Infection of a Lepidopteran Cell Line with Deformed Wing Virus
Abstract
1. Introduction
2. Materials and Methods
2.1. Cells and Viruses
2.2. Cell Harvesting and RNA Extraction
2.3. RT-qPCR
2.4. DWV Replication Test
2.5. Inoculation of A. mellifera Pupa with DWV-P1
3. Results and Discussion
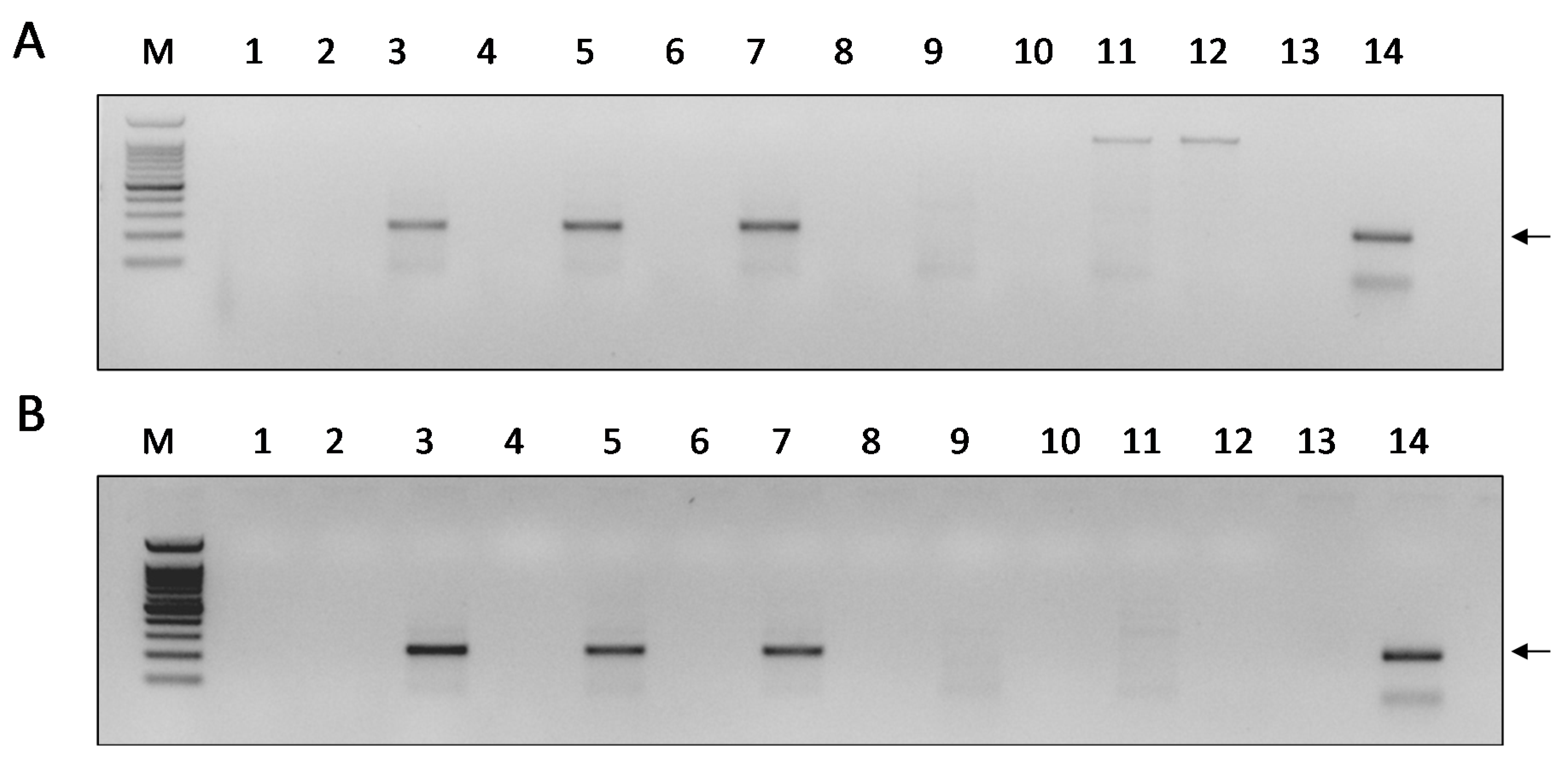
3.1. DWV Replicates in P1 Cells
3.2. Infectivity of DWV-P1
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Beaurepaire, A.; Doublet, V.; De Miranda, J.R.; Piot, N.; Antunez, K.; Campbell, E.; Chantawannakul, P.; Chejanovsky, N.; Gajda, A.; Heerman, M.; et al. Diversity and global distribution of viruses of the western honey bee, Apis mellifera. Insects 2020, 11, 239. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.P.; Siede, R. Honey bee viruses. Adv. Virus Res. 2007, 70, 33–80. [Google Scholar] [CrossRef] [PubMed]
- De Miranda, J.R.; Cordoni, G.; Budge, G. The Acute bee paralysis virus—Kashmir bee virus—Israeli acute paralysis virus complex. J. Invertebr. Pathol. 2009, 103 (Suppl. 1), S30–S47. [Google Scholar] [CrossRef] [PubMed]
- De Miranda, J.R.; Genersch, E. Deformed wing virus. J. Invertebr. Pathol. 2010, 103, S48–S61. [Google Scholar] [CrossRef] [PubMed]
- De Miranda, J.R.; Dainat, B.; Locke, B.; Cordoni, G.; Berthoud, H.; Gauthier, L.; Neumann, P.; Budge, G.E.; Ball, B.V.; Stoltz, D.B. Genetic characterization of slow bee paralysis virus of the honeybee (Apis mellifera L.). J. Gen. Virol. 2010, 91, 2524–2530. [Google Scholar] [CrossRef] [PubMed]
- Ribière, M.; Olivier, V.; Blanchard, P. Chronic bee paralysis: A disease and a virus like no other? J. Invertebr. Pathol. 2010, 103 (Suppl. 1), S120–S131. [Google Scholar] [CrossRef]
- De Miranda, J.R.; Bailey, L.; Ball, B.V.; Blanchard, P.; Budge, G.E.; Chejanovsky, N.; Chen, Y.; Gauthier, L.; Genersch, E.; De Graaf, D.C.; et al. Standard methods for virus research in Apis mellifera. J. Apic. Res. 2013, 52, 1–56. [Google Scholar] [CrossRef]
- Chen, Y.P.; Pettis, J.S.; Corona, M.; Chen, W.P.; Li, C.J.; Spivak, M.; Visscher, P.K.; DeGrandi-Hoffman, G.; Boncristiani, H.; Zhao, Y.; et al. Israeli acute paralysis virus: Epidemiology, pathogenesis and implications for honey bee health. PLoS Pathog. 2014, 10, e1004261. [Google Scholar] [CrossRef]
- Hartmann, U.; Forsgren, E.; Charrière, J.D.; Neumann, P.; Gauthier, L. Dynamics of Apis mellifera filamentous virus (AmFV) infections in honey bees and relationships with other parasites. Viruses 2015, 7, 2654–2667. [Google Scholar] [CrossRef]
- Genersch, E.; Von der Ohe, W.; Kaatz, H.; Schroeder, A.; Otten, C.; Büchler, R.; Berg, S.; Ritter, W.; Mühlen, W.; Gisder, S.; et al. The German bee monitoring project: A long term study to understand periodically high winter losses of honey bee colonies. Apidologie 2010, 41, 332–352. [Google Scholar] [CrossRef]
