Saxifraga spinulosa-Derived Components Rapidly Inactivate Multiple Viruses Including SARS-CoV-2
Abstract
1. Introduction
2. Materials and Methods
2.1. Viruses and Cells
2.2. SS-Derived Samples
2.3. Screening for Virucidal Activity
2.4. Sodium Dodecyl Sulfate–Polyacrylamide Gel Electrophoresis (SDS–PAGE)
2.5. Hemagglutination (HA) and Neuraminidase (NA) Assays
2.6. RT–PCR
2.7. Transmission Electron Microscopy
2.8. Statistical Analysis
3. Results
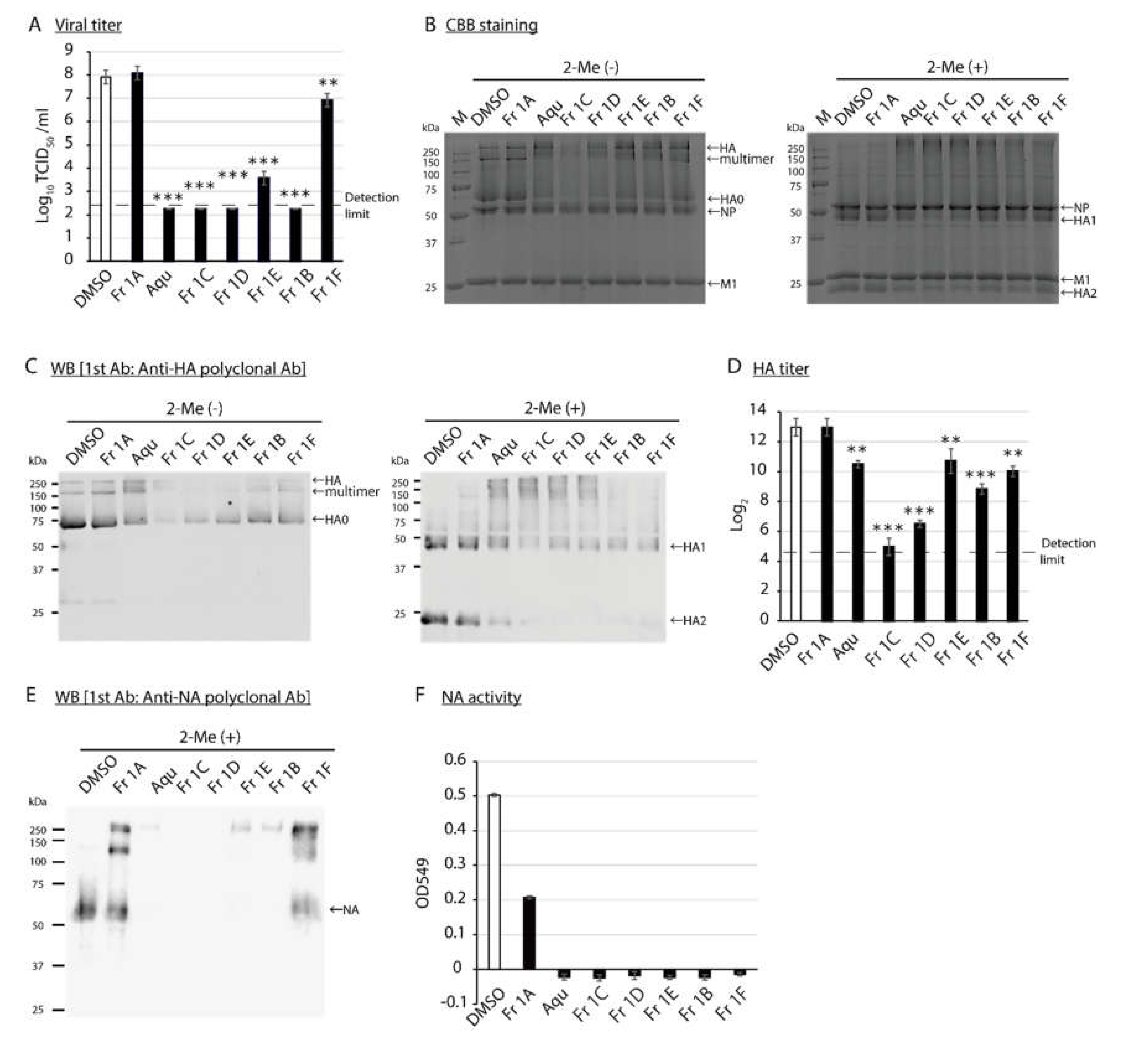
3.1. Identification of SS-Derived Components with Virucidal Activity against IAV, FCV, and MNV
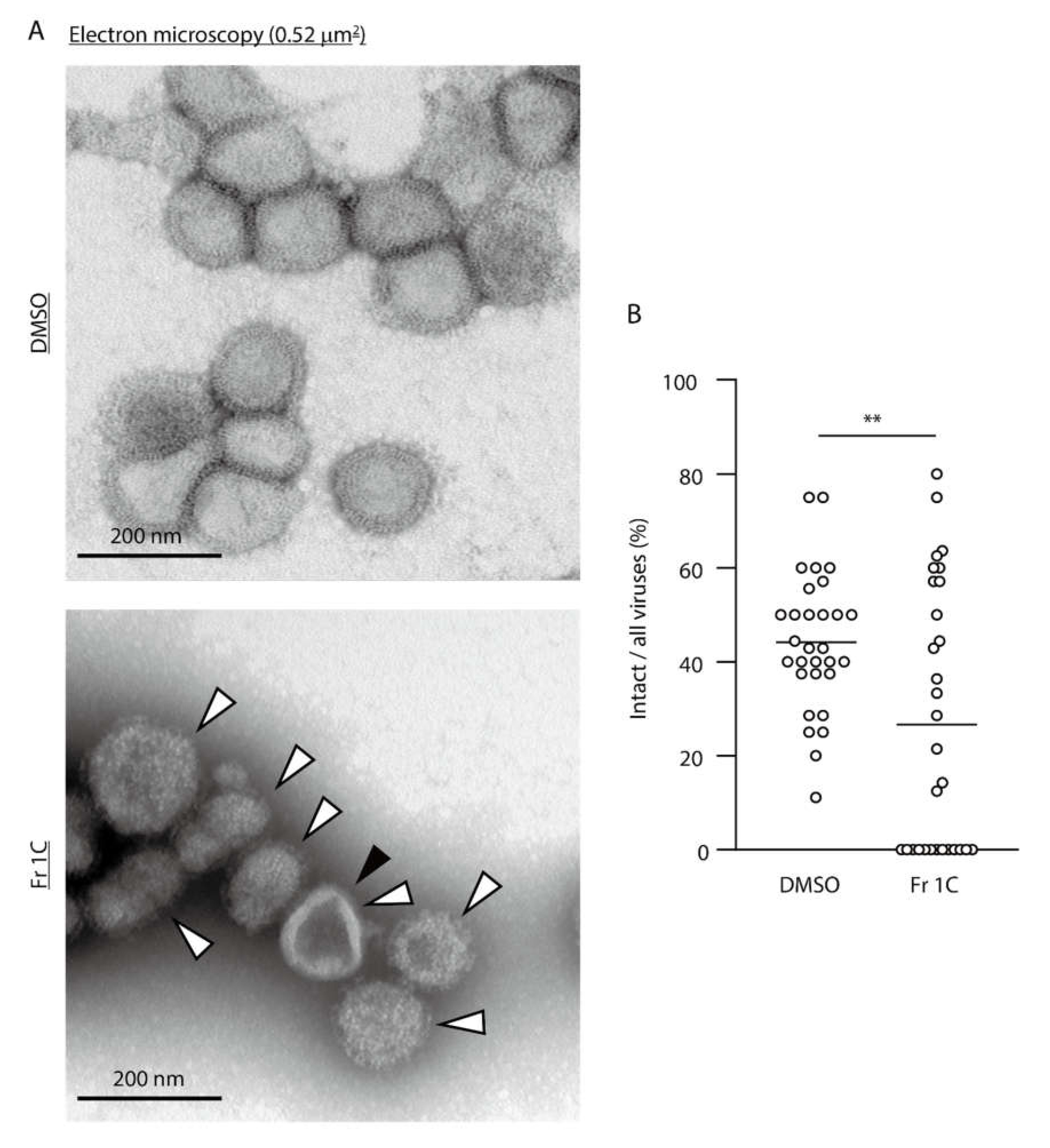
3.2. Impact of SS-Derived Fractions on IAV Proteins and Genome
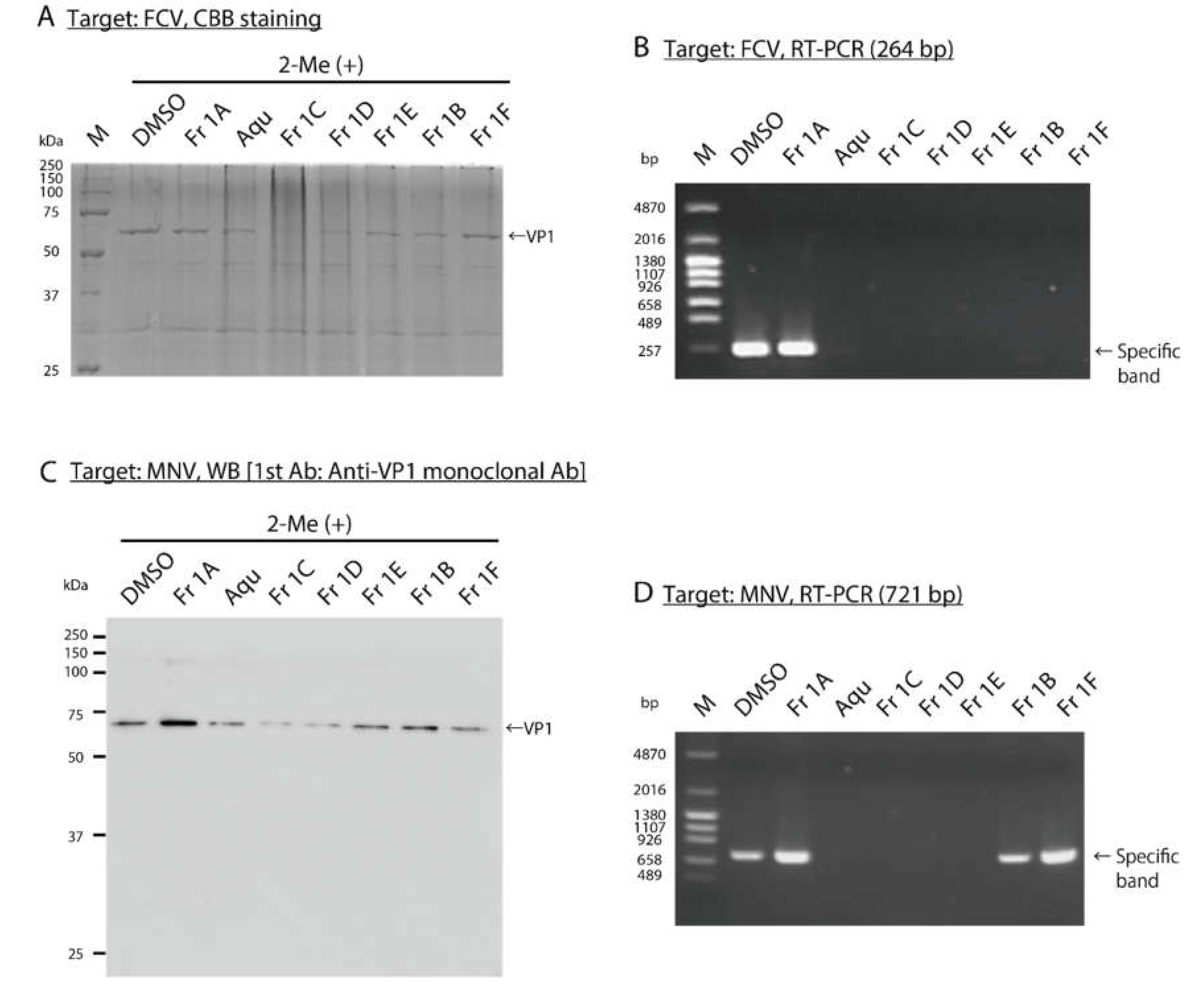
3.3. Impact of SS-Derived Fractions on FCV and MNV Proteins and Genomes
3.4. Identification of SS-Derived Fractions that Promote Rapid Inactivation of SARS-CoV-2
3.5. Impact of SS-Derived Fractions on SARS-CoV-2 Proteins and Genome
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zhu, N.; Zhang, D.; Wang, W.; Li, X.; Yang, B.; Song, J.; Zhao, X.; Huang, B.; Shi, W.; Lu, R.; et al. A novel coronavirus from patients with pneumonia in China, 2019. N. Engl. J. Med. 2020, 382, 727–733. [Google Scholar] [CrossRef]
- Zhou, P.; Yang, X.-L.; Wang, X.G.; Hu, B.; Zhang, L.; Zhang, W.; Si, H.R.; Zhu, Y.; Li, B.; Huang, C.L.; et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 2020, 579, 270–273. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Coronavirus Disease (COVID-2019) Situation Reports. Available online: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports (accessed on 20 June 2020).
- Saunders-Hastings, P.; Krewski, D. Reviewing the history of pandemic influenza: Understanding patterns of emergence and transmission. Pathogens 2016, 5, E66. [Google Scholar] [CrossRef]
- World Health Organization. WHO Estimates of the Global Burden of Foodborne Diseases. Available online: https://www.who.int/activities/estimating-the-burden-of-foodborne-diseases (accessed on 21 May 2020).
- Belliot, G.; Lopman, B.A.; Ambert-Balay, K.; Pothier, P. The burden of norovirus gastroenteritis: An important foodborne and healthcare-related infection. Clin. Microbiol. Infect. 2014, 20, 724–730. [Google Scholar] [CrossRef]
- Chen, Y.; Hall, A.J.; Kirk, M.D. Norovirus disease in older adults living in long-term care facilities: Strategies for management. Curr. Geriatr. Rep. 2017, 6, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Cao, R.; Zhang, L.; Yang, X.; Liu, J.; Xu, M.; Shi, Z.; Hu, Z.; Zhong, W.; Xiao, G. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020, 30, 269–271. [Google Scholar] [CrossRef]
