Merkel Cell Polyomavirus (MCPyV) in the Context of Immunosuppression: Genetic Analysis of Noncoding Control Region (NCCR) Variability among a HIV-1-Positive Population
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. MCPyV DNA Extraction and Quantitative Real-Time Polymerase Chain Reaction (qPCR)
2.3. MCPyV Nested PCR
2.4. NCCR Alignment and Analysis of Putative Binding Sites
2.5. Statistical Analysis
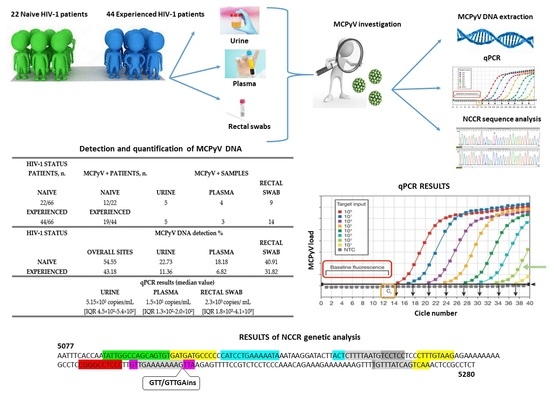
3. Results
3.1. MCPyV DNA Detection and Quantification by Real-Time qPCR Analysis
3.2. Genetic Analysis of NCCR
3.3. Analysis of Putative Binding Sites in NCCR Sequences
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Barth, H.; Solis, M.; Kack-Kack, W.; Soulier, E.; Velay, A.; Fafi-Kremer, S. In Vitro and in Vivo models for the study of Human Polyomavirus infection. Viruses 2016, 8, 292. [Google Scholar] [CrossRef] [PubMed]
- Feng, H.; Shuda, M.; Chang, Y.; Moore, P.S. Clonal integration of a polyomavirus in human Merkel cell carcinoma. Science 2008, 319, 1096–1100. [Google Scholar] [CrossRef] [PubMed]
- Tolstov, Y.L.; Pastrana, D.V.; Feng, H.; Becker, J.C.; Jenkins, F.J.; Moschos, S.; Chang, Y.; Buck, C.B.; Moore, P.S. Human Merkel cell polyomavirus infection II: MCV is a common human infection that can be detected by conformational capsid epitope immunoassays. Int. J. Cancer 2009, 125, 1250–1260. [Google Scholar] [CrossRef] [PubMed]
- Prezioso, C.; Di Lella, F.M.; Rodio, D.M.; Bitossi, C.; Trancassini, M.; Mele, A.; de Vito, C.; Antonelli, G.; Pietropaolo, V. Merkel cell Polyomavirus DNA detection in respiratory samples: Study of a cohort of patients affected by cystic fibrosis. Viruses 2019, 11, 571. [Google Scholar] [CrossRef] [PubMed]
- Zanotta, N.; Delbue, S.; Signorini, L.; Villani, S.; D’Alessandro, S.; Campisciano, G.; Colli, C.; De Seta, F.; Ferrante, P.; Comar, M. Merkel Cell Polyomavirus Is Associated with Anal Infections in Men Who Have Sex with Men. Microorganisms 2019, 7, 54. [Google Scholar] [CrossRef] [PubMed]
- Babakir-Mina, M.; Ciccozzi, M.; Lo Presti, A.; Greco, F.; Perno, C.F.; Ciotti, M. Identification of Merkel Cell Polyomavirus in the lower respiratory tract of Italian patients. J. Med. Virol. 2010, 82, 505–509. [Google Scholar] [CrossRef]
- Iaria, M.; Caccuri, F.; Apostoli, P.; Giagulli, C.; Pelucchi, F.; Padoan, R.F.; Caruso, A.; Fiorentini, S. Detection of KI WU and Merkel cell polyomavirus in respiratory tract of cystic fibrosis patients. Clin. Microbiol. Infect. 2015, 21, e9–e15. [Google Scholar] [CrossRef][Green Version]
- Prezioso, C.; Ciotti, M.; Obregon, F.; Ambroselli, D.; Rodio, D.M.; Cudillo, L.; Gaziev, J.; Mele, A.; Nardi, A.; Favalli, C.; et al. Polyomaviruses shedding in stool of patients with hematological disorders: Detection analysis and study of the non-coding control region’s genetic variability. Med. Microbiol. Immunol. 2019, 208, 845–854. [Google Scholar] [CrossRef]
- Schowalter, R.M.; Buck, C.B. The Merkel cell polyomavirus minor capsid protein. PLoS Pathog. 2013, 9, e1003558. [Google Scholar] [CrossRef]
- Delbue, S.; Franciotta, D.; Giannella, S.; Dolci, M.; Signorini, L.; Ticozzi, R.; D’Alessandro, S.; Campisciano, G.; Comar, M.; Ferrante, P.; et al. Human Polyomaviruses in the cerebrospinal fluid of neurological patients. Microorganisms 2019, 8, 16. [Google Scholar] [CrossRef]
- Ciotti, M.; Prezioso, C.; Pietropaolo, V. An overview on human polyomaviruses biology and related diseases. Future Virol. 2019, 14, 487–501. [Google Scholar] [CrossRef]
- An, P.; Sa’enz Robles, M.T.; Pipas, J.M. Large T antigens of polyomaviruses: Amazing molecular machines. Annu. Rev. Microbiol. 2012, 66, 213–236. [Google Scholar] [CrossRef] [PubMed]
- Hashida, Y.; Higuchi, T.; Matsui, K.; Shibata, Y.; Nakajima, K.; Sano, S.; Daibata, M. Genetic variability of the noncoding control region of cutaneous Merkel cell polyomavirus: Identification of geographically related genotypes. J. Infect. Dis. 2018, 217, 1601–1611. [Google Scholar] [CrossRef] [PubMed]