- Runckel, C.; Flenniken, M.L.; Engel, J.C.; Ruby, J.G.; Ganem, D.; Andino, R.; DeRisi, J.L. Temporal analysis of the honey bee microbiome reveals four novel viruses and seasonal prevalence of known viruses, Nosema, and Crithidia. PLoS ONE 2011, 6, e20656. [Google Scholar] [CrossRef] [PubMed]
- Soroker, V.; Hetzroni, A.; Yakobson, B.; David, D.; David, A.; Voet, H.; Slabezki, Y.; Efrat, H.; Levski, S.; Kamer, Y.; et al. Evaluation of colony losses in Israel in relation to the incidence of pathogens and pests. Apidologie 2011, 42, 192–199. [Google Scholar] [CrossRef]
- Cornman, R.S.; Tarpy, D.R.; Chen, Y.; Jeffreys, L.; Lopez, D.; Pettis, J.S.; VanEngelsdorp, D.; Evans, J.D. Pathogen webs in collapsing honey bee colonies. PLoS ONE 2012, 7, e43562. [Google Scholar] [CrossRef] [PubMed]
- Hou, C.; Rivkin, H.; Slabezki, Y.; Chejanovsky, N. Dynamics of the presence of israeli acute paralysis virus in honey bee colonies with colony collapse disorder. Viruses 2014, 6, 2012–2027. [Google Scholar] [CrossRef] [PubMed]
- Cavigli, I.; Daughenbaugh, K.F.; Martin, M.; Lerch, M.; Banner, K.; Garcia, E.; Brutscher, L.M.; Flenniken, M.L. Pathogen prevalence and abundance in honey bee colonies involved in almond pollination. Apidologie 2016, 47, 251–266. [Google Scholar] [CrossRef] [PubMed]
- Amiri, E.; Meixner, M.; Büchler, R.; Kryger, P. Chronic bee paralysis virus in honeybee queens: Evaluating susceptibility and infection routes. Viruses 2014, 6, 1188–1201. [Google Scholar] [CrossRef]
- Thaduri, S.; Stephan, J.G.; De Miranda, J.R.; Locke, B. Disentangling host-parasite-pathogen interactions in a varroa-resistant honeybee population reveals virus tolerance as an independent, naturally adapted survival mechanism. Sci. Rep. 2019, 9, 1–10. [Google Scholar] [CrossRef]
- Hunter, W.; Ellis, J.; Vanengelsdorp, D.; Hayes, J.; Westervelt, D.; Glick, E.; Williams, M.; Sela, I.; Maori, E.; Pettis, J.; et al. Large-scale field application of RNAi technology reducing Israeli acute paralysis virus disease in honey bees (Apis mellifera, Hymenoptera: Apidae). PLoS Pathog. 2010, 6, e1001160. [Google Scholar] [CrossRef]
- Lamp, B.; Url, A.; Seitz, K.; Eichhorn, J.; Riedel, C.; Sinn, L.J.; Indik, S.; Köglberger, H.; Rümenapf, T. Construction and rescue of a molecular clone of Deformed wing virus (DWV). PLoS ONE 2016, 11, e0164639. [Google Scholar] [CrossRef]
- Ryabov, E.V.; Christmon, K.; Heerman, M.C.; Posada-Florez, F.; Harrison, R.L.; Chen, Y.; Evans, J.D. Development of a honey bee RNA virus vector based on the genome of a deformed wing virus. Viruses 2020, 12, 374. [Google Scholar] [CrossRef]
- Jin, L.; Mehmood, S.; Zhang, G.; Song, Y.; Su, S.; Huang, S.; Huang, H.; Zhang, Y.; Geng, H.; Huang, W.F. Visualizing sacbrood virus of honey bees via transformation and coupling with enhanced green fluorescent protein. Viruses 2020, 12, 224. [Google Scholar] [CrossRef] [PubMed]