- Hoffmann, M.; Kleine-Weber, H.; Schroeder, S.; Krüger, N.; Herrler, T.; Erichsen, S.; Schiergens, T.S.; Herrler, G.; Wu, N.H.; Nitsche, A.; et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell 2020, 181, 271–280. [Google Scholar] [CrossRef] [PubMed]
- Grein, J.; Ohmagari, N.; Shin, D.; Diaz, G.; Asperges, E.; Castagna, A.; Feldt, T.; Green, G.; Green, M.L.; Lescure, F.-X.; et al. Compassionate use of remdesivir for patients with severe Covid-19. N. Engl. J. Med. 2020, in press. [Google Scholar] [CrossRef] [PubMed]
- Hurt, A.C. The epidemiology and spread of drug resistant human influenza viruses. Curr. Opin. Virol. 2014, 8, 22–29. [Google Scholar] [CrossRef]
- Li, T.C.M.; Chan, M.C.W.; Lee, N. Clinical implications of antiviral resistance in influenza. Viruses 2015, 7, 4929–4944. [Google Scholar] [CrossRef]
- Kratzel, A.; Todt, D.; V’kovski, P.; Steiner, S.; Gultom, M.L.; Thao, T.T.N.; Ebert, N.; Holwerda, M.; Steinmann, J.; Niemeyer, D.; et al. Efficient inactivation of SARS-CoV-2 by WHO-recommended hand rub formulations and alcohols. bioRxiv 2020. online ahead of print. [Google Scholar] [CrossRef]
- Lachenmeier, D.W. Safety evaluation of topical applications of ethanol on the skin and inside the oral cavity. J. Occup. Med. Toxicol. 2008, 3, 26. [Google Scholar] [CrossRef] [PubMed]
- Badral, D.; Odonbayar, B.; Murata, T.; Munkhjargal, T.; Tuvshintulga, B.; Igarashi, I.; Suganuma, K.; Inoue, N.; Brantner, A.H.; Odontuya, G.; et al. Flavonoid and galloyl glycosides isolated from Saxifraga spinulosa and their antioxidative and inhibitory activities against species that cause piroplasmosis. J. Nat. Prod. 2017, 80, 2416–2423. [Google Scholar] [CrossRef] [PubMed]
- Zuo, G.Y.; Li, Z.Q.; Chen, L.R.; Xu, X.J. In vitro anti-HCV activities of Saxifraga melanocentra and its related polyphenolic compounds. Antivir. Chem. Chemother. 2005, 16, 393–398. [Google Scholar] [CrossRef]
- Imai, K.; Ogawa, H.; Bui, V.N.; Inoue, H.; Fukuda, J.; Ohba, M.; Yamamoto, Y.; Nakamura, K. Inactivation of high and low pathogenic avian influenza virus H5 subtypes by copper ions incorporated in zeolite-textile materials. Antivir. Res. 2012, 93, 225–233. [Google Scholar] [CrossRef]
- Takeda, Y.; Okuyama, Y.; Nakano, H.; Yaoita, Y.; Machida, K.; Ogawa, H.; Imai, K. Antiviral activities of Hibiscus sabdariffa L. tea extract against human influenza A virus rely largely on acidic pH but partially on a low-pH-independent mechanism. Food Environ. Virol. 2020, 12, 9–19. [Google Scholar] [CrossRef]
