Role of Divalent Cations in HIV-1 Replication and Pathogenicity
Abstract
1. Introduction
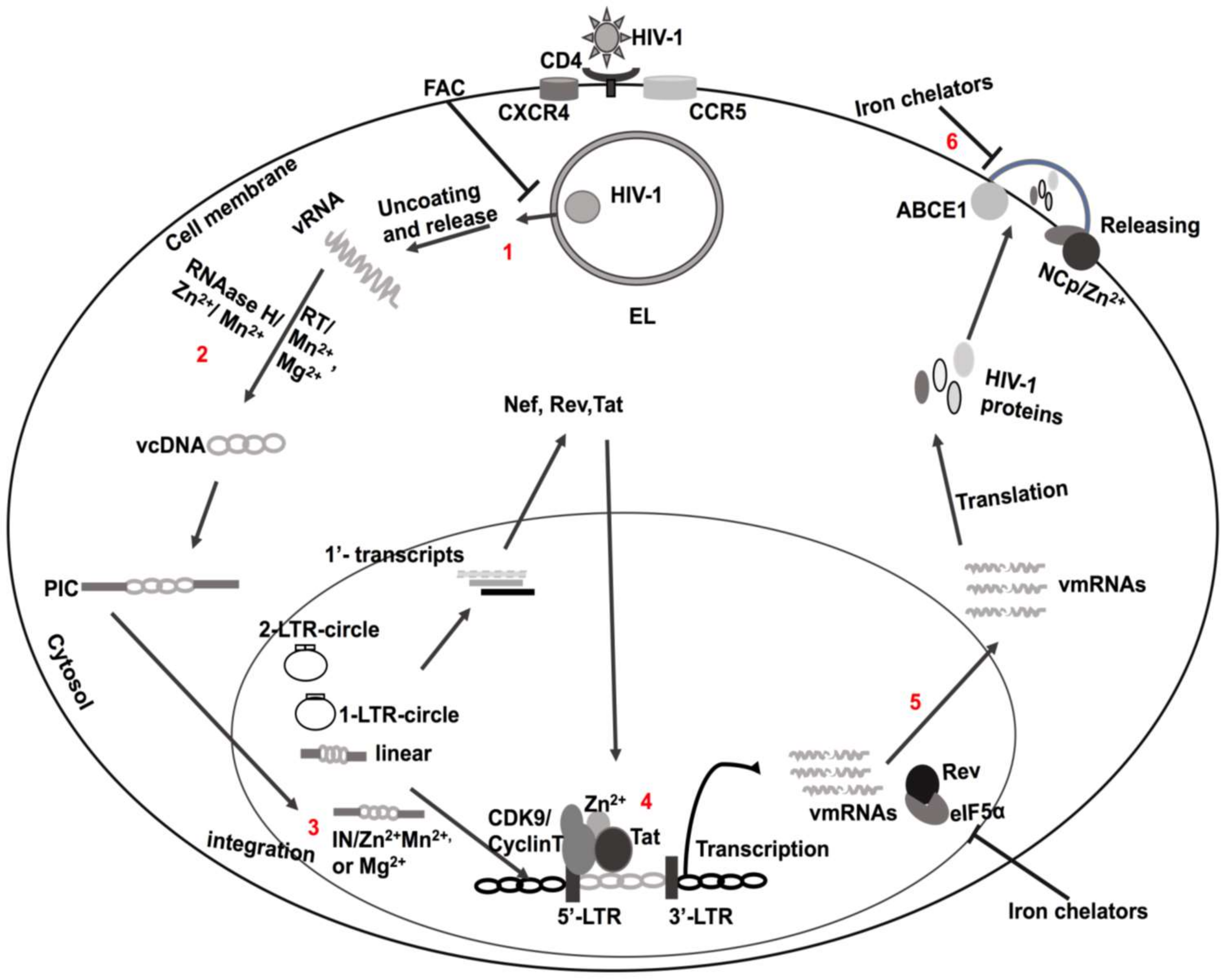
2. HIV-1 Infection and Replication
3. Structural and Functional Domains of HIV-1 Tat
4. Tat-Mediated Activation of Transcription
5. Zinc (Zn2+)
6. Ferrous Iron (Fe2+)
7. Manganese (Mn2+) and Magnesium (Mg2+)
8. Selenium (Se2+)
9. Copper (Cu2+)
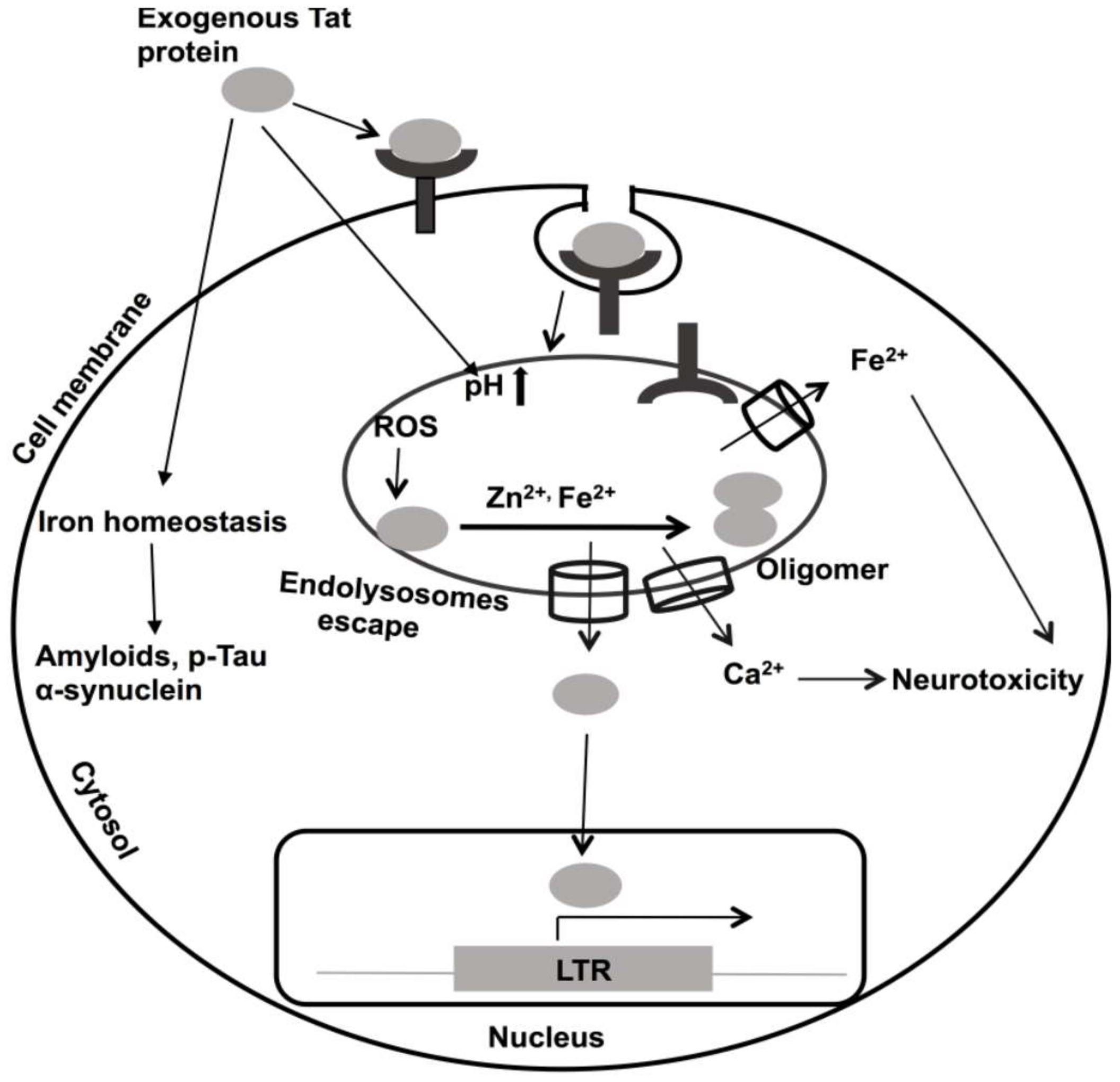
10. Roles of Divalent Cations in HIV-1 Tat-Mediated Pathogenicity
11. Summary
Author Contributions
Funding
Conflicts of Interest
References
- Anastassopoulou, J.; Theophanides, T. The Role of Metal Ions in Biological Systems and Medicine. In Bioinorganic Chemistry: An Inorganic Perspective of Life; Kessissoglou, D.P., Ed.; Springer: Dordrecht, The Netherlands, 1995; pp. 209–2018. [Google Scholar]
- Sigel, A.; Sigel, H.; Sigel, R.K.O. Interactions Between Essential Metal Ions and Human Diseases; Springer: Dordrecht, The Netherlands, 2013. [Google Scholar]
- Delgado, R.; Vergara, C.; Wolff, D. Divalent Cations as Modulators of Neuronal Excitability: Emphasis on Copper and Zinc. Biol. Res. 2006, 39, 173–182. [Google Scholar] [CrossRef][Green Version]
- Bloom, A.J. Metal Regulation of Metabolism. Curr. Opin. Chem. Biol. 2019, 49, 33–38. [Google Scholar] [CrossRef]
- Tan, X. Metalloproteins and Metalloenzymes: Roles and Mechanisms of Metals in Functional Proteins; World Scientific Publishing Company Pte Limited: Singapore, 2018. [Google Scholar]
- Baier, F.; Chen, J.; Solomonson, M.; Strynadka, N.C.J.; Tokuriki, N. Distinct Metal Isoforms Underlie Promiscuous Activity Profiles of Metalloenzymes. ACS Chem. Biol. 2015, 10, 1684–1693. [Google Scholar] [CrossRef] [PubMed]
- Hausinger, R.P. New Metal Cofactors and recent Metallocofactor insights. Curr. Opin. Struct. Biol. 2019, 59, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Pernil, R.; Schleiff, E. Metalloproteins in the Biology of Heterocysts. Life 2019, 9, 32. [Google Scholar] [CrossRef] [PubMed]
- Hoppert, M. Metalloenzymes. In Encyclopedia of Geobiology; Reitner, J., Thiel, V., Eds.; Springer: Dordrecht, The Netherlands, 2011; pp. 558–563. [Google Scholar]
- Knape, M.J.; Herberg, F.W. Metal Coordination in Kinases and Pseudokinases. Biochem. Soc. Trans. 2017, 45, 653–663. [Google Scholar] [CrossRef]
- Walker, G.M.; Duffus, J.H. Magnesium Ions and the Control of the Cell Cycle in Yeast. J. Cell. Sci. 1980, 42, 329–356. [Google Scholar]
- Mackenzie, K.; Foot, N.J.; Anand, S.; Dalton, H.E.; Chaudhary, N.; Collins, B.M.; Mathivanan, S.; Kumar, S. Regulation of the Divalent Metal Ion Transporter via Membrane Budding. Cell. Discov. 2016, 2, 16011. [Google Scholar] [CrossRef]
- Zhang-Keck, Z.Y.; Eckstein, F.; Washington, L.D.; Stallcup, M.R. A Role for Divalent Cations in Specifying the start site for transcription from chromatin templates in vitro. J. Biol. Chem. 1988, 263, 9550–9556. [Google Scholar]
- Chaigne-Delalande, B.; Lenardo, M.J. Divalent cation signaling in immune cells. Trends Immunol. 2014, 35, 332–344. [Google Scholar] [CrossRef]
- Diaz-Ochoa, V.E.; Jellbauer, S.; Klaus, S.; Raffatellu, M. Transition metal ions at the crossroads of mucosal immunity and microbial pathogenesis. Front. Cell Infect. Microbiol. 2014, 4, 2. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Chen, J. The regulation of integrin function by divalent cations. Cell Adh. Migr. 2012, 6, 20–29. [Google Scholar] [CrossRef] [PubMed]
- Stelling, M.P.; Motta, J.M.; Mashid, M.; Johnson, W.E.; Pavão, M.S.; Farrell, N.P. Metal ions and the extracellular matrix in tumor migration. FEBS J. 2019, 286, 2950–2964. [Google Scholar] [CrossRef] [PubMed]
- Hamatake, M.; Iguchi, K.; Hirano, K.; Ishida, R. Zinc Induces Mixed Types of Cell Death, Necrosis, and Apoptosis, in Molt-4 Cells. J. Biochem. 2000, 128, 933–939. [Google Scholar] [CrossRef]
- Liang, Q.; Zhou, B. Copper and Manganese Induce Yeast Apoptosis via Different Pathways. Mol. Biol. Cell. 2007, 18, 4741–4749. [Google Scholar] [CrossRef]
- Yeo, J.E.; Kang, S.K. Selenium effectively inhibits ROS-mediated apoptotic neural precursor cell death in vitro and in vivo in traumatic brain injury. Biochim. Biophys. Acta 2007, 1772, 1199–1210. [Google Scholar] [CrossRef]
- Coffin, A.B.; Reinhart, K.E.; Owens, K.N.; Raible, D.W.; Rubel, E.W. Extracellular divalent cations modulate aminoglycoside-induced hair cell death in the zebrafish lateral line. Hear Res. 2009, 253, 42–51. [Google Scholar] [CrossRef]
- Dribben, W.H.; Eisenman, L.N.; Mennerick, S. Magnesium induces neuronal apoptosis by suppressing excitability. Cell Death Dis. 2010, 1, e63. [Google Scholar] [CrossRef]
- Mou, Y.; Wang, J.; Wu, J.; He, D.; Zhang, C.; Duan, C.; Li, B. Ferroptosis, a new form of cell death: Opportunities and challenges in cancer. J. Hematol. Oncol. 2019, 12, 34. [Google Scholar] [CrossRef]
- P. Aisen, A.; Listowsky, I. Iron Transport and Storage Proteins. Annu. Rev. Biochem. 1980, 49, 357–393. [Google Scholar] [CrossRef]
- Coleman, J.E. ZINC PROTEINS: Enzymes, Storage Proteins, Transcription Factors, and Replication Proteins. Annu. Rev. Biochem. 1992, 61, 897–946. [Google Scholar] [CrossRef] [PubMed]
- Sigel, A.; Sigel, H. Metal ions in biological systems, volume 35: Iron transport and storage microorganisms, plants, and animals. Met. Based Drugs 1998, 5, 262. [Google Scholar] [CrossRef] [PubMed]
- Rolfs, A.; Hediger, M.A. Metal ion transporters in mammals: Structure, function and pathological implications. J. Physiol. 1999, 518, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Eide, D.J. Zinc transporters and the cellular trafficking of zinc. Biochim. Biophys. Acta 2006, 1763, 711–722. [Google Scholar] [CrossRef] [PubMed]
- Prohaska, J.R. Role of copper transporters in copper homeostasis. Am. J. Clin. Nutr. 2008, 88, 826S–829S. [Google Scholar] [CrossRef] [PubMed]
- Zhao, N.; Enns, C.A. Iron transport machinery of human cells: Players and their interactions. Curr. Top. Membr. 2012, 69, 67–93. [Google Scholar]
- Botella, H.; Stadthagen, G.; Lugo-Villarino, G.; de Chastellier, C.; Neyrolles, O. Metallobiology of host–pathogen interactions: An intoxicating new insight. Trends Microbiol. 2012, 20, 106–112. [Google Scholar] [CrossRef]
- Hood, M.I.; Skaar, E.P. Nutritional immunity: Transition metals at the pathogen–host interface. Nat. Rev. Microbiol. 2012, 10, 525–537. [Google Scholar] [CrossRef]
- Veyrier, F.J.; Cellier, M.F. Metal economy in host-microbe interactions. Front. Cell Infect. Microbiol. 2015, 4. [Google Scholar] [CrossRef]
- Skaar, E.P.; Raffatellu, M. Metals in infectious diseases and nutritional immunity. Metallomics 2015, 7, 926–928. [Google Scholar] [CrossRef]
- Weiss, G.; Carver, P.L. Role of divalent metals in infectious disease susceptibility and outcome. Clin. Microbiol. Infect. 2018, 24, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Baum, M.K.; Campa, A.; Lai, S.; Lai, H.; Page, J.B. Zinc Status in Human Immunodeficiency Virus Type 1 Infection and Illicit Drug Use. Clin. Infect. Dis. 2003, 37 (Suppl. 2), S117–S123. [Google Scholar] [CrossRef] [PubMed]
- Irlam, J.J.H.; Visser, M.M.E.; Rollins, N.N.; Siegfried, N. Micronutrient supplementation in children and adults with HIV infection. Cochrane Database Syst. Rev. 2005, 19, CD003650. [Google Scholar]
- Kassu, A.; Yabutani, T.; Mahmud, Z.H.; Mohammad, A.; Nguyen, N.; Huong, B.T.M.; Hailemariam, G.; Diro, E.; Ayele, B.; Wondmikun, Y.; et al. Alterations in serum levels of trace elements in tuberculosis and HIV infections. Eur. J. Clin. Nutr. 2006, 60, 580–586. [Google Scholar] [CrossRef]
- Friis, H. Micronutrient interventions and HIV infection: A review of current evidence. Trop. Med. Int. Health 2007, 11, 1849–1857. [Google Scholar] [CrossRef]
- Banjoko, S.; Oseni, F.; Togun, R.; Onayemi, O.; Emma-Okon, B.; Fakunle, J. Iron status in HIV-1 infection: Implications in disease pathology. BMC Clin. Pathol. 2012, 12, 26. [Google Scholar] [CrossRef]
- Chang, H.-C.; Bayeva, M.; Taiwo, B.; Palella, F.J., Jr.; Hope, T.J.; Ardehali, H. Short communication: High cellular iron levels are associated with increased HIV infection and replication. AIDS Res. Hum. Retroviruses 2015, 31, 305–312. [Google Scholar] [CrossRef]
- Cooper, D.A. Life and death in the cART era. Lancet 2008, 372, 266–267. [Google Scholar] [CrossRef]
- Kwong, P.D.; Wyatt, R.; Robinson, J.; Sweet, R.W.; Sodroski, J.; Hendrickson, W.A. Structure of an HIV gp120 envelope glycoprotein in complex with the CD4 receptor and a neutralizing human antibody. Nature 1998, 393, 648–659. [Google Scholar] [CrossRef]
- Frankel, A.D.; Young, J.A.T. HIV-1: Fifteen Proteins and an RNA. Annu. Rev. Biochem. 1998, 67, 1–25. [Google Scholar] [CrossRef]