- Gusachenko, O.N.; Woodford, L.; Balbirnie-Cumming, K.; Campbell, E.M.; Christie, C.R.; Bowman, A.S.; Evans, D.J. Green bees: Reverse genetic analysis of deformed wing virus transmission, replication, and tropism. Viruses 2020, 12, 532. [Google Scholar] [CrossRef] [PubMed]
- Oldstone, M.B.A.; Levine, A.J. Virology in the Next Millennium. Cell 2000, 100, 139–142. [Google Scholar] [CrossRef][Green Version]
- Carrillo-Tripp, J.; Bonning, B.C.; Miller, W.A. Challenges associated with research on RNA viruses of insects. Curr. Opin. Insect Sci. 2015, 8, 62–68. [Google Scholar] [CrossRef]
- Adelman, Z.N.; Sanchez-Vargas, I.; Travanty, E.A.; Carlson, J.O.; Beaty, B.J.; Blair, C.D.; Olson, K.E. RNA silencing of dengue virus type 2 replication in transformed C6/36 mosquito cells transcribing an inverted-repeat RNA derived from the virus genome. J. Virol. 2002, 76, 12925–12933. [Google Scholar] [CrossRef]
- Swevers, L.; Liu, J.; Smagghe, G. Defense mechanisms against viral infection in Drosophila: RNAi and non-RNAi. Viruses 2018, 10, 230. [Google Scholar] [CrossRef]
- Franco, E.J.; Rodriquez, J.L.; Pomeroy, J.J.; Hanrahan, K.C.; Brown, A.N. The effectiveness of antiviral agents with broad-spectrum activity against chikungunya virus varies between host cell lines. Antivir. Chem. Chemother. 2018, 26, 1–7. [Google Scholar] [CrossRef]
- Goblirsch, M.J.; Spivak, M.S.; Kurtti, T.J. A cell line resource derived from honey bee (Apis mellifera) embryonic tissues. PLoS ONE 2013, 8, e69831. [Google Scholar] [CrossRef]
- Carrillo-Tripp, J.; Dolezal, A.G.; Goblirsch, M.J.; Miller, W.A.; Toth, A.L.; Bonning, B.C. In vivo and in vitro infection dynamics of honey bee viruses. Sci. Rep. 2016, 6, 22265. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Goodman, C.L.; Stanley, D.W.; Bonning, B.C. Cell lines for honey bee virus research. Viruses 2020, 12, 236. [Google Scholar] [CrossRef]
- Xia, X.; Mao, Q.; Wang, H.; Zhou, B.; Wei, T. Replication of Chinese sacbrood virus in primary cell cultures of Asian honeybee (Apis cerana). Arch. Virol. 2014, 159, 3435–3438. [Google Scholar] [CrossRef] [PubMed]
- Genersch, E.; Gisder, S.; Hedtke, K.; Hunter, W.B.; Möckel, N.; Müller, U. Standard methods for cell cultures in Apis mellifera research. J. Apic. Res. 2013, 52, 1–8. [Google Scholar] [CrossRef]
- Gisder, S.; Mockel, N.; Linde, A.; Genersch, E. A cell culture model for Nosema ceranae and Nosema apis allows new insights into the life cycle of these important honey bee-pathogenic microsporidia. Environ. Microbiol. 2011, 13, 404–413. [Google Scholar] [CrossRef]