- Nao, N.; Sato, K.; Yamagishi, J.; Tahara, M.; Nakatsu, Y.; Seki, F.; Katoh, H.; Ohnuma, A.; Shirogane, Y.; Hayashi, M.; et al. Consensus and variations in cell line specificity among human metapneumovirus strains. PLoS ONE 2019, 14, e0215822. [Google Scholar] [CrossRef]
- Kärber, G. Beitrag zur kollektiven behandlung pharmakologischer reihenversuche. Naunyn Schmiedebergs Arch. Exp. Pathol. Pharmakol. 1931, 162, 480–483. [Google Scholar] [CrossRef]
- World Health Organization. WHO Manual on Animal Influenza Diagnosis and Surveillance. Available online: https://apps.who.int/iris/bitstream/handle/10665/68026/WHO_CDS_CSR_NCS_2002.5.pdf?sequence=1&isAllowed=y (accessed on 21 May 2020).
- Kitajima, M.; Oka, T.; Tohya, Y.; Katayama, H.; Takeda, N.; Katayama, K. Development of a broadly reactive nested reverse transcription-PCR assay to detect murine noroviruses, and investigation of the prevalence of murine noroviruses in laboratory mice in Japan. Microbiol. Immunol. 2009, 53, 531–534. [Google Scholar] [CrossRef]
- Furuno, K.; Akasako, T.; Sugihara, N. The contribution of the pyrogallol moiety to the superoxide radical scavenging activity of flavonoids. Biol. Pharm. Bull. 2002, 25, 19–23. [Google Scholar] [CrossRef]
- Haslam, E. Natural polyphenols (vegetable tannins) as drugs: Possible modes of action. J. Nat. Prod. 1996, 59, 205–215. [Google Scholar] [CrossRef] [PubMed]
- Steinmann, J.; Buer, J.; Pietschmann, T.; Steinmann, E. Anti-infective properties of epigallocatechin-3-gallate (EGCG), a component of green tea. Br. J. Pharmacol. 2013, 168, 1059–1073. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Xu, Z.; Zheng, W. A review of the antiviral role of green tea catechins. Molecules 2017, 22, E1337. [Google Scholar] [CrossRef] [PubMed]
- Ide, K.; Kawasaki, Y.; Kawakami, K.; Yamada, H. Anti-influenza virus effects of catechins: A molecular and clinical review. Curr. Med. Chem. 2017, 23, 4473–4783. [Google Scholar] [CrossRef] [PubMed]
- Furushima, D.; Ide, K.; Yamada, H. Effect of tea catechins on influenza infection and the common cold with a focus on epidemiological/clinical studies. Molecules 2018, 23, E1795. [Google Scholar] [CrossRef]
- Nagle, D.G.; Ferreira, D.; Zhou, Y.D. Epigallocatechin-3-gallate (EGCG): Chemical and biomedical perspectives. Phytochemistry 2006, 67, 1849–1855. [Google Scholar] [CrossRef]
