Cocirculation of Swine H1N1 Influenza A Virus Lineages in Germany
Abstract
1. Introduction
- A H1pdmN1pdm variant which emerged in 2014 and lacks cross-reactivity with EA swH1N1 and H1pdmN1pdm, and
- A novel swH1N1 reassortant with the HA gene of the new prevalent Diepholz/2008-like swH1N2 lineage.
2. Materials and Methods
2.1. Study Design
2.2. Cell Lines, Virus Isolation and Virus Amplification
2.3. Nucleic Acid Extraction and PCR Analysis
2.4. Sequence Analysis
2.5. Phylogenetic Analyses
2.6. Nomenclature of S-IAV Lineages and Clades
2.7. Antigenic Analysis
3. Results
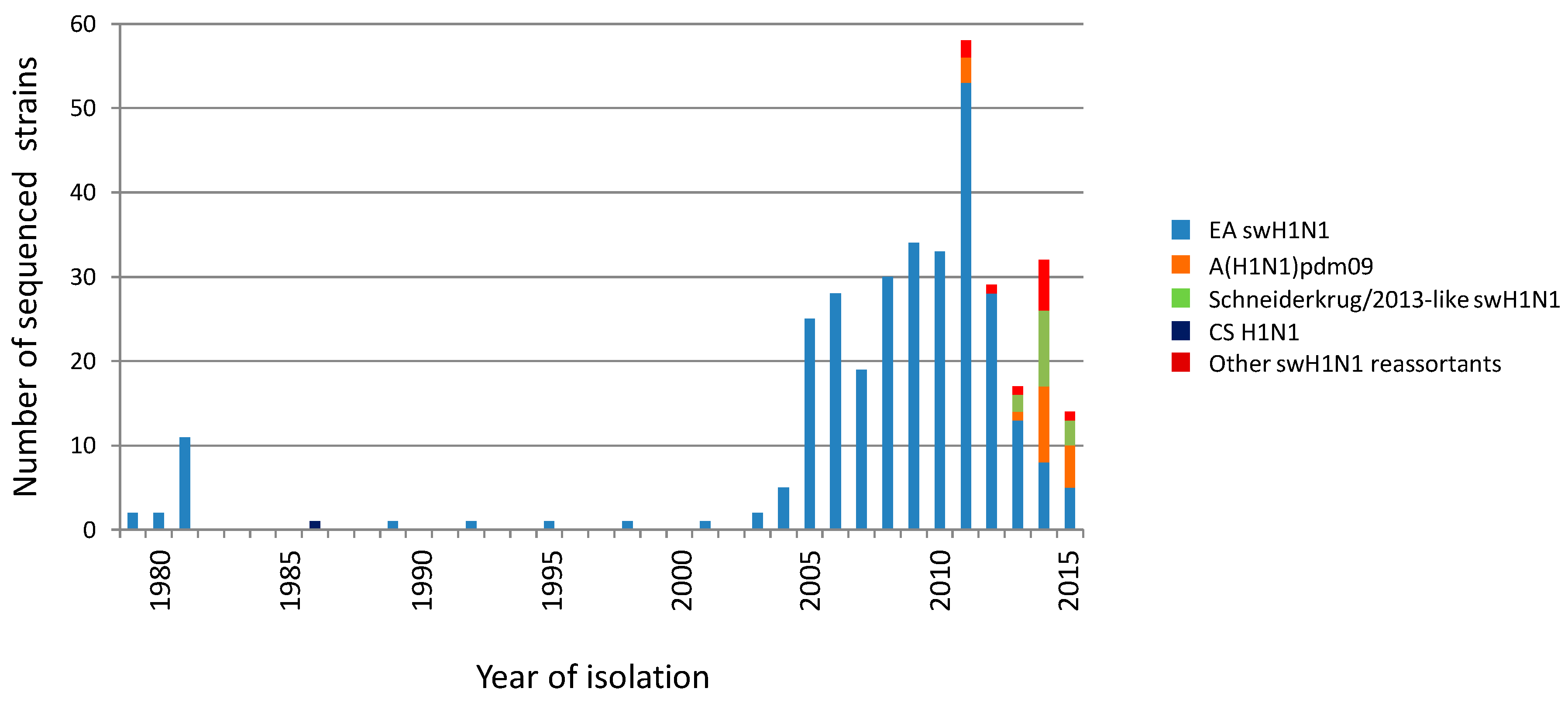
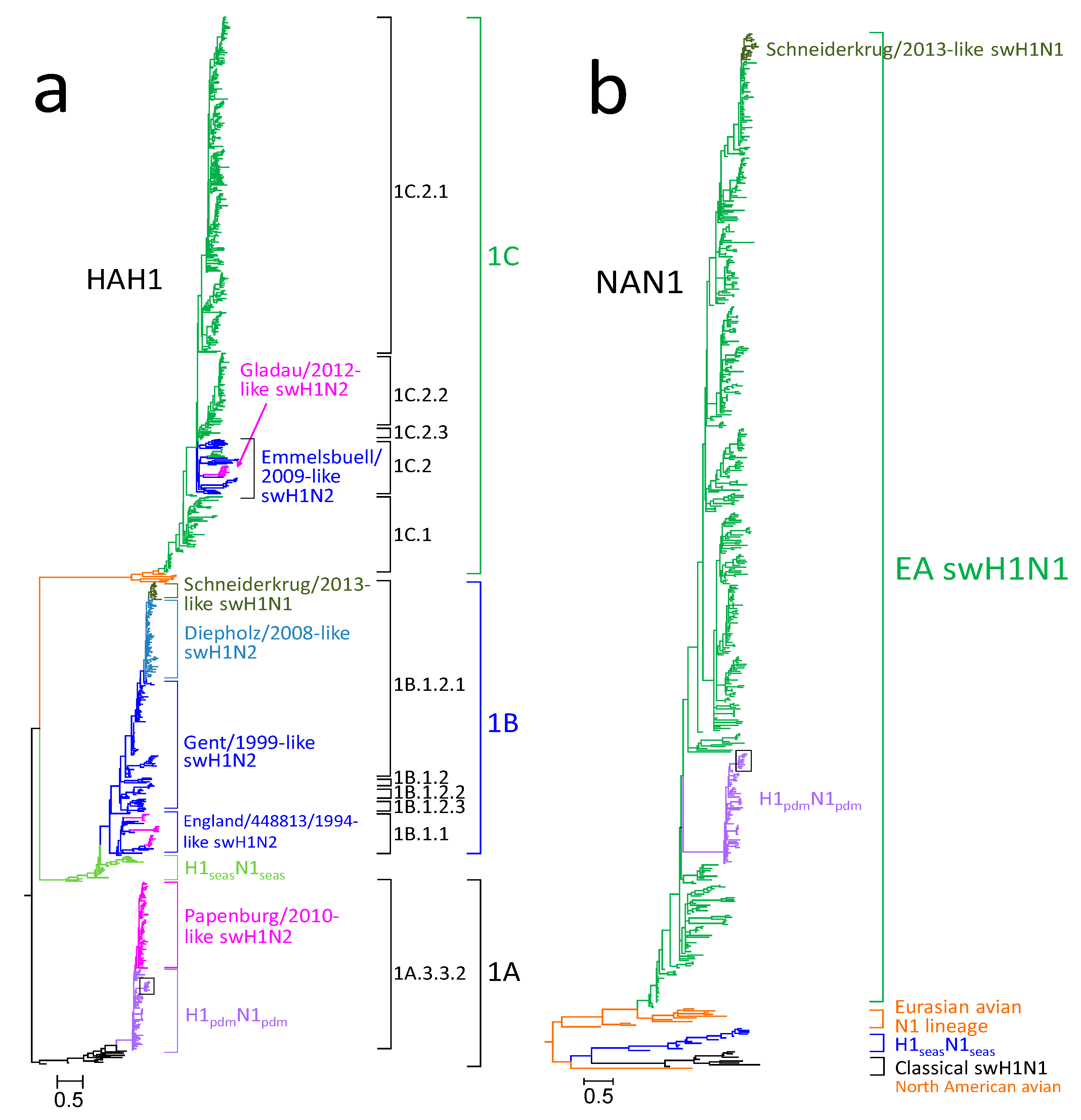
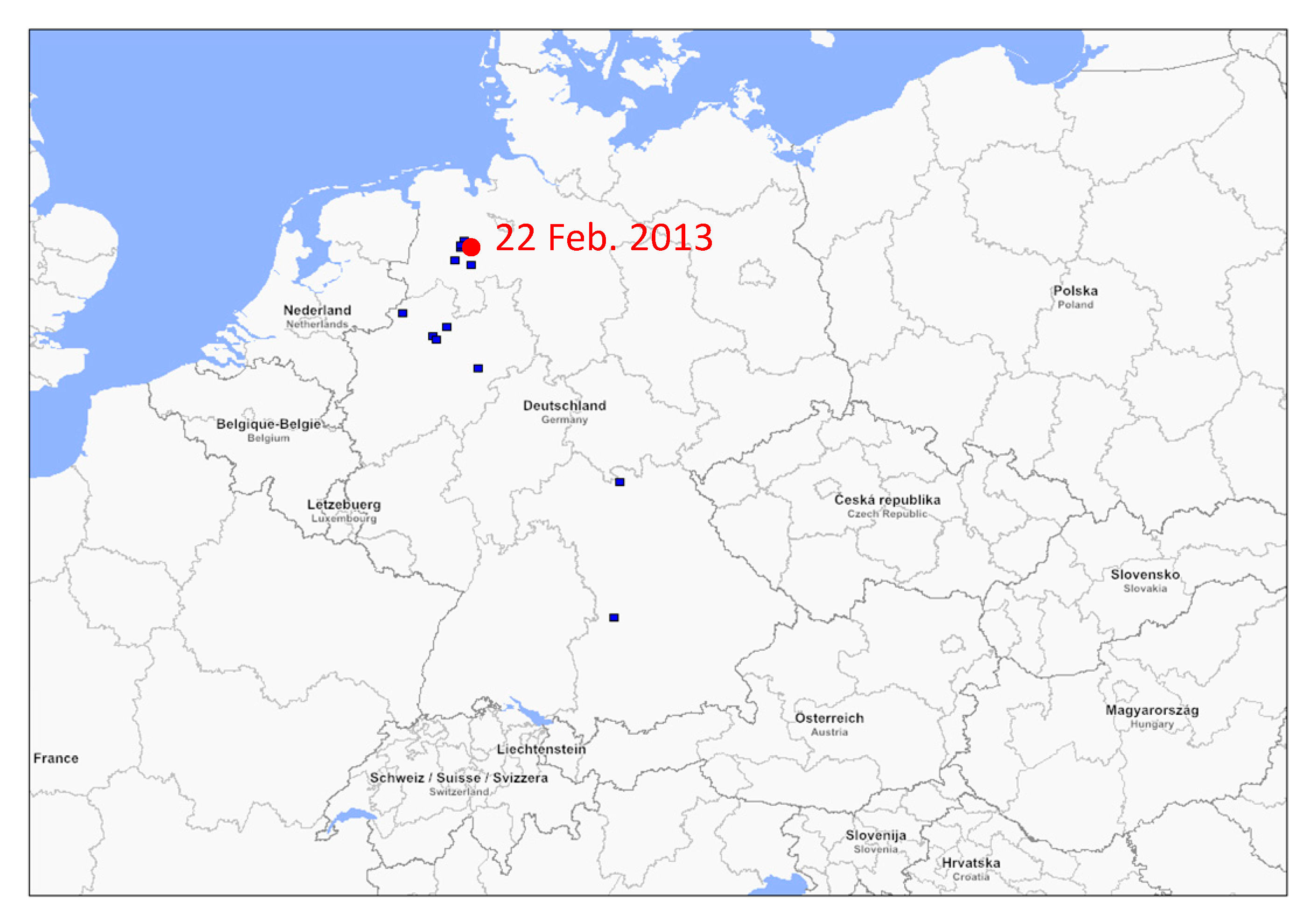
3.1. Phylogenetic Analysis of German swH1N1 Isolates
3.2. Analysis of Antigenic Sites of swH1N1
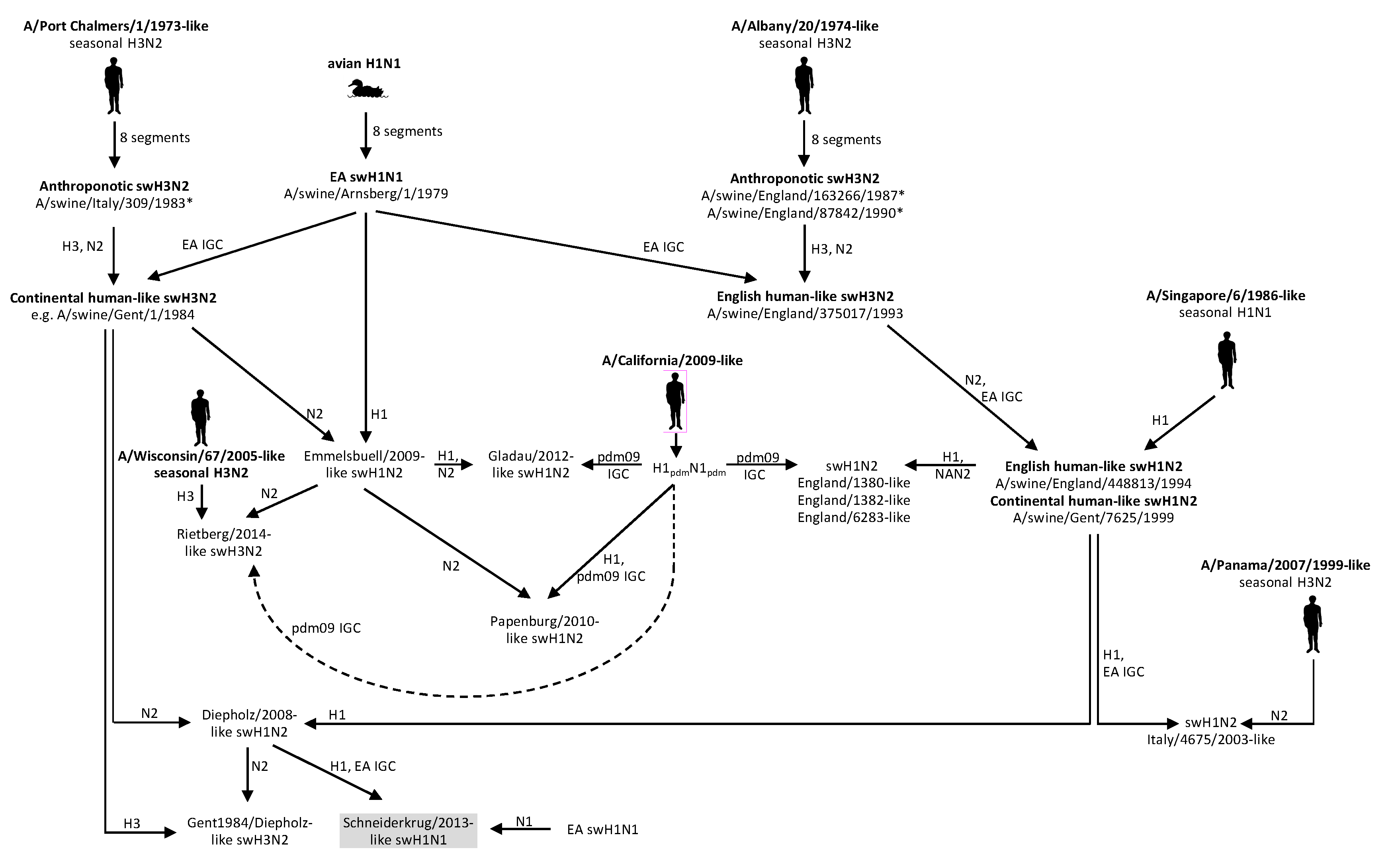
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mena, I.; Nelson, M.I.; Quezada-Monroy, F.; Dutta, J.; Cortes-Fernandez, R.; Lara-Puente, J.H.; Castro-Peralta, F.; Cunha, L.F.; Trovao, N.S.; Lozano-Dubernard, B.; et al. Origins of the 2009 H1N1 influenza pandemic in swine in Mexico. eLife 2016, 5, e16777. [Google Scholar] [CrossRef]
- Pensaert, M.; Ottis, K.; Vandeputte, J.; Kaplan, M.M.; Bachmann, P.A. Evidence for the natural transmission of influenza A virus from wild ducks to swine and its potential importance for man. Bull. WHO 1981, 59, 75–78. [Google Scholar]
- Witte, K.K.; Nienhoff, H.; Ernst, H.; Schmidt, U.; Prager, D. Erstmaliges Auftreten einer durch das Schweineinfluenzavirus verursachten Epizootie in Schweinebeständen der Bundesrepublik Deutschland. Tierärztliche Umsch. 1981, 36, 591–606, [In German]. [Google Scholar]