- Zeichner, S.L. The Molecular Biology of HIV: Insights Into Pathogenesis and Targets for Therapy. Clin. Perinatol. 1994, 21, 39–73. [Google Scholar] [CrossRef]
- Freed, E.O. HIV-1 Replication. Somat. Cell Mol. Genet. 2001, 26, 13–33. [Google Scholar] [CrossRef] [PubMed]
- Campbell, S.M.; Crowe, S.M.; Mak, J. Lipid rafts and HIV-1: From viral entry to assembly of progeny virions. J. Clin. Virol. 2001, 22, 217–227. [Google Scholar] [CrossRef]
- Das, A.T.; Harwig, A.; Berkhout, B. The HIV-1 Tat protein has a versatile role in activating viral transcription. J. Virol. 2011, 85, 9506–9516. [Google Scholar] [CrossRef]
- Liang, C.; Wainberg, M. The role of Tat in HIV-1 replication: An activator and/or a suppressor? AIDS Rev. 2002, 4, 41–49. [Google Scholar]
- Romani, B.; Engelbrecht, S.; Glashoff, R.H. Functions of Tat: The versatile protein of human immunodeficiency virus type 1. J. Gen. Virol. 2010, 91, 1–12. [Google Scholar] [CrossRef]
- Li, L.; Dahiya, S.; Kortagere, S.; Aiamkitsumrit, B.; Cunningham, D.; Pirrone, V.; Nonnemacher, M.R.; Wigdahl, B. Impact of Tat Genetic Variation on HIV-1 Disease. Adv. Virol. 2012, 2012, 123605. [Google Scholar] [CrossRef]
- Rice, A.P. The HIV-1 Tat Protein: Mechanism of Action and Target for HIV-1 Cure Strategies. Curr. Pharm. Des. 2017, 23, 4098–4102. [Google Scholar] [CrossRef]
- Frankel, A.D.; Pabo, C.O. Cellular uptake of the tat protein from human immunodeficiency virus. Cell 1988, 55, 1189–1193. [Google Scholar] [CrossRef]
- Mann, D.A.; Frankel, A.D. Endocytosis and targeting of exogenous HIV-1 Tat protein. EMBO J. 1991, 10, 1733–1739. [Google Scholar] [CrossRef]
- Liu, Y.; Jones, M.; Hingtgen, C.M.; Bu, G.; Laribee, N.; Tanzi, R.E.; Moir, R.D.; Nath, A.; He, J.J. Uptake of HIV-1 tat protein mediated by low-density lipoprotein receptor-related protein disrupts the neuronal metabolic balance of the receptor ligands. Nat. Med. 2000, 6, 1380–1387. [Google Scholar] [CrossRef] [PubMed]
- Kolson, D.L.; Collman, R.; Hrin, R.; Balliet, J.W.; Laughlin, M.; McGann, K.A.; Debouck, C.; Gonzalez-Scarano, F. Human immunodeficiency virus type 1 Tat activity in human neuronal cells: Uptake and trans-activation. J. Gen. Virol 1994, 75, 1927–1934. [Google Scholar] [CrossRef] [PubMed]
- Heaton, R.K.; Clifford, D.B.; Franklin, D.R., Jr.; Woods, S.P.; Ake, C.; Vaida, F.; Ellis, R.J.; Letendre, S.L.; Marcotte, T.D.; Atkinson, J.H.; et al. HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy: CHARTER Study. Neurology 2010, 75, 2087–2096. [Google Scholar] [CrossRef] [PubMed]
- Spudich, S.; Gonzalez-Scarano, F. HIV-1-related central nervous system disease: Current issues in pathogenesis, diagnosis, and treatment. Cold Spring Harb. Perspect. Med. 2012, 2, a007120. [Google Scholar] [CrossRef]
- Bagashev, A.; Sawaya, B.E. Roles and functions of HIV-1 Tat protein in the CNS: An overview. Virol. J. 2013, 10, 358. [Google Scholar] [CrossRef]
- Garcia, J.A.; Harrich, D.; Soultanakis, E.; Wu, F.; Mitsuyasu, R.; Gaynor, R.B. Human immunodeficiency virus type 1 LTR TATA and TAR region sequences required for transcriptional regulation. EMBO J. 1989, 8, 765–778. [Google Scholar] [CrossRef]
- Rana, T.M.; Jeang, K.-T. Biochemical and Functional Interactions between HIV-1 Tat Protein and TAR RNA. Arch. Biochem. Biophys 1999, 365, 175–185. [Google Scholar] [CrossRef]
- Kuppuswamy, M.; Subramanian, T.; Srinivasan, A.; Chinnadurai, G. Multiple functional domains of Tat, the trans-activator of HIV-1, defined by mutational analysis. Nucleic Acids Res. 1989, 17, 3551–3561. [Google Scholar] [CrossRef]
- Jeang, K.T.; Xiao, H.; Rich, E.A. Multifaceted activities of the HIV-1 transactivator of transcription. Tat. J. Biol. Chem. 1999, 274, 28837–28840. [Google Scholar] [CrossRef]
- López-Huertas, M.R.; Callejas, S.; Abia, D.; Mateos, E.; Dopazo, A.; Alcamí, J.; Coiras, M. Modifications in host cell cytoskeleton structure and function mediated by intracellular HIV-1 Tat protein are greatly dependent on the second coding exon. Nucleic Acids Res. 2010, 38, 3287–3307. [Google Scholar] [CrossRef]
- Frankel, A.; Bredt, D.; Pabo, C. Tat protein from human immunodeficiency virus forms a metal-linked dimer. Science 1988, 240, 70–73. [Google Scholar] [CrossRef] [PubMed]
- Kalantari, P.; Ayan, V.; Natarajan, S.K.; Muralidhar, K.; Gandhi, U.H.; Vunta, H.; Henderson, A.J.; Prabhu, K.S. Thioredoxin reductase-1 negatively regulates HIV-1 transactivating protein Tat-dependent transcription in human macrophages. J. Biol. Chem. 2008, 283, 33183–33190. [Google Scholar] [CrossRef] [PubMed]
- Garber, M.E.; Wei, P.; KewalRamani, V.N.; Mayall, T.P.; Herrmann, C.H.; Rice, A.P.; Littman, D.R.; Jones, K.A. The interaction between HIV-1 Tat and human cyclin T1 requires zinc and a critical cysteine residue that is not conserved in the murine CycT1 protein. Genes Dev. 1998, 12, 3512–3527. [Google Scholar] [CrossRef] [PubMed]
- Dingwall, C.; Ernberg, I.; Gait, M.J.; Green, S.M.; Heaphy, S.; Karn, J.; Lowe, A.D.; Singh, M.; Skinner, M.A.; Valerio, R. Human immunodeficiency virus 1 tat protein binds trans-activation-responsive region (TAR) RNA in vitro. Proc. Natl. Acad. Sci. USA 1989, 86, 6925–6929. [Google Scholar] [CrossRef]
- Roy, S.; Delling, U.; Chen, C.H.; Rosen, C.A.; Sonenberg, N. A bulge structure in HIV-1 TAR RNA is required for Tat binding and Tat-mediated trans-activation. Genes Dev. 1990, 4, 1365–1373. [Google Scholar] [CrossRef]
- Weeks, K.M.; Crothers, D.M. RNA recognition by Tat-derived peptides: Interaction in the major groove? Cell 1991, 66, 577–588. [Google Scholar] [CrossRef]
- Hauber, J.; Malim, M.H.; Cullen, B.R. Mutational analysis of the conserved basic domain of human immunodeficiency virus tat protein. J. Virol. 1989, 63, 1181–1187. [Google Scholar] [CrossRef]
- Ruben, S.; Perkins, A.; Purcell, R.; Joung, K.; Sia, R.; Burghoff, R.; Haseltine, W.A.; Rosen, C.A. Structural and functional characterization of human immunodeficiency virus tat protein. J. Virol. 1989, 63, 1–8. [Google Scholar] [CrossRef]
- Schwarze, S.R.; Hruska, K.A.; Dowdy, S.F. Protein transduction: Unrestricted delivery into all cells? Trends Cell Biol. 2000, 10, 290–295. [Google Scholar] [CrossRef]
- Fuchs, S.M.; Raines, R.T. Internalization of cationic peptides: The road less (or more?) traveled. Cell. Mol. Life Sci. 2006, 63, 1819–1822. [Google Scholar] [CrossRef]
- El-Sayed, A.; Futaki, S.; Harashima, H. Delivery of macromolecules using arginine-rich cell-penetrating peptides: Ways to overcome endosomal entrapment. AAPS J. 2009, 11, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Neuveut, C.; Scoggins, R.M.; Camerini, D.; Markham, R.B.; Jeang, K.-T. Requirement for the second coding exon of Tat in the optimal replication of macrophage-tropic HIV-1. J. Biomed. Sci. 2003, 10, 651–660. [Google Scholar] [CrossRef] [PubMed]
- Mahlknecht, U.; Dichamp, I.; Varin, A.; Van Lint, C.; Herbein, G. NF-κB-dependent control of HIV-1 transcription by the second coding exon of Tat in T cells. J. Leukoc. Biol. 2008, 83, 718–727. [Google Scholar] [CrossRef] [PubMed]
- Huh, J.R.; Park, J.M.; Kim, M.; Carlson, B.A.; Hatfield, D.L.; Lee, B.J. Recruitment of TBP or TFIIB to a Promoter Proximal Position Leads to Stimulation of RNA Polymerase II Transcription without Activator Proteins bothin Vivoandin Vitro. Biochem. Biophys. Res. Commun. 1999, 256, 45–51. [Google Scholar] [CrossRef]
- Zhou, M.; Halanski, M.A.; Radonovich, M.F.; Kashanchi, F.; Peng, J.; Price, D.H.; Brady, J.N. Tat Modifies the Activity of CDK9 To Phosphorylate Serine 5 of the RNA Polymerase II Carboxyl-Terminal Domain during Human Immunodeficiency Virus Type 1 Transcription. Mol. Cell Biol. 2000, 20, 5077–5086. [Google Scholar] [CrossRef]
- Ping, Y.H.; Rana, T.M. DSIF and NELF interact with RNA polymerase II elongation complex and HIV-1 Tat stimulates P-TEFb-mediated phosphorylation of RNA polymerase II and DSIF during transcription elongation. J. Biol. Chem. 2001, 276, 12951–12958. [Google Scholar] [CrossRef]
- Parada, C.A.; Roeder, R.G. Enhanced processivity of RNA polymerase II triggered by Tat-induced phosphorylation of its carboxy-terminal domain. Nature 1996, 384, 375–378. [Google Scholar] [CrossRef]
- Cujec, T.P.; Okamoto, H.; Fujinaga, K.; Meyer, J.; Chamberlin, H.; Morgan, D.O.; Peterlin, B.M. The HIV transactivator TAT binds to the CDK-activating kinase and activates the phosphorylation of the carboxy-terminal domain of RNA polymerase II. Genes Dev. 1997, 11, 2645–2657. [Google Scholar] [CrossRef]
- Anne, P.; Longwen, D.; Anil, M.; Cynthia de la, F.; Hong, L.; John, D.W.; Paul, L.; Ajit, K.; Fatah, K. Chromatin Remodeling and Modification during HIV-1 Tat-activated Transcription. Curr. HIV Res. 2003, 1, 343–362. [Google Scholar]
- Price, D.H. P-TEFb, a cyclin-dependent kinase controlling elongation by RNA polymerase II. Mol. Cell Biol. 2000, 20, 2629–2634. [Google Scholar] [CrossRef]