- Liu, J.; Yang, Z.; Wang, S.; Liu, L.; Chen, G.; Wang, L. Exploring the molecular basis of H5N1 hemagglutinin binding with catechins in green tea: A flexible docking and molecular dynamics study. J. Theor. Comput. Chem. 2012, 11, 111–125. [Google Scholar] [CrossRef]
- Nakayama, M.; Suzuki, K.; Toda, M.; Okubo, S.; Hara, Y.; Shimamura, T. Inhibition of the infectivity of influenza virus by tea polyphenols. Antiviral Res. 1993, 21, 289–299. [Google Scholar] [CrossRef]
- Song, J.M.; Lee, K.H.; Seong, B.L. Antiviral effect of catechins in green tea on influenza virus. Antivir. Res. 2005, 62, 66–74. [Google Scholar] [CrossRef]
- Müller, P.; Downard, K.M. Catechin inhibition of influenza neuraminidase and its molecular basis with mass spectrometry. J. Pharm. Biomed. Anal. 2015, 111, 222–230. [Google Scholar] [CrossRef]
- Oxford, J.S.; Lambkin, R.; Guralnik, M.; Rosenbloom, R.A.; Petteruti, M.P.; DiGian, K.; LeFante, C. Preclinical in vitro activity of QR-435 against influenza a virus as a virucide and in paper masks for prevention of viral transmission. Am. J. Ther. 2007, 14, 455–461. [Google Scholar] [CrossRef] [PubMed]
- Ide, K.; Yamada, H.; Kawasaki, Y. Effect of gargling with tea and ingredients of tea on the prevention of influenza infection: A meta-analysis. BMC Public Health 2016, 16, 396. [Google Scholar] [CrossRef] [PubMed]


| Origin (g) | No. | Compound name | Obtained amount (mg) |
|---|---|---|---|
| Fr 1C (5.0 g) | 1 | (2R,3R)-3’-O-β-d-(6’’-O-galloyl)glucopyranosyloxy-5,7,4’,5’- tetrahydroxyflavanonol | 64.5 mg |
| 2 | gallocatechin-3-O-gallate | 88.0 mg | |
| 3 | dihydromyricetin | 2.3 mg | |
| 4 | epigallocatechin-3-O-gallate | 4.8 mg | |
| 5 | catechin | 2.3 mg | |
| 6 | 3-O-(6”-O-galloyl-β-d-glucopyranosyl)gallocatechin | 21.5 mg | |
| 7 | 6’-O-galloyl salidroside | 25.7 mg | |
| 8 | (2R,3R)-dihydromyricetin 3’-O-β-d-glucopyranoside | 3.2 mg | |
| Fr 1D (5.0 g) | 9 | (2R,3R)-3’-O-β-d-(2”,6”-di-O-galloyl)glucopyranosyloxy-5,7,4’,5’- tetrahydroxyflavanonol | 8.0 mg |
| 10 | 8-O-β-d-[6’-O-(3’’-O-methyl)galloyl]glucopyranosyl-p-tyrosol | 7.9 mg | |
| 11 | 4-O-β-d-(6’-O-galloyl)glucopyranosyl-(E)-p-coumaroyl acid | 2.9 mg | |
| 12 | 8-O-β-d-(2’,6’-di-O-galloyl)glucopyranosyl-p-tyrosol | 12.1 mg | |
| 13 | myricetin | 6.2 mg | |
| 14 | rutin | 8.4 mg | |
| 15 | quercetin 3-O-β-d-(6”-O-galloyl)galactopyranoside | 15.6 mg | |
| 16 | myricetin 3-O-β-d-galactopyranoside | 4.6 mg | |
| 17 | quercetin 3-O-β-d-(6”-O-galloyl)glucopyranoside | 2.5 mg | |