- Zell, R.; Scholtissek, C.; Ludwig, S. Genetics, evolution, and the zoonotic capacity of European swine influenza viruses. Curr. Top. Microbiol. Immunol. 2013, 370, 29–55. [Google Scholar] [PubMed]
- Choi, Y.K.; Pascua, P.N.Q.; Song, M.S. Swine influenza viruses: An Asian perspective. Curr. Top. Microbiol. Immunol. 2013, 370, 147–172. [Google Scholar] [PubMed]
- Krumbholz, A.; Lange, J.; Sauerbrei, A.; Groth, M.; Platzer, M.; Kanrai, P.; Pleschka, S.; Scholtissek, C.; Büttner, M.; Dürrwald, R.; et al. The origin of the European avian-like swine influenza viruses. J. Gen. Virol. 2014, 95, 2372–2376. [Google Scholar] [CrossRef] [PubMed]
- Watson, S.; Langat, P.; Reid, S.M.; Lam, T.T.Y.; Cotton, M.; Kelly, M.; Van Reeth, K.; Qiu, Y.; Simon, G.; Bonin, E.; et al. Molecular epidemiology and evolution of influenza viruses circulating within European swine between 2009 and 2013. J. Virol. 2015, 89, 9920–9931. [Google Scholar] [CrossRef]
- Zell, R.; Groth, M.; Krumbholz, A.; Lange, J.; Philipps, A.; Dürrwald, R. Displacement of the Gent/1999 human-like swine H1N2 influenza A virus lineage by novel reassortant H1N2 clades in Gemany. Arch. Virol. 2020, 165, 55–67. [Google Scholar] [CrossRef] [PubMed]
- Moreno, A.; Chiapponi, C.; Boniotti, M.B.; Sozzi, E.; Foni, E.; Barbieri, I.; Zanoni, M.G.; Faccini, S.; Lelli, D.; Cordioli, P. Genomic characterization of H1N2 swine influenza viruses in Italy. Vet. Microbiol. 2012, 156, 265–276. [Google Scholar] [CrossRef]
- Hofshagen, M.; Gjerset, B.; Er, C.; Tarpai, A.; Brun, E.; Dannevig, B.; Bruheim, T.; Fostad, I.G.; Iversen, B.; Hungnes, O.; et al. Pandemic influenza A(H1N1)v: Human to pig transmission in Norway? Eurosurveillance 2009, 14, 19406. [Google Scholar] [CrossRef][Green Version]