- Moens, U.; Johansen, T.; Johnsen, J.I.; Seternes, O.M.; Traavik, T. Noncoding control region of naturally occurring BK virus variants: Sequence comparison and functional analysis. Virus Genes 1995, 10, 261–275. [Google Scholar] [CrossRef]
- White, M.K.; Safak, M.; Khalili, K. Regulation of gene expression in primate polyomaviruses. J. Virol. 2009, 83, 10846–10856. [Google Scholar] [CrossRef]
- Helle, F.; Brochot, E.; Handala, L.; Martin, E.; Castelain, S.; Francois, C.; Duverlie, G. Biology of the BKPyV: An update. Viruses 2017, 9, 327. [Google Scholar] [CrossRef]
- Pietropaolo, V.; Prezioso, C.; Bagnato, F.; Antonelli, G. John Cunningham virus: An overview on biology and disease of the etiological agent of the progressive multifocal leukoencephalopathy. New Microbiol. 2018, 41, 179–186. [Google Scholar]
- Rubinstein, R.; Schoonakker, B.C.; Harley, E.H. Recurring theme of changes in the transcriptional control region of BK virus during adaptation to cell culture. J. Virol. 1991, 65, 1600–1604. [Google Scholar] [CrossRef]
- Johnsen, J.I.; Seternes, O.M.; Johansen, T.; Moens, U.; Mantyjarvi, R.; Traavik, T. Subpopulations of non-coding control region variants within a cell culture-passaged stock of BK virus: Sequence comparisons and biological characteristics. J. Gen. Virol. 1995, 76, 1571–1581. [Google Scholar] [CrossRef]
- Prezioso, C.; Scribano, D.; Bellizzi, A.; Anzivino, E.; Rodio, D.M.; Trancassini, M.; Palamara, A.T.; Pietropaolo, V. Efficient propagation of archetype JC polyomavirus in COS-7 cells: Evaluation of rearrangements within the NCCR structural organization after transfection. Arch. Virol. 2017, 162, 3745–3752. [Google Scholar] [CrossRef]
- Prezioso, C.; Scribano, D.; Rodio, D.M.; Ambrosi, C.; Trancassini, M.; Palamara, A.T.; Pietropaolo, V. COS-7-based model: Methodological approach to study John Cunningham virus replication cycle. Virol. J. 2018, 15, 29. [Google Scholar] [CrossRef] [PubMed]
- Gosert, R.; Rinaldo, C.H.; Funk, G.A.; Egli, A.; Ramos, E.; Drachenberg, C.B.; Hirsch, H.H. Polyomavirus BK with rearranged noncoding control region emerge in vivo in renal transplant patients and increase viral replication and cytopathology. J. Exp. Med. 2008, 205, 841–852. [Google Scholar] [CrossRef] [PubMed]
- Olsen, G.H.; Hirsch, H.H.; Rinaldo, C.H. Functional analysis of polyomavirus BK non-coding control region quasispecies from kidney transplant recipients. J. Med. Virol. 2009, 81, 1959–1967. [Google Scholar] [CrossRef] [PubMed]
- Gosert, R.; Kardas, P.; Major, E.O.; Hirsch, H.H. Rearranged JC virus noncoding control regions found in progressive multifocal leukoencephalopathy patient samples increase virus early gene expression and replication rate. J. Virol. 2010, 84, 10448–10456. [Google Scholar] [CrossRef]
- Saiki, R.K.; Bugawan, T.L.; Horn, G.T.; Mullis, K.B.; Erlich, H.A. Analysis of enzymatically amplified beta-globin and HLA-DQ alpha DNA with allele-specific oligonucleotide probes. Nature 1986, 324, 163–166. [Google Scholar] [CrossRef]
- Imajoh, M.; Hashida, Y.; Nemoto, Y.; Oguri, H.; Maeda, N.; Furihata, M.; Fukaya, T.; Daibata, M. Detection of Merkel cell polyomavirus in cervical squamous cell carcinomas and adenocarcinomas from Japanese patients. Virol. J. 2012, 9, 154. [Google Scholar] [CrossRef]
- Hashida, Y.; Imajoh, M.; Nemoto, Y.; Kamioka, M.; Taniguchi, A.; Taguchi, T.; Kume, M.; Orihashi, K.; Daibata, M. Detection of Merkel cell polyomavirus with a tumour-specific signature in non-small cell lung cancer. Br. J. Cancer 2013, 108, 629–637. [Google Scholar] [CrossRef]
- ClustalW2-Multiple Sequence Alignment. Available online: http://www.ebi.ac.uk/clustalw/ (accessed on 17 March 2020).
- TFBIND. Available online: http://tfbind.hgc.jp/ (accessed on 17 March 2020).
