External Quality Assessment Program for Next-Generation Sequencing-Based HIV Drug Resistance Testing: Logistical Considerations
Abstract
1. Introduction
2. EQA for Sanger Sequencing-Based HIVDR Testing
3. EQA, a Challenging but Essential Component in the Generalized Implementation of Next Generation Sequencing HIVDR Testing
4. EQA Strategies for NGS HIVDR Assays: Logistic Challenges and Considerations
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sciacovelli, L.; Secchiero, S.; Zardo, L.; Zaninotto, M.; Plebani, M. External Quality Assessment: An effective tool for Clinical Governance in laboratory medicine. Clin. Chem. Lab. Med. 2006, 44, 740–749. [Google Scholar] [CrossRef] [PubMed]
- WHO. Laboratory Quality Management System Training Toolkit; WHO Lyon Office: Lyon, France, 2005.
- Schneider, F.; Maurer, C.; Friedberg, R.C. International Organization for Standardization (ISO) 15189. Ann. Lab. Med. 2017, 37, 365–370. [Google Scholar] [CrossRef] [PubMed]
- CLSI. Harmonized Terminology Database. 2019. Available online: https://clsi.org/ (accessed on 20 April 2020).
- ISO/IEC. ISO/IEC Guideline 43-1: Proficiency Testing by Interlaboratory Comparisons-Part 1: Development and Operation of Proficiency Testing Schemes; ISO/IEC: Genève, Switzerland, 1997. [Google Scholar]
- Miller, W.G.; Jones, G.R.; Horowitz, G.L.; Weykamp, C. Proficiency testing/external quality assessment: Current challenges and future directions. Clin. Chem. 2011, 57, 1670–1680. [Google Scholar] [CrossRef]
- Clutter, D.S.; Jordan, M.R.; Bertagnolio, S.; Shafer, R.W. HIV-1 drug resistance and resistance testing. Infect. Genet. Evol. 2016, 46, 292–307. [Google Scholar] [CrossRef]
- Parkin, N.; Bremer, J.; Bertagnolio, S. Genotyping external quality assurance in the World Health Organization HIV drug resistance laboratory network during 2007–2010. Clin. Infect. Dis. 2012, 54 (Suppl. S4), S266–S272. [Google Scholar] [CrossRef]
- Pandit, A.; Mackay, W.G.; Steel, C.; van Loon, A.M.; Schuurman, R. HIV-1 drug resistance genotyping quality assessment: Results of the ENVA7 Genotyping Proficiency Programme. J. Clin. Virol. 2008, 43, 401–406. [Google Scholar] [CrossRef]
- Saeng-Aroon, S.; Saipradit, N.; Loket, R.; Klamkhai, N.; Boonmuang, R.; Kaewprommal, P.; Prommajan, K.; Takeda, N.; Sungkanuparph, S.; Shioda, T.; et al. External Quality Assessment Scheme for HIV-1 Drug-Resistance Genotyping in Thailand. AIDS Res. Hum. Retrovir. 2018, 34, 1028–1035. [Google Scholar] [CrossRef]
- Yoshida, S.; Hattori, J.; Matsuda, M.; Okada, K.; Kazuyama, Y.; Hashimoto, O.; Ibe, S.; Fujisawa, S.; Chiba, H.; Tatsumi, M.; et al. Japanese external quality assessment program to standardize HIV-1 drug-resistance testing (JEQS2010 program) using in vitro transcribed RNA as reference material. AIDS Res. Hum. Retrovir. 2015, 31, 318–325. [Google Scholar] [CrossRef]
- Land, S.; Cunningham, P.; Zhou, J.; Frost, K.; Katzenstein, D.; Kantor, R.; Chen, Y.M.; Oka, S.; DeLong, A.; Sayer, D.; et al. TREAT Asia Quality Assessment Scheme (TAQAS) to standardize the outcome of HIV genotypic resistance testing in a group of Asian laboratories. J. Virol. Methods 2009, 159, 185–193. [Google Scholar] [CrossRef]
- Huang, D.D.; Bremer, J.W.; Brambilla, D.J.; Palumbo, P.E.; Aldrovandi, G.; Eshleman, S.; Brown, C.; Fiscus, S.; Frenkel, L.; Hamdan, H.; et al. Model for assessment of proficiency of human immunodeficiency virus type 1 sequencing-based genotypic antiretroviral assays. J. Clin. Microbiol. 2005, 43, 3963–3970. [Google Scholar] [CrossRef]
- WHO. WHO/HIVResNet HIV Drug Resistance Laboratory Operational Framework; World Health Organization: Geneva, Switzerland, 2017.