- Welsh, M.D.; Baird, P.M.; Guelbenzu-Gonzalo, M.P.; Hanna, A.; Reid, S.M.; Essen, S.; Russell, C.; Thomas, S.; Barrass, L.; McNeilly, F.; et al. Initial incursion of pandemic (H1N1) 2009 influenza A virus into European pigs. Vet. Rec. 2010, 166, 642–645. [Google Scholar] [CrossRef]
- Moreno, A.; Di Trani, L.; Alborali, L.; Vaccari, G.; Barbieri, I.; Falcone, E.; Sozzi, E.; Puzelli, S.; Ferri, G.; Cordioli, P. First pandemic H1N1 outbreak from a pig farm in Italy. Open Virol. J. 2010, 4, 52–56. [Google Scholar] [CrossRef]
- Lange, J.; Groth, M.; Schlegel, M.; Krumbholz, A.; Wieczorek, K.; Ulrich, R.; Köppen, S.; Schulz, K.; Appl, D.; Selbitz, H.J.; et al. Reassortment of the pandemic (H1N1) 2009 virus and establishment of a novel porcine H1N2 influenza virus lineage in Germany. Vet. Microbiol. 2013, 167, 345–356. [Google Scholar] [CrossRef] [PubMed]
- Sokolov, I.; Kurskaya, O.; Leonov, S.; Kabilov, M.; Alikina, T.; Alekseev, A.; Yushkov, Y.; Saito, T.; Uchida, Y.; Mine, J.; et al. Novel reassortant H1N1 swine influenza virus detected in pig population in Russia. Emerg. Microbes Inf. 2019, 8, 1. [Google Scholar]
- Dürrwald, R.; Krumbholz, A.; Baumgarte, S.; Schlegel, M.; Vahlenkamp, T.W.; Selbitz, H.J.; Wutzler, P.; Zell, R. Swine influenza A vaccines, pandemic (H1N1) 2009 virus, and cross-reactivity. Emerg. Infect. Dis. 2010, 16, 1029–1030. [Google Scholar] [CrossRef] [PubMed]
- Harder, T.C.; Grosse Beilage, E.; Lange, E.; Meiners, C.; Döhring, S.; Pesch, S.; Noe, T.; Grund, C.; Beer, M.; Starick, E. Expanded cocirculation of stable subtypes, emerging lineages, and new sporadic reassortants of porcine influenza viruses in swine populations in Northwest Germany. J. Virol. 2013, 87, 10460–10476. [Google Scholar] [CrossRef] [PubMed]