- Raj, G.V.; Khalili, K. Transcriptional regulation: Lessons from the human neurotropic polyomavirus, JCV. Virology 1995, 213, 283–291. [Google Scholar] [CrossRef]
- Engels, E.A.; Frisch, M.; Goedert, J.J.; Biggar, R.J.; Miller, R.W. Merkel cell carcinoma and HIV infection. Lancet 2002, 359, 497–498. [Google Scholar] [CrossRef]
- Clarke, C.A.; Robbins, H.A.; Tatalovich, Z.; Lynch, C.F.; Pawlish, K.S.; Finch, J.L.; Hernandez, B.Y.; Fraumeni, J.F., Jr.; Madeleine, M.M.; Engels, E.A. Risk of Merkel cell carcinoma after solid organ transplantation. J. Natl. Cancer Inst. 2015, 107, dju382. [Google Scholar] [CrossRef]
- Rotondo, J.C.; Bononi, I.; Puozzo, A.; Govoni, M.; Foschi, V.; Lanza, G.; Gafà, R.; Gaboriaud, P.; Touzé, F.A.; Selvatici, R.; et al. Merkel cell carcinomas arising in autoimmune disease affected patients treated with biologic drugs, including anti-TNF. Clin. Cancer Res. 2017, 23, 3929–3934. [Google Scholar] [CrossRef]
- McIlroy, D.; Halary, F.; Bressollette-Bodin, C. Intra-patient viral evolution in polyomavirus-related diseases. Phil. Trans. R. Soc. B 2019, 374, 20180301. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, R.H.; DeCaprio, J.A. Merkel Cell Carcinoma in the HIV-1/AIDS Patient. Cancer Treat Res. 2019, 177, 211–229. [Google Scholar] [PubMed]
- Vahabpour, R.; Nasimi, M.; Naderi, N.; Salehi-Vaziri, M.; Mohajel, N.; Sadeghi, F.; Keyvani, H.; Monavari, S.H. Merkel cell polyomavirus IgG antibody levels are associated with progression to AIDS among HIV-infected individuals. Arch. Virol. 2017, 162, 963–969. [Google Scholar] [CrossRef] [PubMed]
- Fukumoto, H.; Sato, Y.; Hasegawa, H.; Katano, H. Frequent detection of Merkel cell polyomavirus DNA in sera of HIV-1-positive patients. Virol. J. 2013, 10, 84. [Google Scholar] [CrossRef]
- Torres, C.; Barrios, M.E.; Cammarata, R.V.; Cisterna, D.M.; Estrada, T.; Martini Novas, S.; Cahn, P.; Blanco Fernández, M.D.; Mbayed, V.A. High diversity of human polyomaviruses in environmental and clinical samples in Argentina: Detection of JC, BK, Merkel-cell, Malawi, and human 6 and 7 polyomaviruses. Sci. Total Environ. 2016, 542, 192–202. [Google Scholar] [CrossRef]
- Wieland, U.; Mauch, C.; Kreuter, A.; Krieg, T.; Pfister, H. Merkel cell polyomavirus DNA in persons without Merkel cell carcinoma. Emerg. Infect. Dis. 2009, 15, 1496–1498. [Google Scholar] [CrossRef]
- Moens, U.; Krumbholz, A.; Ehlers, B.; Zell, R.; Johne, R.; Calvignac-Spencer, S.; Lauber, C. Biology, evolution, and medical importance of polyomaviruses: An update. Infect. Genet. Evol. 2017, 54, 18–38. [Google Scholar] [CrossRef]
- Peng, J.; Li, K.; Zhang, C.; Gao, L.; Jin, Q. Human papillomavirus and polyomavirus coinfections among Chinese men who have sex with men. J. Infect. 2016, 72, 118–120. [Google Scholar] [CrossRef]
- Vergori, A.; Garbuglia, A.R.; Piselli, P.; Del Nonno, F.; Sias, C.; Lupi, F.; Lapa, D.; Baiocchini, A.; Cimaglia, C.; Gentile, M.; et al. Oral human Papillomavirus DNA detection in HIV-positive men: Prevalence, predictors, and co-occurrence at anal site. BMC Infect. Dis. 2018, 18, 25. [Google Scholar] [CrossRef]
- Signorini, L.; Belingheri, M.; Ambrogi, F.; Pagani, E.; Binda, S.; Ticozzi, R.; Ferraresso, M.; Ghio, L.; Giacon, B.; Ferrante, P.; et al. High frequency of Merkel cell polyomavirus DNA in the urine of kidney transplant recipients and healthy controls. J. Clin. Virol. 2014, 61, 565–570. [Google Scholar] [CrossRef] [PubMed]
