Immunity. Front. Microbiol. 2012, 3, 97. [Google Scholar] [CrossRef] [PubMed]
- Sanz-Ramos, M.; Stoye, J.P. Capsid-binding retrovirus restriction factors: Discovery, restriction specificity and implications for the development of novel therapeutics. J. Gen. Virol. 2013, 94, 2587–2598. [Google Scholar] [CrossRef]
- Pertel, T.; Hausmann, S.; Morger, D.; Züger, S.; Guerra, J.; Lascano, J.; Reinhard, C.; Santoni, F.A.; Uchil, P.D.; Chatel, L.; et al. TRIM5 is an innate immune sensor for the retrovirus capsid lattice. Nature 2011, 472, 361–365. [Google Scholar] [CrossRef]
- Fletcher, A.J.; Vaysburd, M.; Maslen, S.; Zeng, J.; Skehel, J.M.; Towers, G.J.; James, L.C. Trivalent RING Assembly on Retroviral Capsids Activates TRIM5 Ubiquitination and Innate Immune Signaling. Cell Host Microbe 2018, 24, 761–775.e6. [Google Scholar] [CrossRef]
- Yap, M.W.; Nisole, S.; Lynch, C.; Stoye, J.P. Trim5 protein restricts both HIV-1 and murine leukemia virus. Proc. Natl. Acad. Sci. USA 2004, 101, 10786–10791. [Google Scholar] [CrossRef]
- Kutluay, S.B.; Pérez-Caballero, D.; Bieniasz, P.D. Fates of Retroviral Core Components during Unrestricted and TRIM5-Restricted Infection. PLOS Pathog. 2013, 9, e1003214. [Google Scholar] [CrossRef]
- Tareen, S.U.; Sawyer, S.L.; Malik, H.S.; Emerman, M. An expanded clade of rodent Trim5 genes. Virology 2009, 385, 473–483. [Google Scholar] [CrossRef]
- Chang, T.H.; Yoshimi, R.; Ozato, K. Tripartite Motif (TRIM) 12c, a Mouse Homolog of TRIM5, Is a Ubiquitin Ligase That Stimulates Type I IFN and NF-kappaB Pathways along with TNFR-Associated Factor 6. J. Immunol. 2015, 195, 5367–5379. [Google Scholar] [CrossRef]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moran, E.A.; Ross, S.R. Insights into Sensing of Murine Retroviruses. Viruses 2020, 12, 836. https://doi.org/10.3390/v12080836
Moran EA, Ross SR. Insights into Sensing of Murine Retroviruses. Viruses. 2020; 12(8):836. https://doi.org/10.3390/v12080836
Chicago/Turabian StyleMoran, Eileen A., and Susan R. Ross. 2020. "Insights into Sensing of Murine Retroviruses" Viruses 12, no. 8: 836. https://doi.org/10.3390/v12080836
APA StyleMoran, E. A., & Ross, S. R. (2020). Insights into Sensing of Murine Retroviruses. Viruses, 12(8), 836. https://doi.org/10.3390/v12080836