- Simon, G.; Larsen, L.E.; Dürrwald, R.; Foni, E.; Harder, T.; Van Reeth, K.; Markowska-Daniel, I.; Reid, S.M.; Dan, A.; Maldonado, J.; et al. European suveillance network for influenza in pigs: Surveillance programs, diagnostic tools and swine influenza virus subtypes identified in 14 European countries from 2010 to 2013. PLoS ONE 2014, 9, e115815. [Google Scholar] [CrossRef]
- Lange, J.; Groth, M.; Kanrai, P.; Pleschka, S.; Scholtissek, C.; Dürrwald, R.; Platze, M.; Sauerbrei, A.; Zell, R. Circulation of classical swine influenza virus in Europe between the wars? Arch. Virol. 2014, 159, 1467–1473. [Google Scholar] [CrossRef]
- Fouchier, R.A.; Bestebroer, T.M.; Herfst, S.; Van Der Kemp, L.; Rimmelzwaan, G.F.; Osterhaus, A.D. Detection of influenza A viruses from different species by PCR amplification of conserved sequences in the matrix gene. J. Clin. Microbiol. 2000, 38, 4096–4101. [Google Scholar] [CrossRef]
- Zell, R.; Bergmann, S.; Krumbholz, A.; Wutzler, P.; Dürrwald, R. Ongoing evolution of swine influenza viruses: A novel reassortant. Arch. Virol. 2008, 153, 2085–2092. [Google Scholar] [CrossRef]
- Hoffmann, E.; Stech, J.; Guan, Y.; Webster, R.G.; Perez, D.R. Universal primer set for the full-length amplification of all influenza A viruses. Arch. Virol. 2001, 146, 2275–2289. [Google Scholar] [CrossRef] [PubMed]
- Stech, J.; Stech, O.; Herwig, A.; Altmeppen, H.; Hundt, J.; Gohrbandt, S.; Kreibich, A.; Weber, S.; Klenk, H.D.; Mettenleiter, T.C. Rapid and reliable universal cloning of influenza A virus genes by target-primed plasmid amplification. Nucl. Acids Res. 2008, 36, e139. [Google Scholar] [CrossRef]