- Romano, G.; Kasten, M.; Falco, G.; Micheli, P.; Khalili, K.; Giordano, A. Regulatory functions of Cdk9 and of cyclin T1 in HIV tat transactivation pathway gene expression. J. Cell Biochem. 2000, 75, 357–368. [Google Scholar] [CrossRef]
- Zhu, Y.; Pe’ery, T.; Peng, J.; Ramanathan, Y.; Marshall, N.; Marshall, T.; Amendt, B.; Mathews, M.B.; Price, D.H. Transcription elongation factor P-TEFb is required for HIV-1 tat transactivation in vitro. Genes Dev. 1997, 11, 2622–2632. [Google Scholar] [CrossRef] [PubMed]
- Yankulov, K.; Bentley, D. Transcriptional control: Tat cofactors and transcriptional elongation. Curr. Biol. 1998, 8, R447–R449. [Google Scholar] [CrossRef][Green Version]
- Zhou, Q.; Chen, D.; Pierstorff, E.; Luo, K. Transcription elongation factor P-TEFb mediates Tat activation of HIV-1 transcription at multiple stages. EMBO J. 1998, 17, 3681–3691. [Google Scholar] [CrossRef] [PubMed]
- Schulte, A.; Czudnochowski, N.; Barboric, M.; Schönichen, A.; Blazek, D.; Peterlin, B.M.; Geyer, M. Identification of a Cyclin T-Binding Domain in Hexim1 and Biochemical Analysis of Its Binding Competition with HIV-1 Tat. J. Biol. Chem. 2005, 280, 24968–24977. [Google Scholar] [CrossRef] [PubMed]
- Barboric, M.; Yik, J.H.N.; Czudnochowski, N.; Yang, Z.; Chen, R.; Contreras, X.; Geyer, M.; Matija Peterlin, B.; Zhou, Q. Tat competes with HEXIM1 to increase the active pool of P-TEFb for HIV-1 transcription. Nucleic Acids Res. 2007, 35, 2003–2012. [Google Scholar] [CrossRef]
- Karn, J. The molecular biology of HIV latency: Breaking and restoring the Tat-dependent transcriptional circuit. Curr. Opin. HIV AIDS 2011, 6, 4–11. [Google Scholar] [CrossRef]
- Kamori, D.; Ueno, T. HIV-1 Tat and Viral Latency: What We Can Learn from Naturally Occurring Sequence Variations. Front. Microbiol. 2017, 8, 80. [Google Scholar] [CrossRef]
- Donahue, D.A.; Kuhl, B.D.; Sloan, R.D.; Wainberg, M.A. The Viral Protein Tat Can Inhibit the Establishment of HIV-1 Latency. J. Virol. 2012, 86, 3253–3263. [Google Scholar] [CrossRef]
- Khoury, G.; Mota, T.M.; Li, S.; Tumpach, C.; Lee, M.Y.; Jacobson, J.; Harty, L.; Anderson, J.L.; Lewin, S.R.; Purcell, D.F.J. HIV latency reversing agents act through Tat post translational modifications. Retrovirology 2018, 15, 36. [Google Scholar] [CrossRef]
- Huang, H.; Zhou, Z.-H.; Adhikari, R.; Yamada, K.M.; Dhawan, S. Defective iron homeostasis in human immunodeficiency virus type-1 latency. Curr. Trends Immunol. 2016, 17, 125–131. [Google Scholar] [PubMed]
- Shankaran, P.; Madlenakova, M.; Hajkova, V.; Jilich, D.; Svobodova, I.; Horinek, A.; Fujikura, Y.; Melkova, Z. Effects of Heme degradation products on reactivation of latent HIV-1. Acta Virol. 2017, 61, 86–96. [Google Scholar] [CrossRef] [PubMed][Green Version]
- McCall, K.A.; Huang, C.; Fierke, C.A. Function and Mechanism of Zinc Metalloenzymes. J. Nutr. 2000, 130, 1437S–1446S. [Google Scholar] [CrossRef] [PubMed]
- Maret, W. Zinc biochemistry: From a single zinc enzyme to a key element of life. Adv. Nutr. 2013, 4, 82–91. [Google Scholar] [CrossRef] [PubMed]
- Kaur, K.; Gupta, R.; Saraf, S.A.; Saraf, S.K. Zinc: The Metal of Life. Compr. Rev. Food Sci. Food Saf. 2014, 13, 358–376. [Google Scholar] [CrossRef]
- Jacob, C.; Maret, W.; Vallee, B.L. Control of zinc transfer between thionein, metallothionein, and zinc proteins. Proc. Natl. Acad. Sci. USA 1998, 95, 3489–3494. [Google Scholar] [CrossRef]
- Frederickson, C.J.; Suh, S.W.; Silva, D.; Frederickson, C.J.; Thompson, R.B. Importance of Zinc in the Central Nervous System: The Zinc-Containing Neuron. J. Nutr. 2000, 130, 1471S–1483S. [Google Scholar] [CrossRef]
- Hirano, T.; Murakami, M.; Fukada, T.; Nishida, K.; Yamasaki, S.; Suzuki, T. Roles of Zinc and Zinc Signaling in Immunity: Zinc as an Intracellular Signaling Molecule. Adv. Immunol. 2008, 97, 149–176. [Google Scholar]
- Hojyo, S.; Fukada, T. Roles of Zinc Signaling in the Immune System. J. Immunol. Res. 2016, 2016, 21. [Google Scholar] [CrossRef]
- Sprietsma, J.E. Zinc-controlled Th1/Th2 switch significantly determines development of diseases. Med. Hypotheses 1997, 49, 1–14. [Google Scholar] [CrossRef]
- Subramanian Vignesh, K.; Landero Figueroa, J.A.; Porollo, A.; Caruso, J.A.; Deepe, G.S., Jr. Zinc sequestration: Arming phagocyte defense against fungal attack. PLoS Pathog. 2013, 9, e1003815. [Google Scholar]
- Subramanian Vignesh, K.; Deepe, G.S. Immunological orchestration of zinc homeostasis: The battle between host mechanisms and pathogen defenses. Arch. Biochem. Biophys. 2016, 611, 66–78. [Google Scholar] [CrossRef]
- Djoko, K.Y.; Ong, C.-L.Y.; Walker, M.J.; McEwan, A.G. The Role of Copper and Zinc Toxicity in Innate Immune Defense against Bacterial Pathogens. J. Biol. Chem. 2015, 290, 18954–18961. [Google Scholar] [CrossRef] [PubMed]
- Takihara, H.; Cosentino, M.J.; Cockett, A.T.K. Zinc sulfate therapy for infertile male with or without varicocelectomy. Urology 1987, 29, 638–641. [Google Scholar] [CrossRef]
- Yakoob, M.Y.; Theodoratou, E.; Jabeen, A.; Imdad, A.; Eisele, T.P.; Ferguson, J.; Jhass, A.; Rudan, I.; Campbell, H.; Black, R.E.; et al. Preventive zinc supplementation in developing countries: Impact on mortality and morbidity due to diarrhea, pneumonia and malaria. BMC Public Health 2011, 11, S23. [Google Scholar] [CrossRef] [PubMed]
- Prasad, A.S. Discovery of Human Zinc Deficiency: Its Impact on Human Health and Disease. Adv. Nutr. 2013, 4, 176–190. [Google Scholar] [CrossRef] [PubMed]
- Skrovanek, S.; DiGuilio, K.; Bailey, R.; Huntington, W.; Urbas, R.; Mayilvaganan, B.; Mercogliano, G.; Mullin, J.M. Zinc and gastrointestinal disease. World J. Gastrointest Pathophysiol. 2014, 5, 496–513. [Google Scholar] [CrossRef]
- Livingstone, C. Zinc. Nutr. Clin. Pract. 2015, 30, 371–382. [Google Scholar]
- Sprietsma, J. Zinkdeficiëntie predisponeert tot wel of niet virusgeı̈nduceerde (auto-) immuunziekten zoals AIDS en kanker.‘Zinc deficiency predisposes to (auto) immune diseases, whether or not virus-induced, such as AIDS and cancer. Tijdschrift Integrale Geneeskunde 1993, 9, 253–273. [Google Scholar]
- Sprietsma, J. Nutriënten in AIDS-therapie [Nutrients in AIDS therapy]. Orthomoleculair 1993, 11, 72–79. [Google Scholar]
- Mocchegiani, E.; Veccia, S.; Ancarani, F.; Scalise, G.; Fabris, N. Benefit of oral zinc supplementation as an adjunct to zidovudine (AZT) therapy against opportunistic infections in aids. Int. J. Immunopharmacol. 1995, 17, 719–727. [Google Scholar] [CrossRef]
- Baum, M.K.; Shor-Posner, G.; Campa, A. Zinc Status in Human Immunodeficiency Virus Infection. J. Nutr. 2000, 130, 1421S–1423S. [Google Scholar] [CrossRef] [PubMed]
- Wellinghausen, N.; Kern, W.V.; Jöchle, W.; Kern, P. Zinc serum level in human immunodeficiency virus-infected patients in relation to immunological status. Biol. Trace. Elem. Res. 2000, 73, 139–149. [Google Scholar] [CrossRef]
- Odeh, M. The role of zinc in acquired immunodeficiency syndrome. J. Intern Med. 1992, 231, 463–469. [Google Scholar] [CrossRef]
- Falutz, J.; Tsoukas, C.; Gold, P. Zinc as a Cofactor in Human Immunodeficiency Virus—Induced Immunosuppression. JAMA 1988, 259, 2850–2851. [Google Scholar] [CrossRef]
- Graham, N.; Sorensen, D.; Odaka, N.; Brookmeyer, R.; Chan, D.; Willett, W.C.; Morris, J.S.; Saah, A.J. Relationship of serum copper and zinc levels to HIV-1 seropositivity and progression to AIDS. J. Acquir. Immune. Defic. Syndr. 1991, 4, 976–980. [Google Scholar]
- Baum, M.K.; Shor-Posner, G.; Lu, Y.; Rosner, B.; Sauberlich, H.E.; Fletcher, M.A.; Szapocznik, J.; Eisdorfer, C.; Buring, J.E.; Hennekens, C.H. Micronutrients and HIV-1 disease progression. AIDS 1995, 9, 1051–1056. [Google Scholar] [CrossRef]
- Baum, M.K.; Shor-Posner, G.; Lai, S.; Zhang, G.; Lai, H.; Fletcher, M.A.; Sauberlich, H.; Page, J.B. High Risk of HIV-Related Mortality Is Associated With Selenium Deficiency. J. Acquir. Immune. Defic. Syndr. 1997, 15, 370–374. [Google Scholar] [CrossRef]
- Beutler, B.; Cerami, A. Cachectin: More Than a Tumor Necrosis Factor. N. Engl. J. Med. 1987, 316, 379–385. [Google Scholar] [CrossRef]
- Flieger, D.; Riethmüller, G.; Ziegler-Heitbrock, H. Zn2+ inhibits both tumor necrosis factor-mediated DNA fragmentation and cytolysis. Int. J. Cancer 1989, 44, 315–319. [Google Scholar] [CrossRef]
- Rosenberg, Z.F.; Fauci, A.S. Immunopathogenic mechanisms of HIV infection. Clin. Immunol. Immunopathol. 1989, 50, S149–S156. [Google Scholar] [CrossRef]