- Johnson, J.A.; Li, J.F.; Wei, X.; Lipscomb, J.; Irlbeck, D.; Craig, C.; Smith, A.; Bennett, D.E.; Monsour, M.; Sandstrom, P.; et al. Minority HIV-1 drug resistance mutations are present in antiretroviral treatment-naive populations and associate with reduced treatment efficacy. PLoS Med. 2008, 5, e158. [Google Scholar] [CrossRef] [PubMed]
- Korn, K.; Reil, H.; Walter, H.; Schmidt, B. Quality control trial for human immunodeficiency virus type 1 drug resistance testing using clinical samples reveals problems with detecting minority species and interpretation of test results. J. Clin. Microbiol. 2003, 41, 3559–3565. [Google Scholar] [CrossRef] [PubMed]
- Simen, B.B.; Simons, J.F.; Hullsiek, K.H.; Novak, R.M.; Macarthur, R.D.; Baxter, J.D.; Huang, C.; Lubeski, C.; Turenchalk, G.S.; Braverman, M.S.; et al. Low-abundance drug-resistant viral variants in chronically HIV-infected, antiretroviral treatment-naive patients significantly impact treatment outcomes. J. Infect. Dis. 2009, 199, 693–701. [Google Scholar] [CrossRef] [PubMed]
- Ji, H.; Li, Y.; Graham, M.; Liang, B.B.; Pilon, R.; Tyson, S.; Peters, G.; Tyler, S.; Merks, H.; Bertagnolio, S.; et al. Next-generation sequencing of dried blood spot specimens: A novel approach to HIV drug-resistance surveillance. Antivir. Ther. 2011, 16, 871–878. [Google Scholar] [CrossRef]
- Lapointe, H.R.; Dong, W.; Lee, G.Q.; Bangsberg, D.R.; Martin, J.N.; Mocello, A.R.; Boum, Y.; Karakas, A.; Kirkby, D.; Poon, A.F.; et al. HIV drug resistance testing by high-multiplex “wide” sequencing on the MiSeq instrument. Antimicrob. Agents Chemother. 2015, 59, 6824–6833. [Google Scholar] [CrossRef]
- Inzaule, S.C.; Ondoa, P.; Peter, T.; Mugyenyi, P.N.; Stevens, W.S.; de Wit, T.F.R.; Hamers, R.L. Affordable HIV drug-resistance testing for monitoring of antiretroviral therapy in sub-Saharan Africa. Lancet Infect. Dis. 2016, 16, e267–e275. [Google Scholar] [CrossRef]
- Lee, E.R.; Gao, F.; Sandstrom, P.; Ji, H. External quality assessment for next-generation sequencing-based HIV drug resistance testing: Unique requirements and challenges. Viruses 2020, 12, 550. [Google Scholar]
- Lee, E.R.; Parkin, N.; Jennings, C.; Brumme, C.J.; Enns, E.; Casadella, M.; Howison, M.; Coetzer, M.; Avila-Rios, S.; Capina, R.; et al. Performance comparison of next generation sequencing analysis pipelines for HIV-1 drug resistance testing. Sci. Rep. 2020, 10, 1634. [Google Scholar] [CrossRef]
- Lee, E.R.; Enns, E.; Parkin, N.; Brumme, C.J.; Casadella, M.; Howison, M.; Avila Rios, S.; Jennings, R.; Capina, R.; Marinier, E.; et al. Characterization and data assessment of next generation sequencing-based genotyping using existing HIV-1 drug resistance proficiency panels. In Proceedings of the XXVII International HIV Drug Resistance and Treatment Strategies Workshop, Johannesburg, South Africa, 22–23 October 2018. [Google Scholar]
- VQA. VQA HIV Gene Sequencing Proficiency Testing Scoring Criteria and Policies. 2014. Available online: https://www.hanc.info/labs/labresources/vqaResources/Pages/default.aspx (accessed on 20 April 2020).