- Groth, M.; Lange, J.; Kanrai, P.; Pleschka, S.; Scholtissek, C.; Krumbholz, A.; Platzer, M.; Sauerbrei, A.; Zell, R. The genome of an influenza virus from a pilot whale: Relation to influenza viruses of gulls and marine mammals. Inf. Genet. Evol. 2014, 24, 183–186. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Peterson, D.; Peterson, N. MEGA5: Molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol. Biol. Evol. 2011, 28, 2731–2739. [Google Scholar] [CrossRef] [PubMed]
- Ronquist, F.; Huelsenbeck, J.P. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 2003, 19, 1572–1574. [Google Scholar] [CrossRef] [PubMed]
- Drummond, A.J.; Suchard, M.A.; Xie, D.; Rambaut, A. Bayesian phylogenetics with BEAUTi and the BEAST 1.7. Mol. Biol. Evol. 2012, 29, 1969–1973. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Anderson, T.K.; Macken, C.A.; Lewis, N.S.; Scheuermann, R.H.; Van Reeth, K.; Brown, I.H.; Swenson, S.L.; Simon, G.; Saito, T.; Berhane, Y.; et al. A phylogeny-based global nomenclature system and automated annotation tool for H1 hemagglutinin genes from swine influenza A viruses. mSphere 2016, 1, e00275-16. [Google Scholar] [CrossRef]
- Rose, N.; Herve, S.; Eveno, E.; Barbier, N.; Eono, F.; Dorenlor, V.; Andraud, M.; Camsusou, C.; Madec, F.; Simon, G. Dynamics of influenza A virus infections in permanently infected pig farms: Evidence of recurrent infections, circulation of several swine influenza viruses and reassortment events. Vet. Res. 2013, 44, 72. [Google Scholar] [CrossRef]
- Ryt-Hansen, P.; Larsen, I.; Kristensen, C.S.; Krog, J.S.; Wachek, S.; Larsen, L.E. Longitudinal field studies reveal early infection and persistence of influenza A virus in piglets despite the presence of maternally derived antibodies. Vet. Res. 2019, 50, 36. [Google Scholar] [CrossRef]