- Ajuh, E.T.; Wu, Z.; Kraus, E.; Weissbach, F.H.; Bethge, T.; Gosert, R.; Fischer, N.; Hirsch, H.H. Novel human Polyomavirus noncoding control regions differ in bidirectional gene expression according to host cell, Large T-antigen expression, and clinically occurring rearrangements. J. Virol. 2018, 92, e02231-17. [Google Scholar] [CrossRef] [PubMed]
- Markowitz, R.B.; Tolbert, S.; Dynan, W.S. Promoter evolution in BK virus: Functional elements are created at sequence junctions. J. Virol. 1990, 64, 2411–2415. [Google Scholar] [CrossRef] [PubMed]
- Safak, M.; Gallia, G.L.; Khalili, K. A 23-bp sequence element from human neurotropic JC virus is responsive to NF-kappa B subunits. Virology 1999, 262, 178–189. [Google Scholar] [CrossRef] [PubMed]
- Priftakis, P.; Bogdanovic, G.; Kokhaei, P.; Mellstedt, H.; Dalianis, T. BK virus (BKV) quantification in urine samples of bone marrow transplanted patients is helpful for diagnosis of hemorrhagic cystitis, although wide individual variations exist. J. Clin. Virol. 2003, 26, 71–77. [Google Scholar] [CrossRef]
 AP-1: 5104–5114 nucleotide position (5′-GATGATGCCCC-3′); 5164–5172 nucleotide position (5′-CTTTGTAAG-3′); 5257–5265 nucleotide position (5′-GTTTATCAG-3′); 5261–5269 nucleotide position (5′-ATCAGTCAA-3′);
AP-1: 5104–5114 nucleotide position (5′-GATGATGCCCC-3′); 5164–5172 nucleotide position (5′-CTTTGTAAG-3′); 5257–5265 nucleotide position (5′-GTTTATCAG-3′); 5261–5269 nucleotide position (5′-ATCAGTCAA-3′);  NF1: 5088–5105 nucleotide position (5′-TATTGGCCAGCAGTGTG-3′);
NF1: 5088–5105 nucleotide position (5′-TATTGGCCAGCAGTGTG-3′);  TST1: 5200–5214 nucleotide position (5′-GTTGAAAAAAAGTTA-3′);
TST1: 5200–5214 nucleotide position (5′-GTTGAAAAAAAGTTA-3′);  OCT1: 5116–5129 nucleotide position (5′-CATCCTGAAAAATA-3′); 5143–5146 nucleotide position (5′-ACTCTTTTAATG-3′);
OCT1: 5116–5129 nucleotide position (5′-CATCCTGAAAAATA-3′); 5143–5146 nucleotide position (5′-ACTCTTTTAATG-3′);  NFκB: 5188–5197 nucleotide position (5′-CGGGCCTCCC-3′);
NFκB: 5188–5197 nucleotide position (5′-CGGGCCTCCC-3′);  TATA-like sequences: 5146–5160 nucleotide position (5′-CTTTTAATGTCCTCC-3′); 5202–5212 nucleotide position (5′-TGAAAAAAAG-3′); 5256–5265 nucleotide position (5′-TGTTTATCAG-3′).
TATA-like sequences: 5146–5160 nucleotide position (5′-CTTTTAATGTCCTCC-3′); 5202–5212 nucleotide position (5′-TGAAAAAAAG-3′); 5256–5265 nucleotide position (5′-TGTTTATCAG-3′).
 AP-1: 5104–5114 nucleotide position (5′-GATGATGCCCC-3′); 5164–5172 nucleotide position (5′-CTTTGTAAG-3′); 5257–5265 nucleotide position (5′-GTTTATCAG-3′); 5261–5269 nucleotide position (5′-ATCAGTCAA-3′);
AP-1: 5104–5114 nucleotide position (5′-GATGATGCCCC-3′); 5164–5172 nucleotide position (5′-CTTTGTAAG-3′); 5257–5265 nucleotide position (5′-GTTTATCAG-3′); 5261–5269 nucleotide position (5′-ATCAGTCAA-3′);  NF1: 5088–5105 nucleotide position (5′-TATTGGCCAGCAGTGTG-3′);
NF1: 5088–5105 nucleotide position (5′-TATTGGCCAGCAGTGTG-3′);  TST1: 5200–5214 nucleotide position (5′-GTTGAAAAAAAGTTA-3′);
TST1: 5200–5214 nucleotide position (5′-GTTGAAAAAAAGTTA-3′);  OCT1: 5116–5129 nucleotide position (5′-CATCCTGAAAAATA-3′); 5143–5146 nucleotide position (5′-ACTCTTTTAATG-3′);
OCT1: 5116–5129 nucleotide position (5′-CATCCTGAAAAATA-3′); 5143–5146 nucleotide position (5′-ACTCTTTTAATG-3′);  NFκB: 5188–5197 nucleotide position (5′-CGGGCCTCCC-3′);
NFκB: 5188–5197 nucleotide position (5′-CGGGCCTCCC-3′);  TATA-like sequences: 5146–5160 nucleotide position (5′-CTTTTAATGTCCTCC-3′); 5202–5212 nucleotide position (5′-TGAAAAAAAG-3′); 5256–5265 nucleotide position (5′-TGTTTATCAG-3′).