- Dowd, P.S.; Kelleher, J.; Guillou, P. T-lymphocyte subsets and interleukin-2 production in zinc-deficient rats. Br. J. Nutr. 1986, 55, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Hart, P.H.; Vitti, G.F.; Burgess, D.R.; Whitty, G.A.; Piccoli, D.S.; Hamilton, J.A. Potential antiinflammatory effects of interleukin 4: Suppression of human monocyte tumor necrosis factor alpha, interleukin 1, and prostaglandin E2. Proc. Natl. Acad. Sci. USA 1989, 86, 3803–3807. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, Z.F.; Fauci, A.S. Immunopathogenic mechanisms of HIV infection: Cytokine induction of HIV expression. Immunol. Today 1990, 11, 176–180. [Google Scholar] [CrossRef]
- Favier, A.; Sappey, C.; Leclerc, P.; Faure, P.; Micoud, M. Antioxidant status and lipid peroxidation in patients infected with HIV. Chem. Biol. Interact. 1994, 91, 165–180. [Google Scholar] [CrossRef]
- Ivanov, A.V.; Valuev-Elliston, V.T.; Ivanova, O.N.; Kochetkov, S.N.; Starodubova, E.S.; Bartosch, B.; Isaguliants, M.G. Oxidative Stress during HIV Infection: Mechanisms and Consequences. Oxid. Med. Cell Longev. 2016, 2016, 8910396. [Google Scholar] [CrossRef]
- Edeas, M.A.; Peltier, E.; Claise, C.; Khalfoun, Y.; Lindenbaum, A. Immunocytochemical study of uptake of exogenous carrier-free copper-zinc superoxide dismutase by peripheral blood lymphocytes. Cell. Mol. Biol. (Noisy-le-grand) 1996, 42, 1137–1143. [Google Scholar]
- Zheng, R.; Jenkins, T.M.; Craigie, R. Zinc folds the N-terminal domain of HIV-1 integrase, promotes multimerization, and enhances catalytic activity. Proc. Natl. Acad. Sci. USA 1996, 93, 13659–13664. [Google Scholar] [CrossRef]
- Cai, M.; Zheng, R.; Caffrey, M.; Craigie, R.; Clore, G.M.; Gronenborn, A.M. Solution structure of the N-terminal zinc binding domain of HIV-1 integrase. Nat. Struct. Biol. 1997, 4, 567–577. [Google Scholar] [CrossRef]
- Lee, S.P.; Xiao, J.; Knutson, J.R.; Lewis, M.S.; Han, M.K. Zn2+ Promotes the Self-Association of Human Immunodeficiency Virus Type-1 Integrase in Vitro. Biochemistry 1997, 36, 173–180. [Google Scholar] [CrossRef]
- Asante-Appiah, E.; Skalka, A.M. HIV-1 Integrase: Structural Organization, Conformational Changes, and Catalysis. Adv. Virus Res. 1999, 52, 351–369. [Google Scholar] [PubMed]
- Dorfman, T.; Luban, J.; Goff, S.P.; Haseltine, W.A.; Göttlinger, H.G. Mapping of functionally important residues of a cysteine-histidine box in the human immunodeficiency virus type 1 nucleocapsid protein. J. Virol. 1993, 67, 6159–6169. [Google Scholar] [CrossRef] [PubMed]
- Buckman, J.S.; Bosche, W.J.; Gorelick, R.J. Human Immunodeficiency Virus Type 1 Nucleocapsid Zn2+ Fingers Are Required for Efficient Reverse Transcription, Initial Integration Processes, and Protection of Newly Synthesized Viral DNA. J. Virol. 2003, 77, 1469–1480. [Google Scholar] [CrossRef] [PubMed]
- Lee, N.; Gorelick, R.J.; Musier-Forsyth, K. Zinc finger-dependent HIV-1 nucleocapsid protein–TAR RNA interactions. Nucleic Acids Res. 2003, 31, 4847–4855. [Google Scholar] [CrossRef][Green Version]
- Grigorov, B.; Décimo, D.; Smagulova, F.; Péchoux, C.; Mougel, M.; Muriaux, D.; Darlix, J.-L. Intracellular HIV-1 Gag localization is impaired by mutations in the nucleocapsid zinc fingers. Retrovirology 2007, 4, 54. [Google Scholar] [CrossRef]
- Mujeeb, A.; Ulyanov, N.B.; Georgantis, S.; Smirnov, I.; Chung, J.; Parslow, T.G.; James, T.L. Nucleocapsid protein-mediated maturation of dimer initiation complex of full-length SL1 stemloop of HIV-1: Sequence effects and mechanism of RNA refolding. Nucleic Acids Res. 2007, 35, 2026–2034. [Google Scholar] [CrossRef]
- Ali, L.M.; Rizvi, T.A.; Mustafa, F. Cross- and Co-Packaging of Retroviral RNAs and Their Consequences. Viruses 2016, 8, 276. [Google Scholar] [CrossRef]
- Lingappa, J.R.; Dooher, J.E.; Newman, M.A.; Kiser, P.K.; Klein, K.C. Basic Residues in the Nucleocapsid Domain of Gag Are Required for Interaction of HIV-1 Gag with ABCE1 (HP68), a Cellular Protein Important for HIV-1 Capsid Assembly. J. Biol. Chem. 2006, 281, 3773–3784. [Google Scholar] [CrossRef]
- Dussupt, V.; Javid, M.P.; Abou-Jaoudé, G.; Jadwin, J.A.; de La Cruz, J.; Nagashima, K.; Bouamr, F. The Nucleocapsid Region of HIV-1 Gag Cooperates with the PTAP and LYPXnL Late Domains to Recruit the Cellular Machinery Necessary for Viral Budding. PLoS Pathog. 2009, 5, e1000339. [Google Scholar] [CrossRef]
- Muriaux, D.; Darlix, J.-L. Properties and functions of the nucleocapsid protein in virus assembly. RNA Biol. 2010, 7, 744–753. [Google Scholar] [CrossRef]
- Rice, A.P.; Carlotti, F. Mutational analysis of the conserved cysteine-rich region of the human immunodeficiency virus type 1 Tat protein. J. Virol. 1990, 64, 1864–1868. [Google Scholar] [CrossRef] [PubMed]
- Fridell, R.A.; Harding, L.S.; Bogerd, H.P.; Cullen, B.R. Identification of a Novel Human Zinc Finger Protein That Specifically Interacts with the Activation Domain of Lentiviral Tat Proteins. Virology 1995, 209, 347–357. [Google Scholar] [CrossRef] [PubMed]
- Albini, A.; Benelli, R.; Giunciuglio, D.; Cai, T.; Mariani, G.; Ferrini, S.; Noonan, D.M. Identification of a Novel Domain of HIV Tat Involved in Monocyte Chemotaxis. J. Biol. Chem. 1998, 273, 15895–15900. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.-W.; Wang, K.-T. Structural Characterization of the Metal Binding Site in the Cysteine-Rich Region of HIV-1 Tat Protein. Biochem. Biophys. Res. Commun. 1996, 227, 615–621. [Google Scholar] [CrossRef]
- Song, L.; Nath, A.; Geiger, J.D.; Moore, A.; Hochman, S. Human Immunodeficiency Virus Type 1 Tat Protein Directly Activates Neuronal N-methyl-D-aspartate Receptors at an Allosteric Zinc-Sensitive Site. J. Neurovirol. 2003, 9, 399–403. [Google Scholar] [CrossRef]
- Egelé, C.; Barbier, P.; Didier, P.; Piémont, E.; Allegro, D.; Chaloin, O.; Muller, S.; Peyrot, V.; Mély, Y. Modulation of microtubule assembly by the HIV-1 Tat protein is strongly dependent on zinc binding to Tat. Retrovirology 2008, 5, 62. [Google Scholar] [CrossRef]
- Chandra, T.; Maier, W.; König, H.G.; Hirzel, K.; Kögel, D.; Schüler, T.; Chandra, A.; Demirhan, I.; Laube, B. Molecular interactions of the type 1 human immunodeficiency virus transregulatory protein Tat with N-methyl-d-aspartate receptor subunits. Neuroscience 2005, 134, 145–153. [Google Scholar] [CrossRef]
- Haughey, N.J.; Nath, A.; Mattson, M.P.; Slevin, J.T.; Geiger, J.D. HIV-1 Tat through phosphorylation of NMDA receptors potentiates glutamate excitotoxicity. J. Neurochem. 2001, 78, 457–467. [Google Scholar] [CrossRef]
- Misumi, S.; Takamune, N.; Ohtsubo, Y.; Waniguchi, K.; Shoji, S. Zn2+ Binding to Cysteine-Rich Domain of Extracellular Human Immunodeficiency Virus Type 1 Tat Protein Is Associated with Tat Protein-Induced Apoptosis. AIDS Res. Hum. Retroviruses 2004, 20, 297–304. [Google Scholar] [CrossRef]
- Luo, K.; Xiao, Z.; Ehrlich, E.; Yu, Y.; Liu, B.; Zheng, S.; Yu, X.-F. Primate lentiviral virion infectivity factors are substrate receptors that assemble with cullin 5-E3 ligase through a HCCH motif to suppress APOBEC3G. Proc. Natl. Acad. Sci. USA 2005, 102, 11444–11449. [Google Scholar] [CrossRef]
- Wang, X.; Ao, Z.; Chen, L.; Kobinger, G.; Peng, J.; Yao, X. The Cellular Antiviral Protein APOBEC3G Interacts with HIV-1 Reverse Transcriptase and Inhibits Its Function during Viral Replication. J. Virol. 2012, 86, 3777–3786. [Google Scholar] [CrossRef] [PubMed]
- Paul, I.; Cui, J.; Maynard, E.L. Zinc binding to the HCCH motif of HIV-1 virion infectivity factor induces a conformational change that mediates protein-protein interactions. Proc. Natl. Acad. Sci. USA 2006, 103, 18475–18480. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Gao, G. ZAP-mediated mRNA degradation. RNA Biol. 2008, 5, 65–67. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Chen, G.; Lv, F.; Wang, X.; Ji, X.; Xu, Y.; Sun, J.; Wu, L.; Zheng, Y.-T.; Gao, G. Zinc-finger antiviral protein inhibits HIV-1 infection by selectively targeting multiply spliced viral mRNAs for degradation. Proc. Natl. Acad. Sci. USA 2011, 108, 15834–15839. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Wang, X.; Tu, F.; Wang, Q.; Fan, Z.; Gao, G. TRIM25 Is Required for the Antiviral Activity of Zinc Finger Antiviral Protein. J. Virol. 2017, 91, e00088-17. [Google Scholar] [CrossRef] [PubMed]
- Cairo, G.; Bernuzzi, F.; Recalcati, S. A precious metal: Iron, an essential nutrient for all cells. Genes Nutr. 2006, 1, 25–39. [Google Scholar] [CrossRef] [PubMed]
- Dlouhy, A.C.; Outten, C.E. The iron metallome in eukaryotic organisms. Met. Ions Life Sci. 2013, 12, 241–278. [Google Scholar]
- Ganz, T. Molecular Control of Iron Transport. J. Am. Soc. Nephrol. 2007, 18, 394–400. [Google Scholar] [CrossRef]
- Ganz, T. Systemic Iron Homeostasis. Physiol. Rev. 2013, 93, 1721–1741. [Google Scholar] [CrossRef]
- Anderson, G.J.; Vulpe, C.D. The Cellular Physiology of Iron. In Iron Deficiency and Overload: From Basic Biology to Clinical Medicine; Yehuda, S., Mostofsky, D.I., Eds.; Humana Press: Totowa, NJ, USA, 2010; pp. 3–29. [Google Scholar]