- Caton, A.J.; Brownlee, G.G. The antigenic structure of the influenza virus A/PR/8/34 hemagglutinin (H1 subtype). Cell 1982, 31, 417–427. [Google Scholar] [CrossRef]
- Brownlee, G.G.; Fodor, E. The predicted antigenicity of the haemagglutinin of the 1918 Spanish influenza pandemic suggests an avian origin. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2001, 356, 1871–1876. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Owino, S.O.; Crevar, C.J.; Carter, D.M.; Ross, T.M. N-linked glycans and K147 residue on hemagglutinin synergize to elicit broadly reactive H1N1 influenza virus antibodies. J. Virol. 2020, 94, e01432-19. [Google Scholar] [CrossRef]
- Linster, M.; van Boheemen, S.; de Graaf, M.; Schrauwen, E.J.A.; Lexmond, P.; Mänz, B.; Bestebroer, T.M.; Baumann, J.; van Riel, D.; Rimmelzwaan, G.F.; et al. Identification, characterization, and natural selection of mutations driving airborne transmission of A/H5N1 virus. Cell 2014, 157, 329–339. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Lee, I.; Kim, J.I.; Bae, J.Y.; Yoo, K.; Kim, J.; Nam, M.; Park, M.; Yun, S.H.; Cho, Y.S.; et al. Effects of HA and NA glycosylation pattern changes on the transmission of avian influenza A(H7N9) virus in guinea pigs. Biochem. Biophys. Res. Commun. 2016, 479, 192–197. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, J.; Torremorell, M.; Craft, M.E. Mathematical modeling of influenza A virus dynamics within swine farms and the effects of vaccination. PLoS ONE 2014, 9, e106177. [Google Scholar] [CrossRef]
- Cador, C.; Rose, N.; Willem, L.; Andraud, M. Maternally derived immunity extends swine influenza A virus persistence within farrow-to-finisch pig farms: Insights from a stochastic event-driven metapopulation model. PLoS ONE 2016, 11, e0163672. [Google Scholar] [CrossRef]