TATA-like sequences: 5146–5160 nucleotide position (5′-CTTTTAATGTCCTCC-3′); 5202–5212 nucleotide position (5′-TGAAAAAAAG-3′); 5256–5265 nucleotide position (5′-TGTTTATCAG-3′).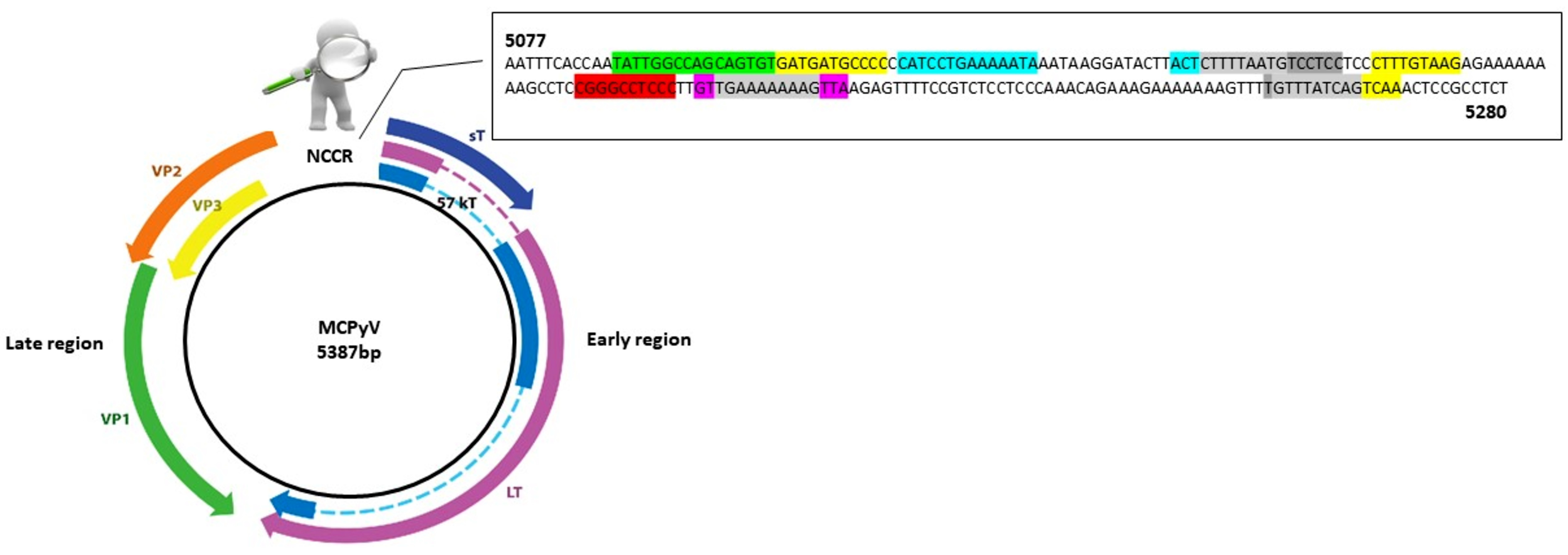
 AP-1: 5104–5114 nucleotide position (5′-GATGATGCCCC-3′); 5164–5172 nucleotide position (5′-CTTTGTAAG-3′); 5257–5265 nucleotide position (5′-GTTTATCAG-3′); 5261–5269 nucleotide position (5′-ATCAGTCAA-3′);
AP-1: 5104–5114 nucleotide position (5′-GATGATGCCCC-3′); 5164–5172 nucleotide position (5′-CTTTGTAAG-3′); 5257–5265 nucleotide position (5′-GTTTATCAG-3′); 5261–5269 nucleotide position (5′-ATCAGTCAA-3′);  NF1: 5088–5105 nucleotide position (5′-TATTGGCCAGCAGTGTG-3′);
NF1: 5088–5105 nucleotide position (5′-TATTGGCCAGCAGTGTG-3′);  TST1: 5200–5214 nucleotide position (5′-GTTGAAAAAAAGTTA-3′);
TST1: 5200–5214 nucleotide position (5′-GTTGAAAAAAAGTTA-3′);  OCT1: 5116–5129 nucleotide position (5′-CATCCTGAAAAATA-3′); 5143–5146 nucleotide position (5′-ACTCTTTTAATG-3′);
OCT1: 5116–5129 nucleotide position (5′-CATCCTGAAAAATA-3′); 5143–5146 nucleotide position (5′-ACTCTTTTAATG-3′);  NFκB: 5188–5197 nucleotide position (5′-CGGGCCTCCC-3′);
NFκB: 5188–5197 nucleotide position (5′-CGGGCCTCCC-3′);  TATA-like sequences: 5146–5160 nucleotide position (5′-CTTTTAATGTCCTCC-3′); 5202–5212 nucleotide position (5′-TGAAAAAAAG-3′); 5256–5265 nucleotide position (5′-TGTTTATCAG-3′); E: experienced HIV-1-positive patients; N: naive HIV-1-positive patients.
TATA-like sequences: 5146–5160 nucleotide position (5′-CTTTTAATGTCCTCC-3′); 5202–5212 nucleotide position (5′-TGAAAAAAAG-3′); 5256–5265 nucleotide position (5′-TGTTTATCAG-3′); E: experienced HIV-1-positive patients; N: naive HIV-1-positive patients.
 AP-1: 5104–5114 nucleotide position (5′-GATGATGCCCC-3′); 5164–5172 nucleotide position (5′-CTTTGTAAG-3′); 5257–5265 nucleotide position (5′-GTTTATCAG-3′); 5261–5269 nucleotide position (5′-ATCAGTCAA-3′);
AP-1: 5104–5114 nucleotide position (5′-GATGATGCCCC-3′); 5164–5172 nucleotide position (5′-CTTTGTAAG-3′); 5257–5265 nucleotide position (5′-GTTTATCAG-3′); 5261–5269 nucleotide position (5′-ATCAGTCAA-3′);  NF1: 5088–5105 nucleotide position (5′-TATTGGCCAGCAGTGTG-3′);
NF1: 5088–5105 nucleotide position (5′-TATTGGCCAGCAGTGTG-3′);  TST1: 5200–5214 nucleotide position (5′-GTTGAAAAAAAGTTA-3′);
TST1: 5200–5214 nucleotide position (5′-GTTGAAAAAAAGTTA-3′);  OCT1: 5116–5129 nucleotide position (5′-CATCCTGAAAAATA-3′); 5143–5146 nucleotide position (5′-ACTCTTTTAATG-3′);
OCT1: 5116–5129 nucleotide position (5′-CATCCTGAAAAATA-3′); 5143–5146 nucleotide position (5′-ACTCTTTTAATG-3′);  NFκB: 5188–5197 nucleotide position (5′-CGGGCCTCCC-3′);
NFκB: 5188–5197 nucleotide position (5′-CGGGCCTCCC-3′);  TATA-like sequences: 5146–5160 nucleotide position (5′-CTTTTAATGTCCTCC-3′); 5202–5212 nucleotide position (5′-TGAAAAAAAG-3′); 5256–5265 nucleotide position (5′-TGTTTATCAG-3′); E: experienced HIV-1-positive patients; N: naive HIV-1-positive patients.