- Anderson, G.J.; Frazer, D.M. Current understanding of iron homeostasis. Am. J. Clin. Nutr. 2017, 106 (Suppl. 6), 1559S–1566S. [Google Scholar] [CrossRef]
- Sharp, P.; Srai, S.-K. Molecular mechanisms involved in intestinal iron absorption. World J. Gastroenterol. 2007, 13, 4716–4724. [Google Scholar] [CrossRef] [PubMed]
- Graham, R.M.; Reutens, G.M.; Herbison, C.E.; Delima, R.D.; Chua, A.C.G.; Olynyk, J.K.; Trinder, D. Transferrin receptor 2 mediates uptake of transferrin-bound and non-transferrin-bound iron. J. Hepatol. 2008, 48, 327–334. [Google Scholar] [CrossRef] [PubMed]
- Mayle, K.M.; Le, A.M.; Kamei, D.T. The intracellular trafficking pathway of transferrin. Biochim. Biophys. Acta 2012, 1820, 264–281. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.; Schroeder, B.; Chen, J.; Schott, M.B.; McNiven, M.A. The Endocytic Fate of the Transferrin Receptor Is Regulated by c-Abl Kinase. J. Biol. Chem. 2016, 291, 16424–16437. [Google Scholar] [CrossRef]
- Fernández, B.; Fdez, E.; Gómez-Suaga, P.; Gil, F.; Molina-Villalba, I.; Ferrer, I.; Patel, S.; Churchill, G.C.; Hilfiker, S. Iron overload causes endolysosomal deficits modulated by NAADP-regulated 2-pore channels and RAB7A. Autophagy 2016, 12, 1487–1506. [Google Scholar] [CrossRef]
- Lambe, T.; Simpson, R.J.; Dawson, S.; Bouriez-Jones, T.; Crockford, T.L.; Lepherd, M.; Latunde-Dada, G.O.; Robinson, H.; Raja, K.B.; Campagna, D.R.; et al. Identification of a Steap3 endosomal targeting motif essential for normal iron metabolism. Blood 2009, 113, 1805–1808. [Google Scholar] [CrossRef]
- Tabuchi, M.; Yoshimori, T.; Yamaguchi, K.; Yoshida, T.; Kishi, F. Human NRAMP2/DMT1, Which Mediates Iron Transport across Endosomal Membranes, Is Localized to Late Endosomes and Lysosomes in HEp-2 Cells. J. Biol. Chem. 2000, 275, 22220–22228. [Google Scholar] [CrossRef]
- Dong, X.-P.; Cheng, X.; Mills, E.; Delling, M.; Wang, F.; Kurz, T.; Xu, H. The type IV mucolipidosis-associated protein TRPML1 is an endolysosomal iron release channel. Nature 2008, 455, 996. [Google Scholar] [CrossRef]
- Winterbourn, C.C. Toxicity of iron and hydrogen peroxide: The Fenton reaction. Toxicol. Lett. 1995, 82-83, 969–974. [Google Scholar] [CrossRef]
- Hemnani, T.; Parihar, M. Reactive oxygen species and oxidative DNA damage. Indian. J. Physiol. Pharmacol. 1998, 42, 440–452. [Google Scholar]
- Cejas, P.; Casado, E.; Belda-Iniesta, C.; De Castro, J.; Espinosa, E.; Redondo, A.; Sereno, M.; García-Cabezas, M.Á.; Juan, A.F.V.; Domínguez-Cáceres, A.; et al. Implications of Oxidative Stress and Cell Membrane Lipid Peroxidation in Human Cancer (Spain). Cancer Causes Control 2004, 15, 707–719. [Google Scholar] [CrossRef] [PubMed]
- Lei, P.; Bai, T.; Sun, Y. Mechanisms of Ferroptosis and Relations With Regulated Cell Death: A Review. Front. Physiol. 2019, 10. [Google Scholar] [CrossRef] [PubMed]
- Stoyanovsky, D.A.; Tyurina, Y.Y.; Shrivastava, I.; Bahar, I.; Tyurin, V.A.; Protchenko, O.; Jadhav, S.; Bolevich, S.B.; Kozlov, A.V.; Vladimirov, Y.A.; et al. Iron catalysis of lipid peroxidation in ferroptosis: Regulated enzymatic or random free radical reaction? Free Radic. Biol. Med. 2019, 133, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Birben, E.; Sahiner, U.M.; Sackesen, C.; Erzurum, S.; Kalayci, O. Oxidative Stress and Antioxidant Defense. World Allergy Organ. J. 2012, 5, 9–19. [Google Scholar] [CrossRef]
- Kurutas, E.B. The importance of antioxidants which play the role in cellular response against oxidative/nitrosative stress: Current state. Nutr. J. 2016, 15, 71. [Google Scholar] [CrossRef]
- Drakesmith, H.; Prentice, A. Viral infection and iron metabolism. Nat. Rev. Microbiol. 2008, 6, 541–552. [Google Scholar] [CrossRef]
- Nekhai, S.; Kumari, N.; Dhawan, S. Role of cellular iron and oxygen in the regulation of HIV-1 infection. Future Virol. 2013, 8, 301–311. [Google Scholar] [CrossRef]
- Boelaert, J.R.; Weinberg, G.A.; Weinberg, E.D. Altered iron metabolism in HIV infection: Mechanisms, possible consequences, and proposals for management. Infect. Agents Dis. 1996, 5, 36–46. [Google Scholar]
- Gordeuk, V.R.; Delanghe, J.R.; Langlois, M.R.; Boelaert, J.R. Iron status and the outcome of HIV infection: An overview. J. Clin. Virol. 2001, 20, 111–115. [Google Scholar] [CrossRef]
- Drakesmith, H.; Chen, N.; Ledermann, H.; Screaton, G.; Townsend, A.; Xu, X.-N. HIV-1 Nef down-regulates the hemochromatosis protein HFE, manipulating cellular iron homeostasis. Proc. Natl. Acad. Sci. USA 2005, 102, 11017–11022. [Google Scholar] [CrossRef]
- Rawat, R.; Humphrey, J.H.; Ntozini, R.; Mutasa, K.; Iliff, P.J.; Stoltzfus, R.J. Elevated iron stores are associated with HIV disease severity and mortality among postpartum women in Zimbabwe. Public Health Nutr. 2009, 12, 1321–1329. [Google Scholar] [CrossRef] [PubMed]
- McDermid, J.M.; Jaye, A.; Schim van der Loeff, M.F.; Todd, J.; Bates, C.; Austin, S.; Jeffries, D.; Awasana, A.A.; Whittle, H.C.; Prentice, A.M. Elevated Iron Status Strongly Predicts Mortality in West African Adults With HIV Infection. J. Acquir. Immune Defic. Syndr. 2007, 46, 498–507. [Google Scholar] [CrossRef] [PubMed]
- Barthelme, D.; Scheele, U.; Dinkelaker, S.; Janoschka, A.; MacMillan, F.; Albers, S.-V.; Driessen, A.J.M.; Stagni, M.S.; Bill, E.; Meyer-Klaucke, W.; et al. Structural Organization of Essential Iron-Sulfur Clusters in the Evolutionarily Highly Conserved ATP-binding Cassette Protein ABCE1. J. Biol. Chem. 2007, 282, 14598–14607. [Google Scholar] [CrossRef] [PubMed]
- Zimmerman, C.; Klein, K.C.; Kiser, P.K.; Singh, A.R.; Firestein, B.L.; Riba, S.C.; Lingappa, J.R. Identification of a host protein essential for assembly of immature HIV-1 capsids. Nature 2002, 415, 88–92. [Google Scholar] [CrossRef] [PubMed]
- Schatz, O.; Oft, M.; Dascher, C.; Schebesta, M.; Rosorius, O.; Jaksche, H.; Dobrovnik, M.; Bevec, D.; Hauber, J. Interaction of the HIV-1 Rev cofactor eukaryotic initiation factor 5A with ribosomal protein L5. Proc. Natl. Acad. Sci. USA 1998, 95, 1607–1612. [Google Scholar] [CrossRef] [PubMed]
- Modem, S.; Thipparthi, R.R. Cellular Proteins and HIV-1 Rev Function. Curr. HIV Res. 2009, 7, 91–100. [Google Scholar]
- Liu, J.; Henao-Mejia, J.; Liu, H.; Zhao, Y.; He, J.J. Translational Regulation of HIV-1 Replication by HIV-1 Rev Cellular Cofactors Sam68, eIF5A, hRIP, and DDX3. J. Neuroimmune Pharmacol. 2011, 6, 308–321. [Google Scholar] [CrossRef]
- Hoque, M.; Hanauske-Abel, H.M.; Palumbo, P.; Saxena, D.; D’Alliessi Gandolfi, D.; Park, M.H.; Pe’ery, T.; Mathews, M.B. Inhibition of HIV-1 gene expression by Ciclopirox and Deferiprone, drugs that prevent hypusination of eukaryotic initiation factor 5A. Retrovirology 2009, 6, 90. [Google Scholar] [CrossRef]
- Sattentau, Q.J.; Stevenson, M. Macrophages and HIV-1: An Unhealthy Constellation. Cell Host Microbe. 2016, 19, 304–310. [Google Scholar] [CrossRef]
- Knutson, M.D.; Oukka, M.; Koss, L.M.; Aydemir, F.; Wessling-Resnick, M. Iron release from macrophages after erythrophagocytosis is up-regulated by ferroportin 1 overexpression and down-regulated by hepcidin. Proc. Natl. Acad. Sci. USA 2005, 102, 1324–1328. [Google Scholar] [CrossRef]
- Beaumont, C. Multiple regulatory mechanisms act in concert to control ferroportin expression and heme iron recycling by macrophages. Haematologica 2010, 95, 1233–1236. [Google Scholar] [CrossRef] [PubMed]
- Finn, A.V.; Nakano, M.; Polavarapu, R.; Karmali, V.; Saeed, O.; Zhao, X.; Yazdani, S.; Otsuka, F.; Davis, T.; Habib, A.; et al. Hemoglobin Directs Macrophage Differentiation and Prevents Foam Cell Formation in Human Atherosclerotic Plaques. J. Am. Coll. Cardiol. 2012, 59, 166–177. [Google Scholar] [CrossRef] [PubMed]
- Schimanski, L.M.; Drakesmith, H.; Merryweather-Clarke, A.T.; Viprakasit, V.; Edwards, J.P.; Sweetland, E.; Bastin, J.M.; Cowley, D.; Chinthammitr, Y.; Robson, K.J.H.; et al. In vitro functional analysis of human ferroportin (FPN) and hemochromatosis-associated FPN mutations. Blood 2005, 105, 4096–4102. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Kashanchi, F.; Foster, A.; Rotimi, J.; Turner, W.; Gordeuk, V.R.; Nekhai, S. Hepcidin induces HIV-1 transcription inhibited by ferroportin. Retrovirology 2010, 7, 104. [Google Scholar] [CrossRef]
- Jison, M.L.; Munson, P.J.; Barb, J.J.; Suffredini, A.F.; Talwar, S.; Logun, C.; Raghavachari, N.; Beigel, J.H.; Shelhamer, J.H.; Danner, R.L.; et al. Blood mononuclear cell gene expression profiles characterize the oxidant, hemolytic, and inflammatory stress of sickle cell disease. Blood 2004, 104, 270–280. [Google Scholar] [CrossRef]
- Kroot, J.J.C.; Laarakkers, C.M.M.; Kemna, E.H.J.M.; Biemond, B.J.; Swinkels, D.W. Regulation of serum hepcidin levels in sickle cell disease. Haematologica 2009, 94, 885–887. [Google Scholar] [CrossRef]