- Pitzer, V.E.; Aguas, R.; Riley, S.; Loeffen, W.L.A.; Wood, J.L.N.; Grenfell, B.T. High turnover drives prolonged persistence of influenza in managed pig herds. J. R. Soc. Interface 2016, 13, 20160138. [Google Scholar] [CrossRef]
- Cador, C.; Andraud, M.; Willem, L.; Rose, N. Control of endemic swine flu persistence in farrow-to-finish pig farms: A stochastic metapopulation modeling assessment. Vet. Res. 2017, 48, 58. [Google Scholar] [CrossRef]

| EA swH1N1 | Sporadic, Anthropogenic H1pdmN1pdm | Papenburg/2010-Like H1pdmN2 | Wachtum/2014-Like H1pdmN1pdm | |
|---|---|---|---|---|
| Immune Sera | ||||
| EA swH1N1 | 197 | <20 | <20 | <20 |
| anthropogenic H1pdmN1pdm | 21 | 106 | 34 | <20 |
| Papenburg/2010-like H1pdmN2 | <20 | 49 | 485 | <20 |
| Wachtum/2014-like H1pdmN1pdm | <20 | <20 | <20 | 160 |
| Hyper Immune Sera | ||||
| EA swH1N1 | 2560 | 80 | 160 | 80 |
| anthropogenic H1pdmN1pdm | 640 | 2560 | 640 | 320 |
| Papenburg/2010-like H1pdmN2 | 80 | 320 | 2560 | 20 |
| Wachtum/2014-like H1pdmN1pdm | 160 | 40 | 20 | 2560 |
| Amino Acid Position of Glycosylation Sequons (N-X-S/T) | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 27–29 | 28–30 | 40–42 | 101–103 | 104–106 | 136–138 | 142–144 1 | 172–174 1 | 177–179 1 | 179–181 2 | 202–204 3 | 212–214 | 286–288 | 291–293 | 293–295 | 304–306 | 498–500 | 557–559 | |
| Lineage 1A | ||||||||||||||||||
| anthroponotic H1pdmN1pdm | + | + | + | - | + | - | - | - | - | - | - | - | - | - | + | + | + | + |
| Wachtum/2014-like swH1pdmN1pdm | + | + | + | - | + | - | - | - | - | + | - | - | - | - | + | + | + | + |
| Papenburg/2010-like swH1N2 | + | + | + | - | + | - | - | - | - | - | + | - | - | - | + | + | + | + |
| Lineage 1B | ||||||||||||||||||