TATA-like sequences: 5146–5160 nucleotide position (5′-CTTTTAATGTCCTCC-3′); 5202–5212 nucleotide position (5′-TGAAAAAAAG-3′); 5256–5265 nucleotide position (5′-TGTTTATCAG-3′); E: experienced HIV-1-positive patients; N: naive HIV-1-positive patients.
 AP-1: 5104–5114 nucleotide position (5′-GATGATGCCCC-3′); 5164–5172 nucleotide position (5′-CTTTGTAAG-3′); 5257–5265 nucleotide position (5′-GTTTATCAG-3′); 5261–5269 nucleotide position (5′-ATCAGTCAA-3′);
AP-1: 5104–5114 nucleotide position (5′-GATGATGCCCC-3′); 5164–5172 nucleotide position (5′-CTTTGTAAG-3′); 5257–5265 nucleotide position (5′-GTTTATCAG-3′); 5261–5269 nucleotide position (5′-ATCAGTCAA-3′);  NF1: 5088–5105 nucleotide position (5′-TATTGGCCAGCAGTGTG-3′);
NF1: 5088–5105 nucleotide position (5′-TATTGGCCAGCAGTGTG-3′);  TST1: 5200–5214 nucleotide position (5′-GTTGAAAAAAAGTTA-3′);
TST1: 5200–5214 nucleotide position (5′-GTTGAAAAAAAGTTA-3′);  OCT1: 5116–5129 nucleotide position (5′-CATCCTGAAAAATA-3′); 5143–5146 nucleotide position (5′-ACTCTTTTAATG-3′);
OCT1: 5116–5129 nucleotide position (5′-CATCCTGAAAAATA-3′); 5143–5146 nucleotide position (5′-ACTCTTTTAATG-3′);  NFκB: 5188–5197 nucleotide position (5′-CGGGCCTCCC-3′);
NFκB: 5188–5197 nucleotide position (5′-CGGGCCTCCC-3′);  TATA-like sequences: 5146–5160 nucleotide position (5′-CTTTTAATGTCCTCC-3′); 5202–5212 nucleotide position (5′-TGAAAAAAAG-3′); 5256–5265 nucleotide position (5′-TGTTTATCAG-3′); E: experienced HIV-1-positive patients; N: naive HIV-1-positive patients.
TATA-like sequences: 5146–5160 nucleotide position (5′-CTTTTAATGTCCTCC-3′); 5202–5212 nucleotide position (5′-TGAAAAAAAG-3′); 5256–5265 nucleotide position (5′-TGTTTATCAG-3′); E: experienced HIV-1-positive patients; N: naive HIV-1-positive patients.
 AP-1: 5104–5114 nucleotide position (5′-GATGATGCCCC-3′); 5164–5172 nucleotide position (5′-CTTTGTAAG-3′); 5257–5265 nucleotide position (5′-GTTTATCAG-3′); 5261–5269 nucleotide position (5′-ATCAGTCAA-3′);
AP-1: 5104–5114 nucleotide position (5′-GATGATGCCCC-3′); 5164–5172 nucleotide position (5′-CTTTGTAAG-3′); 5257–5265 nucleotide position (5′-GTTTATCAG-3′); 5261–5269 nucleotide position (5′-ATCAGTCAA-3′);  NF1: 5088–5105 nucleotide position (5′-TATTGGCCAGCAGTGTG-3′);
NF1: 5088–5105 nucleotide position (5′-TATTGGCCAGCAGTGTG-3′);  TST1: 5200–5214 nucleotide position (5′-GTTGAAAAAAAGTTA-3′);
TST1: 5200–5214 nucleotide position (5′-GTTGAAAAAAAGTTA-3′);  OCT1: 5116–5129 nucleotide position (5′-CATCCTGAAAAATA-3′); 5143–5146 nucleotide position (5′-ACTCTTTTAATG-3′);
OCT1: 5116–5129 nucleotide position (5′-CATCCTGAAAAATA-3′); 5143–5146 nucleotide position (5′-ACTCTTTTAATG-3′);  NFκB: 5188–5197 nucleotide position (5′-CGGGCCTCCC-3′);
NFκB: 5188–5197 nucleotide position (5′-CGGGCCTCCC-3′);  TATA-like sequences: 5146–5160 nucleotide position (5′-CTTTTAATGTCCTCC-3′); 5202–5212 nucleotide position (5′-TGAAAAAAAG-3′); 5256–5265 nucleotide position (5′-TGTTTATCAG-3′); E: experienced HIV-1-positive patients; N: naive HIV-1-positive patients.
TATA-like sequences: 5146–5160 nucleotide position (5′-CTTTTAATGTCCTCC-3′); 5202–5212 nucleotide position (5′-TGAAAAAAAG-3′); 5256–5265 nucleotide position (5′-TGTTTATCAG-3′); E: experienced HIV-1-positive patients; N: naive HIV-1-positive patients.