- Bagasra, O.; Steiner, R.M.; Ballas, S.K.; Castro, O.; Dornadula, G.; Embury, S.; Jungkind, D.; Bobroski, L.; Kutlar, A.; Burchott, S. Viral Burden and Disease Progression in HIV-1–Infected Patients with Sickle Cell Anemia. Am. J. Hematol. 1998, 59, 199–207. [Google Scholar] [CrossRef]
- Nouraie, M.; Nekhai, S.; Gordeuk, V.R. Sickle cell disease is associated with decreased HIV but higher HBV and HCV comorbidities in US hospital discharge records: A cross-sectional study. Sex Transm. Infect. 2012, 88, 528–533. [Google Scholar] [CrossRef]
- Taylor VI, J.G.; Ackah, D.; Cobb, C.; Orr, N.; Percy, M.J.; Sachdev, V.; Machado, R.; Castro, O.; Kato, G.J.; Chanock, S.J.; et al. Mutations and polymorphisms in hemoglobin genes and the risk of pulmonary hypertension and death in sickle cell disease. Am. J. Hematol. 2008, 83, 6–14. [Google Scholar] [CrossRef]
- Ferreira, A.; Marguti, I.; Bechmann, I.; Jeney, V.; Chora, Â.; Palha, N.R.; Rebelo, S.; Henri, A.; Beuzard, Y.; Soares, M.P. Sickle Hemoglobin Confers Tolerance to Plasmodium Infection. Cell 2011, 145, 398–409. [Google Scholar] [CrossRef]
- Debebe, Z.; Ammosova, T.; Jerebtsova, M.; Kurantsin-Mills, J.; Niu, X.; Charles, S.; Richardson, D.R.; Ray, P.E.; Gordeuk, V.R.; Nekhai, S. Iron chelators ICL670 and 311 inhibit HIV-1 transcription. Virology 2007, 367, 324–333. [Google Scholar] [CrossRef] [PubMed]
- Debebe, Z.; Ammosova, T.; Breuer, D.; Lovejoy, D.B.; Kalinowski, D.S.; Karla, P.K.; Kumar, K.; Jerebtsova, M.; Ray, P.; Kashanchi, F.; et al. Iron Chelators of the Di-2-pyridylketone Thiosemicarbazone and 2-Benzoylpyridine Thiosemicarbazone Series Inhibit HIV-1 Transcription: Identification of Novel Cellular Targets—Iron, Cyclin-Dependent Kinase (CDK) 2, and CDK9. Mol. Pharmacol. 2011, 79, 185–196. [Google Scholar] [CrossRef] [PubMed]
- St., Gelais, C.; de Silva, S.; Hach, J.C.; White, T.E.; Diaz-Griffero, F.; Yount, J.S.; Wu, L. Identification of Cellular Proteins Interacting with the Retroviral Restriction Factor SAMHD1. J. Virol. 2014, 88, 5834–5844. [Google Scholar] [CrossRef] [PubMed]
- Goldstone, D.C.; Ennis-Adeniran, V.; Hedden, J.J.; Groom, H.C.T.; Rice, G.I.; Christodoulou, E.; Walker, P.A.; Kelly, G.; Haire, L.F.; Yap, M.W.; et al. HIV-1 restriction factor SAMHD1 is a deoxynucleoside triphosphate triphosphohydrolase. Nature 2011, 480, 379–382. [Google Scholar] [CrossRef] [PubMed]
- Laguette, N.; Sobhian, B.; Casartelli, N.; Ringeard, M.; Chable-Bessia, C.; Ségéral, E.; Yatim, A.; Emiliani, S.; Schwartz, O.; Benkirane, M. SAMHD1 is the dendritic- and myeloid-cell-specific HIV-1 restriction factor counteracted by Vpx. Nature 2011, 474, 654–657. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Li, C.; Huang, J.; Cung, T.; Seiss, K.; Beamon, J.; Carrington, M.F.; Porter, L.C.; Burke, P.S.; Yang, Y.; et al. CD4+ T cells from elite controllers resist HIV-1 infection by selective upregulation of p21. J. Clin. Investig. 2011, 121, 1549–1560. [Google Scholar] [CrossRef] [PubMed]
- Choi, B.H.; Kim, C.G.; Bae, Y.-S.; Lim, Y.; Lee, Y.H.; Shin, S.Y. p21Waf1/Cip1 Expression by Curcumin in U-87MG Human Glioma Cells: Role of Early Growth Response-1 Expression. Cancer Res. 2008, 68, 1369–1377. [Google Scholar] [CrossRef]
- Saletta, F.; Suryo Rahmanto, Y.; Noulsri, E.; Richardson, D.R. Iron Chelator-Mediated Alterations in Gene Expression: Identification of Novel Iron-Regulated Molecules That Are Molecular Targets of Hypoxia-Inducible Factor-1α and p53. Mol. Pharmacol. 2010, 77, 443–458. [Google Scholar] [CrossRef]
- Kaul, D.K.; Fabry, M.E.; Suzuka, S.M.; Zhang, X. Antisickling fetal hemoglobin reduces hypoxia-inducible factor-1α expression in normoxic sickle mice: Microvascular implications. Am. J. Physiol. Heart Circ. Physiol. 2013, 304, H42–H50. [Google Scholar] [CrossRef]
- Devadas, K.; Dhawan, S. Hemin Activation Ameliorates HIV-1 Infection via Heme Oxygenase-1 Induction. J. Immunol. 2006, 176, 4252–4257. [Google Scholar] [CrossRef]
- Devadas, K.; Hewlett, I.K.; Dhawan, S. Lipopolysaccharide suppresses HIV-1 replication in human monocytes by protein kinase C-dependent heme oxygenase-1 induction. J. Leukoc. Biol. 2010, 87, 915–924. [Google Scholar] [CrossRef] [PubMed]
- Kumari, N.; Ammosova, T.; Diaz, S.; Lin, X.; Niu, X.; Ivanov, A.; Jerebtsova, M.; Dhawan, S.; Oneal, P.; Nekhai, S. Increased iron export by ferroportin induces restriction of HIV-1 infection in sickle cell disease. Blood Adv. 2016, 1, 170–183. [Google Scholar] [CrossRef]
- Berra, E.; Benizri, E.; Ginouvès, A.; Volmat, V.; Roux, D.; Pouysségur, J. HIF prolyl-hydroxylase 2 is the key oxygen sensor setting low steady-state levels of HIF-1alpha in normoxia. EMBO J. 2003, 22, 4082–4090. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Ammosova, T.; Kumari, N.; Nekhai, S. Protein Phosphatase-1-targeted Small Molecules, Iron Chelators and Curcumin Analogs as HIV-1 Antivirals. Curr. Pharm. Des. 2017, 23, 4122–4132. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Chen, R.; Liu, M.; Li, H.; Xue, Y.; Ramey, W.N.; He, N.; Ai, N.; Luo, H.; Zhu, Y.; Zhou, N.; et al. PP2B and PP1α cooperatively disrupt 7SK snRNP to release P-TEFb for transcription in response to Ca2+ signaling. Genes Dev. 2008, 22, 1356–1368. [Google Scholar] [CrossRef]
- Ammosova, T.; Obukhov, Y.; Kotelkin, A.; Breuer, D.; Beullens, M.; Gordeuk, V.R.; Bollen, M.; Nekhai, S. Protein phosphatase-1 activates CDK9 by dephosphorylating Ser175. PLoS ONE 2011, 6, e18985. [Google Scholar] [CrossRef]
- Taylor, C.T.; Furuta, G.T.; Synnestvedt, K.; Colgan, S.P. Phosphorylation-dependent targeting of cAMP response element binding protein to the ubiquitin/proteasome pathway in hypoxia. Proc. Natl. Acad. Sci. USA 2000, 97, 12091–12096. [Google Scholar] [CrossRef]
- Comerford, K.M.; Leonard, M.O.; Cummins, E.P.; Fitzgerald, K.T.; Beullens, M.; Bollen, M.; Taylor, C.T. Regulation of protein phosphatase 1γ activity in hypoxia through increased interaction with NIPP1: Implications for cellular metabolism. J. Cell. Physiol. 2006, 209, 211–218. [Google Scholar] [CrossRef]
- Ammosova, T.; Yedavalli, V.R.; Niu, X.; Jerebtsova, M.; Van Eynde, A.; Beullens, M.; Bollen, M.; Jeang, K.-T.; Nekhai, S. Expression of a protein phosphatase 1 inhibitor, cdNIPP1, increases CDK9 threonine 186 phosphorylation and inhibits HIV-1 transcription. J. Biol. Chem. 2011, 286, 3798–3804. [Google Scholar] [CrossRef]
- Nekhai, S.; Jerebtsova, M.; Jackson, A.; Southerland, W. Regulation of HIV-1 transcription by protein phosphatase 1. Curr. HIV Res. 2007, 5, 3–9. [Google Scholar]
- Tabor, E.; Epstein, J.; Hewlett, I.; Lee, S. Inhibition by desferrioxamine of in-vitro replication of HIV-1. Lancet 1991, 337, 795. [Google Scholar] [CrossRef]
- Van Asbeck, B.S.; Georgiou, N.A.; van der Bruggen, T.; Oudshoorn, M.; Nottet, H.S.L.M.; Marx, J.J.M. Anti-HIV effect of iron chelators: Different mechanisms involved. J. Clin. Virol. 2001, 20, 141–147. [Google Scholar] [CrossRef]
- Ammosova, T.; Berro, R.; Kashanchi, F.; Nekhai, S. RNA interference directed to CDK2 inhibits HIV-1 transcription. Virology 2005, 341, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Guendel, I.; Carpio, L.; Easley, R.; Van Duyne, R.; Coley, W.; Agbottah, E.; Dowd, C.; Kashanchi, F.; Kehn-Hall, K. 9-aminoacridine Inhibition of HIV-1 Tat Dependent Transcription. Virol. J. 2009, 6, 114. [Google Scholar] [CrossRef] [PubMed]
- Coulonval, K.; Bockstaele, L.; Paternot, S.; Roger, P.P. Phosphorylations of Cyclin-dependent Kinase 2 Revisited Using Two-dimensional Gel Electrophoresis. J. Biol. Chem. 2003, 278, 52052–52060. [Google Scholar] [CrossRef] [PubMed]
- Herbeck, J.; Ghorai, S.; Chen, L.; Rinaldo, C.R.; Margolick, J.B.; Detels, R.; Jacobson, L.; Wolinsky, S.; Mullins, J.I. p21WAF1/CIP1 RNA Expression in Highly HIV-1 Exposed, Uninfected Individuals. PLoS ONE 2015, 10, e0119218. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wu, W.; Kehn-Hall, K.; Pedati, C.; Zweier, L.; Castro, I.; Klase, Z.; Dowd, C.S.; Dubrovsky, L.; Bukrinsky, M.; Kashanchi, F. Drug 9AA reactivates p21/Waf1 and Inhibits HIV-1 progeny formation. Virol. J. 2008, 5, 41. [Google Scholar] [CrossRef][Green Version]
- Sáez-Cirión, A.; Hamimi, C.; Bergamaschi, A.; David, A.; Versmisse, P.; Mélard, A.; Boufassa, F.; Barré-Sinoussi, F.; Lambotte, O.; Rouzioux, C.; et al. Restriction of HIV-1 replication in macrophages and CD4+ T cells from HIV controllers. Blood 2011, 118, 955–964. [Google Scholar] [CrossRef]
- Kashanchi, F.; Agbottah, E.T.; Pise-Masison, C.A.; Mahieux, R.; Duvall, J.; Kumar, A.; Brady, J.N. Cell cycle-regulated transcription by the human immunodeficiency virus type 1 Tat transactivator. J. Virol. 2000, 74, 652–660. [Google Scholar] [CrossRef]