- Li, Y.; Jousset, F.-X.; Giraud, C.; Rolling, F.; Quiot, J.M.; Bergoin, M. A titration procedure of the Junonia Cœnia Densovirus and quantitation of transfection by Its cloned genomic DNA in four lepidopteran cell lines. J. Virol. Methods 1996, 57, 47–60. [Google Scholar] [CrossRef]
- Chejanovsky, N.; Gershburg, E. The wild-type Autographa californica nuclear polyhedrosis virus induces apoptosis of Spodoptera littoralis cells. Virology 1995, 209, 519–525. [Google Scholar] [CrossRef][Green Version]
- Levin, S.; Sela, N.; Chejanovsky, N. Two novel viruses associated with the Apis mellifera pathogenic mite Varroa destructor. Sci. Rep. 2016, 6, 37710. [Google Scholar] [CrossRef] [PubMed]
- Zioni, N.; Soroker, V.; Chejanovsky, N. Replication of varroa destructor virus 1 (VDV-1) and a varroa destructor virus 1-deformed wing virus recombinant (VDV-1-DWV) in the head of the honey bee. Virology 2011, 417, 106–112. [Google Scholar] [CrossRef]
- Evans, J.D. Beepath: An ordered quantitative-PCR array for exploring honey bee immunity and disease. J. Invertebr. Pathol. 2006, 93, 135–139. [Google Scholar] [CrossRef]
- Levin, S.; Galbraith, D.A.; Sela, N.; Erez, T.; Grozinger, C.M.; Chejanovsky, N. Presence of apis rhabdovirus-1 in populations of pollinators and their parasites from two continents. Front. Microbiol. 2017, 8, 1–11. [Google Scholar] [CrossRef]
- Ma, H.; Nandakumar, S.; Bae, E.H.; Chin, P.J.; Khan, A.S. The Spodoptera frugiperda Sf9 cell line is a heterogeneous population of rhabdovirus-infected and virus-negative cells: Isolation and characterization of cell clones containing rhabdovirus X-gene variants and virus-negative cell clones. Virology 2019, 536, 125–133. [Google Scholar] [CrossRef]
- Merten, O.W. Virus contaminations of cell cultures—A biotechnological view. Cytotechnology 2002, 39, 91–116. [Google Scholar] [CrossRef] [PubMed]
- Goic, B.; Vodovar, N.; Mondotte, J.A.; Monot, C.; Frangeul, L.; Blanc, H.; Gausson, V.; Vera-Otarola, J.; Cristofari, G.; Saleh, M.C. RNA-mediated interference and reverse transcription control the persistence of RNA viruses in the insect model Drosophila. Nat. Immunol. 2013, 14, 396–403. [Google Scholar] [CrossRef]




© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Erez, T.; Chejanovsky, N. Infection of a Lepidopteran Cell Line with Deformed Wing Virus. Viruses 2020, 12, 739. https://doi.org/10.3390/v12070739
Erez T, Chejanovsky N. Infection of a Lepidopteran Cell Line with Deformed Wing Virus. Viruses. 2020; 12(7):739. https://doi.org/10.3390/v12070739
Chicago/Turabian StyleErez, Tal, and Nor Chejanovsky. 2020. "Infection of a Lepidopteran Cell Line with Deformed Wing Virus" Viruses 12, no. 7: 739. https://doi.org/10.3390/v12070739
APA StyleErez, T., & Chejanovsky, N. (2020). Infection of a Lepidopteran Cell Line with Deformed Wing Virus. Viruses, 12(7), 739. https://doi.org/10.3390/v12070739