 AP-1: 5104–5114 nucleotide position (5′-GATGATGCCCC-3′); 5164–5172 nucleotide position (5′-CTTTGTAAG-3′); 5257–5265 nucleotide position (5′-GTTTATCAG-3′); 5261–5269 nucleotide position (5′-ATCAGTCAA-3′);
AP-1: 5104–5114 nucleotide position (5′-GATGATGCCCC-3′); 5164–5172 nucleotide position (5′-CTTTGTAAG-3′); 5257–5265 nucleotide position (5′-GTTTATCAG-3′); 5261–5269 nucleotide position (5′-ATCAGTCAA-3′);  NF1: 5088–5105 nucleotide position (5′-TATTGGCCAGCAGTGTG-3′);
NF1: 5088–5105 nucleotide position (5′-TATTGGCCAGCAGTGTG-3′);  TST1: 5200–5214 nucleotide position (5′-GTTGAAAAAAAGTTA-3′);
TST1: 5200–5214 nucleotide position (5′-GTTGAAAAAAAGTTA-3′);  OCT1: 5116–5129 nucleotide position (5′-CATCCTGAAAAATA-3′); 5143–5146 nucleotide position (5′-ACTCTTTTAATG-3′);
OCT1: 5116–5129 nucleotide position (5′-CATCCTGAAAAATA-3′); 5143–5146 nucleotide position (5′-ACTCTTTTAATG-3′);  NFκB: 5188–5197 nucleotide position (5′-CGGGCCTCCC-3′);
NFκB: 5188–5197 nucleotide position (5′-CGGGCCTCCC-3′);  TATA-like sequences: 5146–5160 nucleotide position (5′-CTTTTAATGTCCTCC-3′); 5202–5212 nucleotide position (5′-TGAAAAAAAG-3′); 5256–5265 nucleotide position (5′-TGTTTATCAG-3′); E: experienced HIV-1-positive patients; N: naive HIV-1-positive patients.
TATA-like sequences: 5146–5160 nucleotide position (5′-CTTTTAATGTCCTCC-3′); 5202–5212 nucleotide position (5′-TGAAAAAAAG-3′); 5256–5265 nucleotide position (5′-TGTTTATCAG-3′); E: experienced HIV-1-positive patients; N: naive HIV-1-positive patients.
 AP-1: 5104–5114 nucleotide position (5′-GATGATGCCCC-3′); 5164–5172 nucleotide position (5′-CTTTGTAAG-3′); 5257–5265 nucleotide position (5′-GTTTATCAG-3′); 5261–5269 nucleotide position (5′-ATCAGTCAA-3′);
AP-1: 5104–5114 nucleotide position (5′-GATGATGCCCC-3′); 5164–5172 nucleotide position (5′-CTTTGTAAG-3′); 5257–5265 nucleotide position (5′-GTTTATCAG-3′); 5261–5269 nucleotide position (5′-ATCAGTCAA-3′);  NF1: 5088–5105 nucleotide position (5′-TATTGGCCAGCAGTGTG-3′);
NF1: 5088–5105 nucleotide position (5′-TATTGGCCAGCAGTGTG-3′);  TST1: 5200–5214 nucleotide position (5′-GTTGAAAAAAAGTTA-3′);
TST1: 5200–5214 nucleotide position (5′-GTTGAAAAAAAGTTA-3′);  OCT1: 5116–5129 nucleotide position (5′-CATCCTGAAAAATA-3′); 5143–5146 nucleotide position (5′-ACTCTTTTAATG-3′);
OCT1: 5116–5129 nucleotide position (5′-CATCCTGAAAAATA-3′); 5143–5146 nucleotide position (5′-ACTCTTTTAATG-3′);  NFκB: 5188–5197 nucleotide position (5′-CGGGCCTCCC-3′);
NFκB: 5188–5197 nucleotide position (5′-CGGGCCTCCC-3′);  TATA-like sequences: 5146–5160 nucleotide position (5′-CTTTTAATGTCCTCC-3′); 5202–5212 nucleotide position (5′-TGAAAAAAAG-3′); 5256–5265 nucleotide position (5′-TGTTTATCAG-3′); E: experienced HIV-1-positive patients; N: naive HIV-1-positive patients.
TATA-like sequences: 5146–5160 nucleotide position (5′-CTTTTAATGTCCTCC-3′); 5202–5212 nucleotide position (5′-TGAAAAAAAG-3′); 5256–5265 nucleotide position (5′-TGTTTATCAG-3′); E: experienced HIV-1-positive patients; N: naive HIV-1-positive patients.