- Noguera-Julian, M.; Lee, E.R.; Travers, S.; Shafer, R.W.; Kantor, R.; Ji, H. Dry panel for next generation sequencing-based HIV DRT EQA. Viruses 2020. submitted. [Google Scholar]
- Vandamme, A.M.; Camacho, R.J.; Ceccherini-Silberstein, F.; De, L.A.; Palmisano, L.; Paraskevis, D.; Paredes, R.; Poljak, M.; Schmit, J.C.; Soriano, V.; et al. European recommendations for the clinical use of HIV drug resistance testing: 2011 update. AIDS Rev. 2011, 13, 77–108. [Google Scholar]
- Ji, H.; Enns, E.; Brumme, C.J.; Parkin, N.; Howison, M.; Lee, E.R.; Capina, R.; Marinier, E.; Avila-Rios, S.; Sandstrom, P.; et al. Bioinformatic data processing pipelines in support of next-generation sequencing-based HIV drug resistance testing: The Winnipeg Consensus. J. Int. AIDS Soc. 2018, 21, e25193. [Google Scholar] [CrossRef] [PubMed]
- Wensing, A.M.; Calvez, V.; Gunthard, H.F.; Johnson, V.A.; Paredes, R.; Pillay, D.; Shafer, R.W.; Richman, D.D. 2017 Update of the Drug Resistance Mutations in HIV-1. Top. Antivir. Med. 2016, 24, 132–133. [Google Scholar] [PubMed]
- Parkin, N.; Zaccaro, D.; Avila-Rios, S.; Brumme, C.; Hunt, G.; Ji, H.; Kantor, R.; Mbisa, J.L.; Predes, R.; Rivera-Amill, V.; et al. Multi-Laboratory comparison of next-generation to Sanger-based sequencing for HIV-1 drug resistance genotyping. In Proceedings of the XXVII International HIV Drug Resistance and Treatment Strategies Workshop, Johannesburg, South Africa, 22–23 October 2018. [Google Scholar]
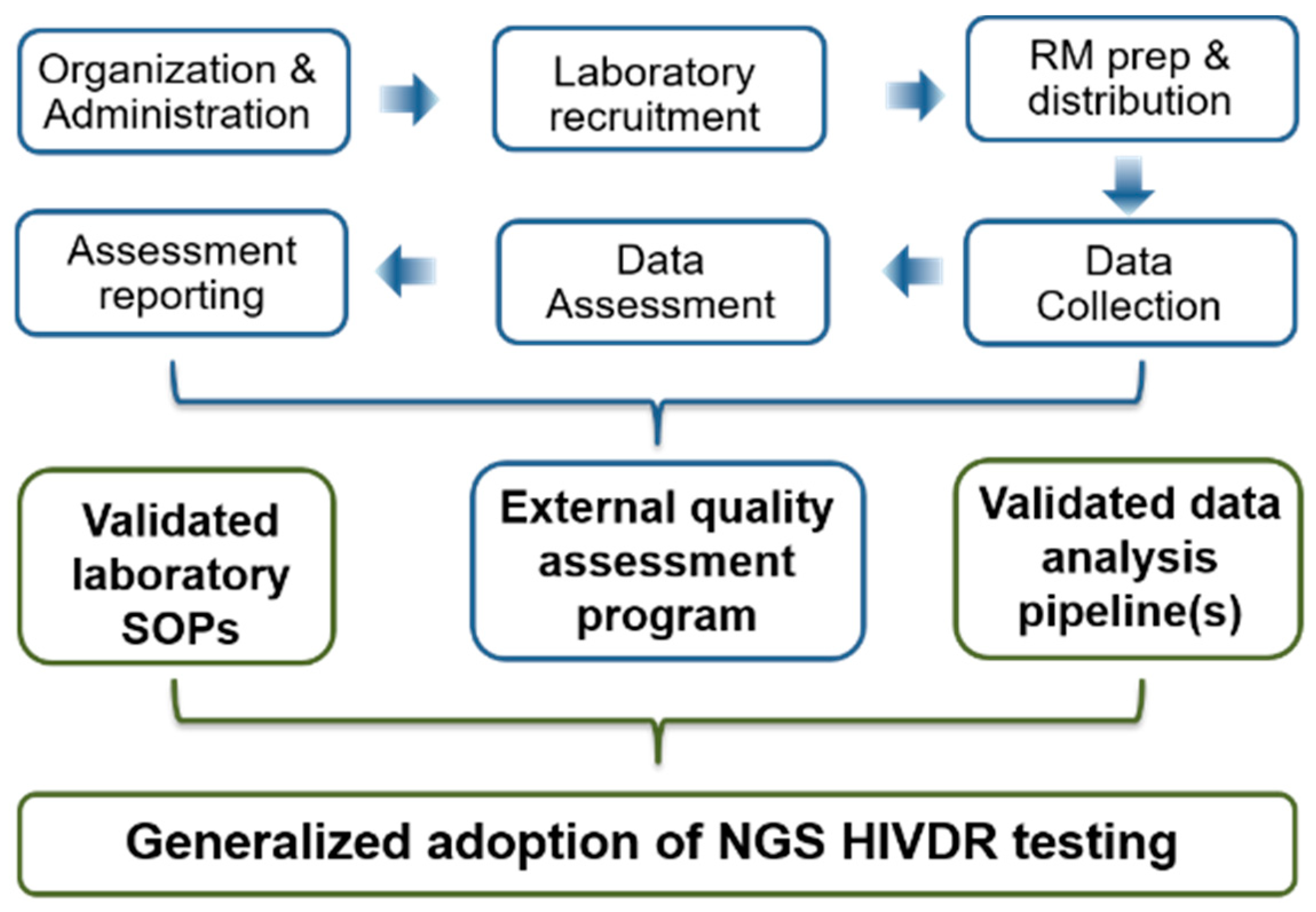
| EQA Tasks | Logistical Issues | Sanger Experiences (VQA as an Example) | NGS HIVDR EQA Considerations and Recommendations |
|---|---|---|---|
| Organization and Administration | Who organizes/operates? |
|
|
| Who participates? |
|
| |
| Who funds? |
|
| |
| Laboratory Recruitment | Recruitment strategies |
|
|
| Basic infrastructure requirements |
|
| |
| Sample processing capacity requirement |
|
| |
| Bioinformatics capacity requirement |
|
| |
| Reference Materials prep and distribution: Wet Panels [21] | Panel design | VQA panels contain five specimens, designed with the following factors:
|
|
| Panel specimen types |
|
* Initial VP panels may focus on plasma specimens at viral loads of ≥1,000 copies/mL. | |
| Panel characterization strategies |
|
| |
| Panel size |
|
| |