| Fr 1E (3.3 g) | 18 | (2S)-3’-O-β-d-(6”-O-galloyl)glucopyranosyloxy-5,7,4’,5’- tetrahydroxyflavanone | 7.1 mg |
| 19 | (2R)-3’-O-β-d-(6”-O-galloyl)glucopyranosyloxy-5,7,4’,5’- tetrahydroxyflavanone | 9.1 mg | |
| 20 | (2S)-3’-O-β-d-(2”,6”-di-O-galloyl)glucopyranosyloxy-5,7,4’,5’- tetrahydroxyflavanone | 9.8 mg | |
| 21 | (2S)-3’-O-β-d-[6”-O-(3’”-O-methyl)galloyl]glucopyranosyloxy-5,7,4’,5’- tetrahydroxyflavanone | 4.6 mg | |
| 22 | naringenin 7-O-β-d-(6”-O-galloyl)glucopyranoside | 3.1 mg | |
| 23 | quercetin | 9.2 mg | |
| 24 | eriodictyol | 11.0 mg | |
| 25 | taxifolin | 2.5 mg | |
| 26 | quercetin 3-O-β-d-glucopyranoside | 1.5 mg | |
| 27 | quercetin 3-O-α-l-rhamnopyranoside | 2.8 mg | |
| 28 | gallic acid | 10.4 mg | |
| 29 | 4-(4’-hydroxyphenyl)-2-butanone 4’-O-β-d-(2”,6”-di-O- galloyl)glucopyranoside | 4.0 mg |
| A Target: IAV, concentration of sample: 25 µg/mL. | ||||
|---|---|---|---|---|
| Extract and fraction name | [DMSO control #) - Sample #)] ± SD | |||
| Reaction time: 24 hrs | 6 hrs | 10 min | 1 min | |
| Aqueous | ≥3.33 ± 1.16* | ≥3.83 ± 1.16* | ≥3.83 ± 0.76* | 2.83 ± 0.29** |
| Fr 1A | 0.67 ± 1.16 | N.T. | N.T. | N.T. |
| Fr 1B | ≥4.17 ± 0.29** | ≥3.83 ± 1.16* | ≥4.33 ± 1.26* | 0.5 ± 0.5 |
| Fr 1C | ≥4.17 ± 0.29** | ≥3.83 ± 1.16* | ≥4.5 ± 1.32* | ≥3.83 ± 0.29** |
| Fr 1D | ≥4.17 ± 0.29** | ≥3.83 ± 1.16* | ≥3.67 ± 0.76* | 1.83 ± 0.29** |
| Fr 1E | ≥4.17 ± 0.29** | ≥3.83 ± 1.16* | ≥4.17 ± 1.26** | 1.1 ± 0.42** |
| Fr 1F | ≥4 ± 0.5** | ≥2.1 ± 1.43* | 0.5 ± 0.5 | N.T. |
| B Target: FCV, concentration of sample: 25 µg/mL. | ||||
| Extract and fraction name | [DMSO control #) - Sample #)] ± SD | |||
| Reaction time: 48 hrs | 6 hrs | 10 min | 1 min | |
| Aqueous | ≥2.83 ± 0.29** | ≥2.83 ± 0.58* | ≥2.83 ± 0.29** | ≥2.83 ± 0.29** |
| Fr 1A | ≥2.83 ± 0.29** | ≥1.75 ± 0.29* | -0.17 ± 0.29 | N.T. |
| Fr 1B | ≥2.5 ± 0.5* | ≥2.67 ± 0.29** | ≥2.67 ± 0.29** | ≥3 ± 0.5** |
| Fr 1C | ≥2.83 ± 0.29** | ≥2.83 ± 0.58* | ≥2.83 ± 0.29** | ≥3 ± 0*** |
| Fr 1D | ≥2.83 ± 0.29** | ≥2.67 ± 0.29** | ≥2.83 ± 0.29** | ≥3 ± 0*** |
| Fr 1E | ≥2.83 ± 0.58* | ≥2.67 ± 0.29** | ≥2.83 ± 0.29** | ≥2.67 ± 0.29** |
| Fr 1F | ≥2.83 ± 0.58* | ≥2.67 ± 0.63** | ≥2.67 ± 0.29** | ≥2.83 ± 0.29** |
| C Target: MNV, concentration of sample: 100 µg/mL. | ||||
| Extract and fraction name | [DMSO control #) - Sample #)] ± SD | |||
| Reaction time: 48 hrs | 6 hrs | 10 min | 1 min | |
| Aqueous | 1.67 ± 0.12*** | 1.88 ± 0.25*** | 0 ± 0.5 | N.T. |
| Fr 1A | 0 ± 0.63 | N.T. | N.T. | N.T. |
| Fr 1B | 1.83 ± 0.58* | 1.88 ± 0.48** | 0.33 ± 0.29 | N.T. |