| Patients Enrolled | Total n. | Gender, n. | Age | HIV-1 RNA load, Range | CD4+ Counts, Range |
|---|---|---|---|---|---|
| Naive | 22 | Male: 16; Female: 6 | Range: 21–68 years old Mean: 39.27 years old | 1.26 × 103–10 × 106 copies/mL | 9–890/mm3 |
| Experienced | 44 | Male: 39; Female: 5 | Range: 21–76 years old Mean: 43.7 years old | TND-27 × 105 copies/mL | 312–1178/mm3 |
| HIV-1 Status Patients, n. | MCPyV+ Patients, n. | MCPyV+ Samples | ||
|---|---|---|---|---|
| Naive | Naive | Urine | Plasma | Rectal Swab |
| 22/66 | 12/22 | 5 | 4 | 9 |
| Experienced | Experienced | |||
| 44/66 | 19/44 | 5 | 3 | 14 |
| HIV-1 Status | MCPyV DNA Detection % | |||
| Overall Sites | Urine | Plasma | Rectal Swab | |
| Naive | 54.55 | 22.73 | 18.18 | 40.91 |
| Experienced | 43.18 | 11.36 | 6.82 | 31.82 |
| qPCR results (median value) | ||||
| Urine | Plasma | Rectal Swab | ||
| 5.15 × 102 copies/mL (IQR 4.5 × 102–5.4 × 102) | 1.5 × 102 copies/mL (IQR1.3 × 102–2.0 × 102) | 2.3 × 103 copies/mL (IQR1.8 × 103–4.1 × 103) | ||
| Patients, n° | N/E | Age | GENDER | NCCR MODIFICATIONS | ||
|---|---|---|---|---|---|---|
| URINE | PLASMA | RECTAL SWAB | ||||
| 4 | E | 29 | M | ∆GCA, ∆CC, insGTT | ||
| 7 | E | 36 | M | ∆GCA, ∆CCC, insGTT | ||
| 8 | E | 46 | M | ∆AA, insGTT | ||
| 11 | E | 55 | M | ∆CC, ∆AA | Not detected | |
| 12 | E | 37 | M | ∆CC, ∆TT, insGTTGA | ||
| 13 | N | 34 | M | ∆CC, ∆AA, insGTTGA | ∆CCC, insGTTGA | |
| 14 | N | 68 | M | ∆GCA, ∆AA, ∆GA, insGTTGA | ||
| 19 | N | 42 | M | ∆CC | Not detected | |
| 21 | N | 36 | M | ∆CC, ∆AA | ∆AA, ∆TTG | |
| 23 | N | 50 | M | ∆ATG, ∆CC, ∆AA | ||
| 26 | E | 41 | F | ∆CC, ∆AA | ∆TGA, ∆ATG, ∆CC, ∆AA | |
| 27 | E | 38 | M | ∆CC, ∆AA | ||
| 29 | E | 38 | M | ∆GA, ∆CC, ∆AA, ins GTT | ||
| 30 | E | 39 | F | ∆CCCCCC | Not detected | |
| 34 | E | 30 | M | ∆CC, ∆AA | ||
| 35 | E | 40 | M | ∆CC | ∆CC, insGTT | |
| 36 | N | 41 | M | ∆AA, ∆TTTGTT | ∆AA, ∆TTTTGTTT, insGTTGA | ∆GA, ∆ATG, 2∆AA, ∆TTTTGTTT, insGTTGA |
| 37 | E | 61 | M | ∆GA, ∆CC, ∆AA, insGTTGA | ||
| 38 | N | 45 | M | ∆AA | ∆CCCC, 2∆AA, insGTT | |
| 39 | E | 25 | M | ∆GCA, ∆CCC, insGTT | ||
| 40 | E | 26 | M | Not detected | ||
| 41 | N | 28 | M | ∆AA | Not detected | |
| 42 | E | 28 | M | Not detected | Not detected | |
| 43 | N | 46 | M | insGTTGA | ||
| 47 | N | 44 | M | Not detected | ||
| 55 | N | 45 | M | ∆ATG, ∆CC | ||
| 57 | E | 34 | M | ∆TGATGA, ∆CC, ∆AA | ||
| 61 | E | 43 | M | ∆GCA, ∆CCAA, insGTT | ||
| 64 | N | 45 | F | Not detected | ∆GA, ∆CC, ∆CT, insGTTGA | |
| 65 | E | 60 | M | Not detected | ∆GA, ∆ATG, ∆CC, ∆AAAAA, insGTTGA | |
| 66 | E | 44 | F | ∆GA, ATG, ∆CC, ∆AAAAA | ||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prezioso, C.; Obregon, F.; Ambroselli, D.; Petrolo, S.; Checconi, P.; Rodio, D.M.; Coppola, L.; Nardi, A.; de Vito, C.; Sarmati, L.; et al. Merkel Cell Polyomavirus (MCPyV) in the Context of Immunosuppression: Genetic Analysis of Noncoding Control Region (NCCR) Variability among a HIV-1-Positive Population. Viruses 2020, 12, 507. https://doi.org/10.3390/v12050507
Prezioso C, Obregon F, Ambroselli D, Petrolo S, Checconi P, Rodio DM, Coppola L, Nardi A, de Vito C, Sarmati L, et al. Merkel Cell Polyomavirus (MCPyV) in the Context of Immunosuppression: Genetic Analysis of Noncoding Control Region (NCCR) Variability among a HIV-1-Positive Population. Viruses. 2020; 12(5):507. https://doi.org/10.3390/v12050507
Chicago/Turabian StylePrezioso, Carla, Francisco Obregon, Donatella Ambroselli, Sara Petrolo, Paola Checconi, Donatella Maria Rodio, Luigi Coppola, Angelo Nardi, Corrado de Vito, Loredana Sarmati, and et al. 2020. "Merkel Cell Polyomavirus (MCPyV) in the Context of Immunosuppression: Genetic Analysis of Noncoding Control Region (NCCR) Variability among a HIV-1-Positive Population" Viruses 12, no. 5: 507. https://doi.org/10.3390/v12050507
APA StylePrezioso, C., Obregon, F., Ambroselli, D., Petrolo, S., Checconi, P., Rodio, D. M., Coppola, L., Nardi, A., de Vito, C., Sarmati, L., Andreoni, M., Palamara, A. T., Ciotti, M., & Pietropaolo, V. (2020). Merkel Cell Polyomavirus (MCPyV) in the Context of Immunosuppression: Genetic Analysis of Noncoding Control Region (NCCR) Variability among a HIV-1-Positive Population. Viruses, 12(5), 507. https://doi.org/10.3390/v12050507