| Schneiderkrug/2014-like swH1N1 | + | + | + | - | - | - | + | - | + | - | - | - | + | - | - | + | + | + |
| Diepholz/2008-like swH1N2 | + | + | + | - | - | - | + | - | + | - | - | - | + | - | - | + | + | + |
| Gent/1999-like swH1N2 | + | + | + | - | - | - | - | + | + | - | - | - | + | - | - | + | + | + |
| England/448813/1994-like swH1N2 | + | + | + | - | - | - | - | - | + | - | - | - | + | - | - | + | + | + |
| Lineage 1C | ||||||||||||||||||
| sublineage 1C.2 (EA swH1N1, swH1N2) | + | + | + | B | B | B | - | - | - | B | - | + | - | + | - | - | + | + |
| sublineage 1C.1, 1991–1998 | + | + | + | - | + | - | - | - | - | B | - | - | - | + | - | - | + | + |
| sublineage 1C.1, 1979–1989 | + | + | + | - | + | - | - | - | - | - | - | - | - | - | - | + | + | + |
| avian H1N1 | + | + | + | - | + | - | - | - | - | - | - | - | - | - | + | + | + | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zell, R.; Groth, M.; Krumbholz, A.; Lange, J.; Philipps, A.; Dürrwald, R. Cocirculation of Swine H1N1 Influenza A Virus Lineages in Germany. Viruses 2020, 12, 762. https://doi.org/10.3390/v12070762
Zell R, Groth M, Krumbholz A, Lange J, Philipps A, Dürrwald R. Cocirculation of Swine H1N1 Influenza A Virus Lineages in Germany. Viruses. 2020; 12(7):762. https://doi.org/10.3390/v12070762
Chicago/Turabian StyleZell, Roland, Marco Groth, Andi Krumbholz, Jeannette Lange, Anja Philipps, and Ralf Dürrwald. 2020. "Cocirculation of Swine H1N1 Influenza A Virus Lineages in Germany" Viruses 12, no. 7: 762. https://doi.org/10.3390/v12070762
APA StyleZell, R., Groth, M., Krumbholz, A., Lange, J., Philipps, A., & Dürrwald, R. (2020). Cocirculation of Swine H1N1 Influenza A Virus Lineages in Germany. Viruses, 12(7), 762. https://doi.org/10.3390/v12070762