| Panel distribution |
|
| |
| HIV gene targets |
|
| |
| HIV DRM MRV frequency range |
|
| |
| Different VLs/mutation loads |
|
| |
| Reference Materials prep and distribution: Dry Panels [25] | Genuine raw sequencing data |
|
|
| Synthetic/in silico datasets |
| Such data may complement datasets derived from real specimens for covering:
| |
| Panel size |
|
| |
| Data access |
|
| |
| Potential application |
| Such panels involve no sample processing in the laboratory and may serve the needs for
| |
| Data Collection | Data submission requirement |
|
|
| Consensus sequence |
|
| |
| HIV DRM / Variation reports |
|
| |
| Raw sequencing data |
|
| |
| Information on the protocols applied |
|
| |
| Data collection approach |
|
| |
| Data Assessment | Guidelines/SOPs |
|
|
| Data assessment parameters [24] |
|
| |
| Scoring strategies |
|
| |
| Assessment Reporting | Files & contents | Report files:
|
|
| Assessment, data distribution and retention/archival |
|
| |
| Other Challenges | Incentives for participation |
|
|
| Program sustainability |
|
| |
| Strategies to facilitate SS to NGS transitioning |
|
|
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ji, H.; Parkin, N.; Gao, F.; Denny, T.; Jennings, C.; Sandstrom, P.; Kantor, R. External Quality Assessment Program for Next-Generation Sequencing-Based HIV Drug Resistance Testing: Logistical Considerations. Viruses 2020, 12, 556. https://doi.org/10.3390/v12050556
Ji H, Parkin N, Gao F, Denny T, Jennings C, Sandstrom P, Kantor R. External Quality Assessment Program for Next-Generation Sequencing-Based HIV Drug Resistance Testing: Logistical Considerations. Viruses. 2020; 12(5):556. https://doi.org/10.3390/v12050556
Chicago/Turabian StyleJi, Hezhao, Neil Parkin, Feng Gao, Thomas Denny, Cheryl Jennings, Paul Sandstrom, and Rami Kantor. 2020. "External Quality Assessment Program for Next-Generation Sequencing-Based HIV Drug Resistance Testing: Logistical Considerations" Viruses 12, no. 5: 556. https://doi.org/10.3390/v12050556
APA StyleJi, H., Parkin, N., Gao, F., Denny, T., Jennings, C., Sandstrom, P., & Kantor, R. (2020). External Quality Assessment Program for Next-Generation Sequencing-Based HIV Drug Resistance Testing: Logistical Considerations. Viruses, 12(5), 556. https://doi.org/10.3390/v12050556