| Fr 1C | ≥2.92 ± 0.97*** | ≥3.13 ± 0.25*** | 2.17 ± 0.29** | 2 ± 0*** |
| Fr 1D | 1.67 ± 0.41*** | ≥2.75 ± 0.65** | 2.17 ± 0.29** | 1.83 ± 0.29** |
| Fr 1E | 1.4 ± 0.65** | 1.38 ± 0.25** | 0.67 ± 0.29 | N.T. |
| Fr 1F | 0.25 ± 0.5 | N.T. | N.T. | N.T. |
| A Target: IAV, concentration of sample: 25 µg/mL. | |||||
|---|---|---|---|---|---|
| Origin | No. | [DMSO control #) - Sample #)] ± SD | |||
| Reaction time: 24 hrs | 6 hrs | 10 min | 1 min | ||
| Fr 1C | 1 | 1.33 ± 0.76 | N.T. | N.T. | N.T. |
| 2 | ≥4 ± 0.5** | ≥2.67 ± 0.76* | ≥1.67 ± 0.93** | 2.17 ± 0.29** | |
| 3 | ≥4 ± 1* | ≥2.5 ± 1.32* | 0.92 ± 0.74* | 1.83 ± 0.76* | |
| 4 | ≥3.33 ± 1.26* | ≥3.83 ± 0.58** | ≥2.83 ± 0.29** | 1.17 ± 0.29* | |
| 5 | 0.75 ± 0.96 | N.T. | N.T. | N.T. | |
| 6 | 0.17 ± 0.76 | N.T. | N.T. | N.T. | |
| 7 | 2.25 ± 0.96* | 1.5 ± 0.91* | 0.5 ± 0.84 | N.T. | |
| 8 | ≥2.88 ± 1.32* | 2 ± 0.71* | 0.33 ± 0.29 | N.T. | |
| Fr 1D | 9 | 2 ± 0.5* | 0.5 ± 0.5 | N.T. | N.T. |
| 10 | 0.83 ± 0.29* | 0.67 ± 0.29 | N.T. | N.T. | |
| 11 | 1.33 ± 0.29* | 0.17 ± 0.29 | N.T. | N.T. | |
| 12 | 1.4 ± 0.96* | −0.33 ± 0.29 | N.T. | N.T. | |
| 13 | ≥1.75 ± 1.13** | −0.33 ± 0.29 | N.T. | N.T. | |
| 14 | −0.17 ± 1.61 | N.T. | N.T. | N.T. | |
| 15 | −0.17 ± 1.61 | N.T. | N.T. | N.T. | |
| 16 | 1.1 ± 0.74* | 0.67 ± 0.29 | N.T. | N.T. | |
| 17 | 0.33 ± 0.29 | N.T. | N.T. | N.T. | |
| Fr 1E | 18 | 0.33 ± 0.76 | N.T. | N.T. | N.T. |
| 19 | 0.17 ± 0.56 | N.T. | N.T. | N.T. | |
| 20 | 1 ± 1.73 | N.T. | N.T. | N.T. | |
| 21 | 0.5 ± 0.5 | N.T. | N.T. | N.T. | |
| 22 | 0.67 ± 0.29 | N.T. | N.T. | N.T. | |
| 23 | 0.67 ± 0.29 | N.T. | N.T. | N.T. | |
| 24 | 0.5 ± 0.32 | N.T. | N.T. | N.T. | |
| 25 | 0.5 ± 0.32 | N.T. | N.T. | N.T. | |
| 26 | −0.5 ± 1.32 | N.T. | N.T. | N.T. | |
| 27 | 0.17 ± 0.29 | N.T. | N.T. | N.T. | |
| 28 | 0.87 ± 1.53 | N.T. | N.T. | N.T. | |
| 29 | 0.5 ± 1.32 | N.T. | N.T. | N.T. | |
| B Target: FCV, concentration of sample: 25 µg/mL. | |||||
| Origin | No. | [DMSO control #) - Sample #)] ± SD | |||
| Reaction time: 48 hrs | 6 hrs | 10 min | 1 min | ||
| Fr 1C | 1 | ≥2.5 ± 0.87* | ≥2.83 ± 0.29** | 0.33 ± 0.29 | N.T. |
| 2 | ≥2.67 ± 0.58* | ≥2.67 ± 0.29** | 2 ± 0*** | ≥2.5 ± 0.5* | |
| 3 | ≥2.75 ± 0.5** | ≥2.83 ± 0.58* | ≥2 ± 0.5* | 2 ± 0*** | |
| 4 | ≥2.67 ± 0.58* | ≥2.83 ± 0.29** | ≥2.17 ± 0.29** | ≥2.5 ± 0.5* | |
| 5 | ≥2.17 ± 0.58* | ≥2 ± 0.5* | −0.33 ± 0.29 | N.T. | |
| 6 | ≥2.67 ± 0.58* | 1.33 ± 0.29* | 0.67 ± 0.29 | N.T. | |
| 7 | ≥2.67 ± 0.58* | ≥3 ± 0.5** | ≥3.5 ± 0.5** | 2.33 ± 0.29** | |