- Deng, L.; Ammosova, T.; Pumfery, A.; Kashanchi, F.; Nekhai, S. HIV-1 Tat Interaction with RNA Polymerase II C-terminal Domain (CTD) and a Dynamic Association with CDK2 Induce CTD Phosphorylation and Transcription from HIV-1 Promoter. J. Biol. Chem. 2002, 277, 33922–33929. [Google Scholar] [CrossRef]
- Nekhai, S.; Zhou, M.; Fernandez, A.; Lane, W.S.; Lamb, N.J.; Brady, J.; Kumar, A. HIV-1 Tat-associated RNA polymerase C-terminal domain kinase, CDK2, phosphorylates CDK7 and stimulates Tat-mediated transcription. Biochem. J. 2002, 364, 649–657. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Li, Z.; Niu, J.; Xu, Y.; Ma, L.; Lu, A.; Wang, X.; Qian, Z.; Huang, Z.; Jin, X.; et al. Antiviral effects of ferric ammonium citrate. Cell Discov. 2018, 4, 14. [Google Scholar] [CrossRef] [PubMed]
- Malina, J.; Hannon, M.J.; Brabec, V. Iron(II) supramolecular helicates interfere with the HIV-1 Tat–TAR RNA interaction critical for viral replication. Sci. Rep. 2016, 6, 29674. [Google Scholar] [CrossRef] [PubMed]
- Mancone, C.; Grimaldi, A.; Refolo, G.; Abbate, I.; Rozera, G.; Benelli, D.; Fimia, G.M.; Barnaba, V.; Tripodi, M.; Piacentini, M.; et al. Iron overload down-regulates the expression of the HIV-1 Rev cofactor eIF5A in infected T lymphocytes. Proteome Sci. 2017, 15, 18. [Google Scholar] [CrossRef]
- Shytaj, I.; Lucic, B.; Forcato, M.; Billingsley, J.; Bosinger, S.; Stanic, M.; Gregoretti, F.; Antonelli, L.; Oliva, G.; Frese, C.; et al. Alterations of redox and iron metabolism accompany development of HIV latency. bioRxiv 2019, 11, e102209. [Google Scholar] [CrossRef]
- Piette, J.; Legrand-Poels, S. HIV-1 reactivation after an oxidative stress mediated by different reactive oxygen species. Chem. Biol. Interact. 1994, 91, 79–89. [Google Scholar] [CrossRef]
- Song, S.; Christova, T.; Perusini, S.; Alizadeh, S.; Bao, R.-Y.; Miller, B.W.; Hurren, R.; Jitkova, Y.; Gronda, M.; Isaac, M.; et al. Wnt Inhibitor Screen Reveals Iron Dependence of β-Catenin Signaling in Cancers. Cancer Res. 2011, 71, 7628–7639. [Google Scholar] [CrossRef]
- Coombs, G.S.; Schmitt, A.A.; Canning, C.A.; Alok, A.; Low, I.C.C.; Banerjee, N.; Kaur, S.; Utomo, V.; Jones, C.M.; Pervaiz, S.; et al. Modulation of Wnt/β-catenin signaling and proliferation by a ferrous iron chelator with therapeutic efficacy in genetically engineered mouse models of cancer. Oncogene 2012, 31, 213–225. [Google Scholar] [CrossRef]
- Holley, A.K.; Bakthavatchalu, V.; Velez-Roman, J.M.; St Clair, D.K. Manganese superoxide dismutase: Guardian of the powerhouse. Int. J. Mol. Sci. 2011, 12, 7114–7162. [Google Scholar] [CrossRef]
- Miriyala, S.; Spasojevic, I.; Tovmasyan, A.; Salvemini, D.; Vujaskovic, Z.; St Clair, D.; Batinic-Haberle, I. Manganese superoxide dismutase, MnSOD and its mimics. Biochim. Biophys Acta 2012, 1822, 794–814. [Google Scholar] [CrossRef]
- Baly, D.L.; Keen, C.L.; Hurley, L.S. Effects of manganese deficiency on pyruvate carboxylase and phosphoenolpyruvate carboxykinase activity and carbohydrate homeostasis in adult rats. Biol. Trace Elem. Res. 1986, 11, 201. [Google Scholar] [CrossRef] [PubMed]
- Wedler, F.C.; Denman, R.B.; Roby, W.G. Glutamine synthetase from ovine brain is a manganese(II) enzyme. Biochemistry 1982, 21, 6389–6396. [Google Scholar] [CrossRef] [PubMed]
- Sidoryk-Wegrzynowicz, M.; Aschner, M. Manganese toxicity in the central nervous system: The glutamine/glutamate-γ-aminobutyric acid cycle. J. Intern. Med. 2013, 273, 466–477. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Yang, X. The Essential Element Manganese, Oxidative Stress, and Metabolic Diseases: Links and Interactions. Oxid. Med. Cell. Longev. 2018, 2018, 7580707. [Google Scholar] [CrossRef]
- Raoul, H.; Mabondzo, A.; Le Naour, R.; Dormont, D. Effect of human immunodeficiency virus type 1 (HIV-1) monocyte-derived macrophages infection on the manganous superoxide dismutase gene expression. Chem. Biol. Interact. 1994, 91, 123–131. [Google Scholar] [CrossRef]
- Raoul, H.; Naour, R.L.; Blond, D.; Dormont, D. HIV Type 1 Infection of Human Macrophages Induces an Upregulation of Manganese Superoxide Dismutase Gene That May Protect Cells from Death. AIDS Res. Hum. Retroviruses 1998, 14, 427–434. [Google Scholar] [CrossRef]
- Sarafianos, S.G.; Marchand, B.; Das, K.; Himmel, D.M.; Parniak, M.A.; Hughes, S.H.; Arnold, E. Structure and Function of HIV-1 Reverse Transcriptase: Molecular Mechanisms of Polymerization and Inhibition. J. Mol. Biol. 2009, 385, 693–713. [Google Scholar] [CrossRef]
- Huang, H.; Chopra, R.; Verdine, G.L.; Harrison, S.C. Structure of a Covalently Trapped Catalytic Complex of HIV-1 Reverse Transcriptase: Implications for Drug Resistance. Science 1998, 282, 1669–1675. [Google Scholar] [CrossRef]
- Tian, L.; Kim, M.-S.; Li, H.; Wang, J.; Yang, W. Structure of HIV-1 reverse transcriptase cleaving RNA in an RNA/DNA hybrid. Proc. Natl. Acad. Sci. USA 2018, 115, 507–512. [Google Scholar] [CrossRef]
- Cirino, N.M.; Cameron, C.E.; Smith, J.S.; Roth, M.J.; Benkovic, S.J.; Le Grice, S.F.J. Divalent cation modulation of the ribonuclease functions of human immunodeficiency virus reverse transcriptase. Biochemistry 1995, 34, 9936–9943. [Google Scholar] [CrossRef]
- Klumpp, K.; Hang, J.Q.; Rajendran, S.; Yang, Y.; Derosier, A.; Wong Kai In, P.; Overton, H.; Parkes, K.E.B.; Cammack, N.; Martin, J.A. Two-metal ion mechanism of RNA cleavage by HIV RNase H and mechanism-based design of selective HIV RNase H inhibitors. Nucleic Acids Res. 2003, 31, 6852–6859. [Google Scholar] [CrossRef] [PubMed]
- Ben-Artzi, H.; Zeelon, E.; Le-Grice, S.F.J.; Gorecki, M.; Panet, A. Characterization of the double stranded RNA dependent RNase activity associated with recombinant reverse transcriptases. Nucleic Acids Res. 1992, 20, 5115–5118. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Goldschmidt, V.; Didierjean, J.; Ehresmann, B.; Ehresmann, C.; Isel, C.; Marquet, R. Mg2+ dependency of HIV-1 reverse transcription, inhibition by nucleoside analogues and resistance. Nucleic Acids Res. 2006, 34, 42–52. [Google Scholar] [CrossRef] [PubMed]
- Schultz, S.J.; Champoux, J.J. RNase H activity: Structure, specificity, and function in reverse transcription. Virus Res. 2008, 134, 86–103. [Google Scholar] [CrossRef] [PubMed]
- Vartanian, J.-P.; Sala, M.; Henry, M.; Wain-Hobson, S.; Meyerhans, A. Manganese cations increase the mutation rate of human immunodeficiency virus type 1 ex vivo. J. Gen. Virol. 1999, 80, 1983–1986. [Google Scholar] [CrossRef] [PubMed]
- Fenstermacher, K.J.; DeStefano, J.J. Mechanism of HIV reverse transcriptase inhibition by zinc: Formation of a highly stable enzyme-(primer-template) complex with profoundly diminished catalytic activity. J. Biol. Chem. 2011, 286, 40433–40442. [Google Scholar] [CrossRef] [PubMed]
- Delelis, O.; Carayon, K.; Saïb, A.; Deprez, E.; Mouscadet, J.-F. Integrase and integration: Biochemical activities of HIV-1 integrase. Retrovirology 2008, 5, 114. [Google Scholar] [CrossRef]
- Craigie, R. The molecular biology of HIV integrase. Future Virol. 2012, 7, 679–686. [Google Scholar] [CrossRef]
- Yi, J.; Asante-Appiah, E.; Skalka, A.M. Divalent Cations Stimulate Preferential Recognition of a Viral DNA End by HIV-1 Integrase. Biochemistry 1999, 38, 8458–8468. [Google Scholar] [CrossRef]
- Thang, K.C.; David, R.D. Structure and Function of HIV-1 Integrase. Curr. Top. Med. Chem. 2004, 4, 965–977. [Google Scholar]
- Asante-Appiah, E.; Seeholzer, S.H.; Skalka, A.M. Structural Determinants of Metal-induced Conformational Changes in HIV-1 Integrase. J. Biol. Chem. 1998, 273, 35078–35087. [Google Scholar] [CrossRef] [PubMed]
- Tchertanov, L.; Mouscadet, J.-F. Target Recognition by Catechols and β-Ketoenols: Potential Contribution of Hydrogen Bonding and Mn/Mg Chelation to HIV-1 Integrase Inhibition. J. Med. Chem. 2007, 50, 1133–1145. [Google Scholar] [CrossRef] [PubMed]
- Barciela, J.; Latorre, C.; García-Martín, S.; Peña, R. A brief study of the role of Selenium as antioxidant. Elec. J. Environ. Agric. Food Chem. 2008, 7, 3151–3155. [Google Scholar]
- Zoidis, E.; Seremelis, I.; Kontopoulos, N.; Danezis, G.P. Selenium-Dependent Antioxidant Enzymes: Actions and Properties of Selenoproteins. Antioxidants 2018, 7, 66. [Google Scholar] [CrossRef]
- Arthur, J.R.; Nicol, F.; Beckett, G.J. The role of selenium in thyroid hormone metabolism and effects of selenium deficiency on thyroid hormone and iodine metabolism. Biol. Trace Elem. Res. 1992, 34, 321–325. [Google Scholar] [CrossRef]
- Dennert, G.; Zwahlen, M.; Brinkman, M.; Vinceti, M.; Zeegers, M.P.A.; Horneber, M. Selenium for preventing cancer. Cochrane Database Syst. Rev. 2011, 29, CD005195. [Google Scholar]
- Johansson, U.; Johnsson, F.; Joelsson, B.; Berglund, M.; Akesson, B. Selenium status in patients with liver cirrhosis and alcoholism. Br. J. Nutr. 1986, 55, 227–233. [Google Scholar] [CrossRef]