| 8 | ≥2.67 ± 0.58* | ≥2.67 ± 0.29** | ≥3.67 ± 0.29** | 1.88 ± 0.29** | |
| Fr 1D | 9 | ≥2.5 ± 0.87* | ≥2.67 ± 0.29** | 1.33 ± 0.29* | 1.5 ± 0.5* |
| 10 | ≥2.25 ± 0.29*** | ≥1.83 ± 0.58* | −0.17 ± 0.29 | N.T. | |
| 11 | ≥2.33 ± 0.58* | ≥2.17 ± 0.58* | 0 ± 0 | N.T. | |
| 12 | ≥2.33 ± 0.58* | 0.83 ± 0.58 | N.T. | N.T. | |
| 13 | ≥2.67 ± 0.58* | ≥2.38 ± 1.03* | −0.17 ± 0.29 | N.T. | |
| 14 | 0.83 ± 0.29* | N.T. | N.T. | N.T. | |
| 15 | ≥2.5 ± 0.5* | 1 ± 1.32 | N.T. | N.T. | |
| 16 | ≥2.33 ± 0.29** | ≥1.75 ± 0.87* | −0.17 ± 0.29 | N.T. | |
| 17 | ≥2.38 ± 0.63** | 0 ± 0 | N.T. | N.T. | |
| Fr 1E | 18 | ≥2.25 ± 0.65** | 0.5 ± 0.71 | N.T. | N.T. |
| 19 | 0.67 ± 1.16 | N.T. | N.T. | N.T. | |
| 20 | ≥2.83 ± 0.58* | ≥1.8 ± 1.3* | −0.17 ± 0.29 | N.T. | |
| 21 | ≥2.25 ± 0.96* | 0.17 ± 0.29 | N.T. | N.T. | |
| 22 | ≥2.17 ± 0.29** | 1.5 ± 0*** | −0.67 ± 0.29 | N.T. | |
| 23 | ≥1.8 ± 1.3* | ≥1.9 ± 0.25* | −0.5 ± 0.5 | N.T. | |
| 24 | ≥2.67 ± 0.58* | ≥1.7 ± 0.98* | −0.67 ± 0.29 | N.T. | |
| 25 | ≥2.67 ± 0.58* | 1.13 ± 1.11 | N.T. | N.T. | |
| 26 | ≥1.83 ± 0.29** | 0.75 ± 0.65 | N.T. | N.T. | |
| 27 | 0.83 ± 0.76 | N.T. | N.T. | N.T. | |
| 28 | ≥1.5 ± 0.71* | 1.67 ± 0.29* | 1.83 ± 0.58* | 1.33 ± 0.29** | |
| 29 | ≥2.33 ± 0.58* | ≥1.9 ± 1.14* | 0.67 ± 0.29 | N.T. | |
| C Target: MNV, concentration of sample: 100 µg/mL. | |||||
| Origin | No. | [DMSO control #) - Sample #)] ± SD | |||
| Reaction time:48 hrs | 6 hrs | 10 min | |||
| Fr 1C | 1 | −0.16 ± 0.58 | N.T. | N.T. | |
| 2 | 1.4 ± 0.55** | 1.25 ± 0.5* | −0.17 ± 0.29 | ||
| 3 | 0.5 ± 0 | N.T. | N.T. | ||
| 4 | 1.1 ± 0.23*** | 1.5 ± 0.41** | 0 ± 0.5 | ||
| 5 | 0 ± 0.5 | N.T. | N.T. | ||
| 6 | 0 ± 0 | N.T. | N.T. | ||
| 7 | 0.33 ± 0.29 | N.T. | N.T. | ||
| 8 | 0.17 ± 0.29 | N.T. | N.T. | ||
| Fr 1D | 9 | 0.33 ± 0.58 | N.T. | N.T. | |
| 10 | 0.33 ± 0.29 | N.T. | N.T. | ||
| 11 | 0 ± 0.5 | N.T. | N.T. | ||
| 12 | −0.63 ± 0.48 | N.T. | N.T. | ||
| 13 | −0.33 ± 0.29 | N.T. | N.T. | ||
| 14 | −0.63 ± 0.48 | N.T. | N.T. | ||
| 15 | −0.63 ± 0.29 | N.T. | N.T. | ||
| 16 | −0.33 ± 0.29 | N.T. | N.T. | ||
| 17 | 0.17 ± 0.29 | N.T. | N.T. | ||
| Fr 1E | 18 | −0.16 ± 0.58 | N.T. | N.T. | |
| 19 | 0 ± 0.41 | N.T. | N.T. | ||
| 20 | 0 ± 0 | N.T. | N.T. | ||
| 21 | 0 ± 0.5 | N.T. | N.T. | ||
| 22 | −0.33 ± 0.29 | N.T. | N.T. | ||
| 23 | −0.5 ± 0.5 | N.T. | N.T. | ||
| 24 | −0.33 ± 0.29 | N.T. | N.T. | ||
| 25 | −0.33 ± 0.29 | N.T. | N.T. | ||
| 26 | −0.33 ± 0.29 | N.T. | N.T. | ||
| 27 | 0 ± 0 | N.T. | N.T. | ||
| 28 | −0.16 ± 0.29 | N.T. | N.T. | ||
| 29 | −0.67 ± 0.29 | N.T. | N.T. | ||
| Extract and Fraction Name | [DMSO control #) - Sample #)] ± SD | ||
|---|---|---|---|
| Reaction time: 1 min | 30 sec | 10 sec | |
| Aqueous | ≥2.25 ± 0.29*** | N.T. | N.T. |
| Fr 1A | 0 ± 0 | N.T. | N.T. |
| Fr 1B | ≥2.17 ± 0.58* | N.T. | N.T. |
| Fr 1C | ≥2.5 ± 0.71** | ≥2.17 ± 0.41*** | ≥2.33 ± 0.41*** |
| Fr 1D | ≥2.25 ± 0.65** | N.T. | N.T. |
| Fr 1E | 2 ± 0.41** | N.T. | N.T. |
| Fr 1F | 0.5 ± 0.71 | N.T. | N.T. |
| Origin | No | [DMSO Control #) - Sample #)] ± SD | Origin | No | [DMSO Control #) - Sample #)] ± SD | Origin | No | [DMSO Control #) - Sample #)] ± SD |
|---|---|---|---|---|---|---|---|---|
| Reaction time: 1 min | Reaction time: 1 min | Reaction time: 1 min | ||||||
| Fr 1C | 1 | 0.5 ± 0.5 | Fr 1D | 9 | 0.63 ± 0.48 | Fr 1E | 18 | 0.17 ± 0.29 |
| 2 | 1.7 ± 0.2*** | 10 | 0.17 ± 0.29 | 19 | 0.33 ± 0.58 | |||
| 3 | 0.13 ± 0.63 | 11 | 0 ± 0.5 | 20 | 0.33 ± 0.29 | |||
| 4 | 1 ± 0.71* | 12 | 0 ± 0.5 | 21 | 0.33 ± 0.29 | |||
| 5 | −0.33 ± 0.58 | 13 | 0 ± 0.87 | 22 | 0.17 ± 0.29 | |||
| 6 | 0.17 ± 0.29 | 14 | −0.17 ± 0.29 | 23 | 0 ± 0.5 | |||
| 7 | 0.38 ± 0.48 | 15 | −0.33 ± 0.58 | 24 | −0.33 ± 0.29 | |||
| 8 | 0.5 ± 0.41 | 16 | −0.33 ± 0.58 | 25 | −0.17 ± 0.29 | |||
| 17 | 0 ± 0 | 26 | −0.5 ± 0.5 | |||||
| 27 | 0 ± 0.5 | |||||||
| 28 | 0.25 ± 0.29 | |||||||
| 29 | 0.33 ± 0.58 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Takeda, Y.; Murata, T.; Jamsransuren, D.; Suganuma, K.; Kazami, Y.; Batkhuu, J.; Badral, D.; Ogawa, H. Saxifraga spinulosa-Derived Components Rapidly Inactivate Multiple Viruses Including SARS-CoV-2. Viruses 2020, 12, 699. https://doi.org/10.3390/v12070699
Takeda Y, Murata T, Jamsransuren D, Suganuma K, Kazami Y, Batkhuu J, Badral D, Ogawa H. Saxifraga spinulosa-Derived Components Rapidly Inactivate Multiple Viruses Including SARS-CoV-2. Viruses. 2020; 12(7):699. https://doi.org/10.3390/v12070699
Chicago/Turabian StyleTakeda, Yohei, Toshihiro Murata, Dulamjav Jamsransuren, Keisuke Suganuma, Yuta Kazami, Javzan Batkhuu, Duger Badral, and Haruko Ogawa. 2020. "Saxifraga spinulosa-Derived Components Rapidly Inactivate Multiple Viruses Including SARS-CoV-2" Viruses 12, no. 7: 699. https://doi.org/10.3390/v12070699
APA StyleTakeda, Y., Murata, T., Jamsransuren, D., Suganuma, K., Kazami, Y., Batkhuu, J., Badral, D., & Ogawa, H. (2020). Saxifraga spinulosa-Derived Components Rapidly Inactivate Multiple Viruses Including SARS-CoV-2. Viruses, 12(7), 699. https://doi.org/10.3390/v12070699