- Avery, J.C.; Hoffmann, P.R. Selenium, Selenoproteins, and Immunity. Nutrients 2018, 10, 1203. [Google Scholar] [CrossRef]
- Campa, A.; Shor-Posner, G.; Indacochea, F.; Zhang, G.; Lai, H.; Asthana, D.; Scott, G.B.; Baum, M.K. Mortality risk in selenium-deficient HIV-positive children. J. Acquir. Immune. Defic. Syndr. Hum. Retrovirol. 1999, 20, 508–513. [Google Scholar] [CrossRef]
- Kupka, R.; Msamanga, G.; Spiegelman, D.; Morris, S.; Mugusi, F.; Hunter, D.; Fawzi, W. Selenium Status Is Associated with Accelerated HIV Disease Progression among HIV-1–Infected Pregnant Women in Tanzania. J. Nutr. 2004, 134, 2556–2560. [Google Scholar] [CrossRef]
- Campa, A.; Martinez, S.S.; Baum, M.K. Selenium in HIV/AIDS. In Selenium: Its Molecular Biology and Role in Human Health; Hatfield, D.L., Schweizer, U., Tsuji, P.A., Gladyshev, V.N., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 333–342. [Google Scholar]
- Look, M.P.; Rockstroh, J.K.; Rao, G.S.; Kreuzer, K.A.; Spengler, U.; Sauerbruch, T. Serum selenium versus lymphocyte subsets and markers of disease progression and inflammatory response in human immunodeficiency virus-1 infection. Biol. Trace Elem. Res. 1997, 56, 31–41. [Google Scholar] [CrossRef] [PubMed]
- Jones, C.Y.; Tang, A.M.; Forrester, J.E.; Huang, J.; Hendricks, K.M.; Knox, T.A.; Spiegelman, D.; Semba, R.D.; Woods, M.N. Micronutrient Levels and HIV Disease Status in HIV-Infected Patients on Highly Active Antiretroviral Therapy in the Nutrition for Healthy Living Cohort. J. Acquir. Immune. Defic. Syndr. 2006, 43, 475–482. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, J.D.; Campa, A.M.; Ondercin, J.P.; Leoung, G.S.; Pless, R.F.; Baum, M.K. Micronutrient Supplementation Increases CD4 Count in HIV-Infected Individuals on Highly Active Antiretroviral Therapy: A Prospective, Double-Blinded, Placebo-Controlled Trial. J. Acquir. Immune. Defic. Syndr 2006, 42, 523–528. [Google Scholar] [CrossRef] [PubMed]
- Hurwitz, B.E.; Klaus, J.R.; Llabre, M.M.; Gonzalez, A.; Lawrence, P.J.; Maher, K.J.; Greeson, J.M.; Baum, M.K.; Shor-Posner, G.; Skyler, J.S.; et al. Suppression of Human Immunodeficiency Virus Type 1 Viral Load With Selenium Supplementation: A Randomized Controlled Trial. Arch. Intern. Med. 2007, 167, 148–154. [Google Scholar] [CrossRef]
- Kamwesiga, J.; Mutabazi, V.; Kayumba, J.; Tayari, J.-C.K.; Uwimbabazi, J.C.; Batanage, G.; Uwera, G.; Baziruwiha, M.; Ntizimira, C.; Murebwayire, A.; et al. Effect of selenium supplementation on CD4+ T-cell recovery, viral suppression and morbidity of HIV-infected patients in Rwanda: A randomized controlled trial. AIDS 2015, 29, 1045–1052. [Google Scholar] [CrossRef]
- Hori, K.; Hatfield, D.; Maldarelli, F.; Lee, B.J.; Clouse, K.A. Selenium Supplementation Suppresses Tumor Necrosis Factor α-Induced Human Immunodeficiency Virus Type 1 Replication in Vitro. AIDS Res. Hum. Retroviruses 1997, 13, 1325–1332. [Google Scholar] [CrossRef]
- Siegfried, N.; Irlam, J.H.; Visser, M.E.; Rollins, N.N. Micronutrient supplementation in pregnant women with HIV infection. Cochrane Database Syst. Rev. 2012, 14, CD009755. [Google Scholar] [CrossRef]
- Grobler, L.; Nagpal, S.; Sudarsanam, T.D.; Sinclair, D. Nutritional supplements for people being treated for active tuberculosis. Cochrane Database Syst. Rev. 2016, 29, CD006086. [Google Scholar] [CrossRef]
- Alim, I.; Caulfield, J.T.; Chen, Y.; Swarup, V.; Geschwind, D.H.; Ivanova, E.; Seravalli, J.; Ai, Y.; Sansing, L.H.; Ste.Marie, E.J.; et al. Selenium Drives a Transcriptional Adaptive Program to Block Ferroptosis and Treat Stroke. Cell 2019, 177, 1262–1279.e25. [Google Scholar] [CrossRef]
- Tainer, J.A.; Getzoff, E.D.; Richardson, J.S.; Richardson, D.C. Structure and mechanism of copper, zinc superoxide dismutase. Nature 1983, 306, 284–287. [Google Scholar] [CrossRef]
- Samanovic, M.I.; Ding, C.; Thiele, D.J.; Darwin, K.H. Copper in Microbial Pathogenesis: Meddling with the Metal. Cell Host Microbe. 2012, 11, 106–115. [Google Scholar] [CrossRef] [PubMed]
- Besold, A.N.; Culbertson, E.M.; Culotta, V.C. The Yin and Yang of copper during infection. J. Biol. Inorg. Chem. 2016, 21, 137–144. [Google Scholar] [CrossRef] [PubMed]
- German, N.; Lüthje, F.; Hao, X.; Rønn, R.; Rensing, C. Chapter Two - Microbial Virulence and Interactions With Metals. In Progress in Molecular Biology and Translational Science; San Francisco, M., San Francisco, B., Eds.; Academic Press: Cambridge, MA, USA, 2016; pp. 27–49. [Google Scholar]
- Sprietsma, J.E. Cysteine, glutathione (GSH) and zinc and copper ions together are effective, natural, intracellular inhibitors of (AIDS) viruses. Med. Hypotheses 1999, 52, 529–538. [Google Scholar] [CrossRef] [PubMed]
- Moreno, T.; Artacho, R.; Navarro, M.; Pérez, A.; Ruiz-López, M.D. Serum copper concentration in HIV-infection patients and relationships with other biochemical indices. Sci. Total Environ. 1998, 217, 21–26. [Google Scholar] [CrossRef]
- Ren, Y.; Smith, A. Mechanism of Metallothionein Gene Regulation by Heme-Hemopexin: ROLES OF PROTEIN KINASE C, REACTIVE OXYGEN SPECIES, AND cis-ACTING ELEMENTS. J. Biol. Chem. 1995, 270, 23988–23995. [Google Scholar] [CrossRef] [PubMed]
- DrÖGe, W.; Gross, A.; Hack, V.; Kinscherf, R.; Schykowski, M.; Bockstette, M.; Mihm, S.; Galter, D. Role of Cysteine and Glutathione in HIV Infection and Cancer Cachexia: Therapeutic Intervention with N-Acetylcysteine. In Advances in Pharmacology; Sies, H., Ed.; Academic Press: Cambridge, MA, USA.
- Staal, F.J.T.; Ela, S.W.; Roederer, M.; Anderson, M.T.; Herzenberg, L.A.; Herzenberg, L.A. Glutathione deficiency and human immunodeficiency virus infection. Lancet 1992, 339, 909–912. [Google Scholar] [CrossRef]
- Dröge, W.; Eck, H.P.; Mihm, S. HIV-induced cysteine deficiency and T-cell dysfunction—A rationale for treatment with N-acetylcysteine. Immunol. Today 1992, 13, 211–214. [Google Scholar] [CrossRef]
- Zhang, Z.Y.; Reardon, I.M.; Hui, J.O.; O’Connell, K.L.; Poorman, R.A.; Tomasselli, A.G.; Heinrikson, R.L. Zinc inhibition of renin and the protease from human immunodeficiency virus type 1. Biochemistry 1991, 30, 8717–8721. [Google Scholar] [CrossRef]
- Karlström, A.R.; Levine, R.L. Copper inhibits the protease from human immunodeficiency virus 1 by both cysteine-dependent and cysteine-independent mechanisms. Proc. Natl. Acad. Sci. USA 1991, 88, 5552–5556. [Google Scholar] [CrossRef]
- Miesel, R.; Mahmood, N.; Weser, U. Activity of Cu2Zn2 superoxide dismutase against the human immunodeficiency virus type 1. Redox Rep. 1995, 1, 99–103. [Google Scholar] [CrossRef]
- Agrawal, L.; Louboutin, J.-P.; Strayer, D.S. Preventing HIV-1 tat-induced neuronal apoptosis using antioxidant enzymes: Mechanistic and therapeutic implications. Virology 2007, 363, 462–472. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, L.; Louboutin, J.P.; Reyes, B.A.S.; Van Bockstaele, E.J.; Strayer, D.S. Antioxidant enzyme gene delivery to protect from HIV-1 gp120-induced neuronal apoptosis. Gene Ther. 2006, 13, 1645–1656. [Google Scholar] [CrossRef] [PubMed]
- Hodek, J.; Zajícová, V.; Lovětinská-Šlamborová, I.; Stibor, I.; Müllerová, J.; Weber, J. Protective hybrid coating containing silver, copper and zinc cations effective against human immunodeficiency virus and other enveloped viruses. BMC Microbiol. 2016, 16 (Suppl. 1), 56. [Google Scholar] [CrossRef] [PubMed]
- Borkow, G.; Sidwell, R.W.; Smee, D.F.; Barnard, D.L.; Morrey, J.D.; Lara-Villegas, H.H.; Shemer-Avni, Y.; Gabbay, J. Neutralizing viruses in suspensions by copper oxide-based filters. Antimicrob. Agents Chemother. 2007, 51, 2605–2607. [Google Scholar] [CrossRef] [PubMed]
- Borkow, G.; Lara, H.H.; Covington, C.Y.; Nyamathi, A.; Gabbay, J. Deactivation of human immunodeficiency virus type 1 in medium by copper oxide-containing filters. Antimicrob. Agents Chemother. 2008, 52, 518–525. [Google Scholar] [CrossRef] [PubMed]
- Borkow, G.; Covington, C.Y.; Gautam, B.; Anzala, O.; Oyugi, J.; Juma, M.; Abdullah, M.S. Prevention of Human Immunodeficiency Virus Breast milk Transmission with Copper Oxide: Proof-of-Concept Study. Breastfeed Med. 2011, 6, 165–170. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Li, G.; Steiner, J.; Nath, A. Role of Tat Protein in HIV Neuropathogenesis. Neurotox Res. 2009, 16, 205–220. [Google Scholar] [CrossRef]
- Ngwainmbi, J.; De, D.D.; Smith, T.H.; El-Hage, N.; Fitting, S.; Kang, M.; Dewey, W.L.; Hauser, K.F.; Akbarali, H.I. Effects of HIV-1 Tat on enteric neuropathogenesis. J. Neurosci. 2014, 34, 14243–14251. [Google Scholar] [CrossRef]
- Sabatier, J.M.; Vives, E.; Mabrouk, K.; Benjouad, A.; Rochat, H.; Duval, A.; Hue, B.; Bahraoui, E. Evidence for neurotoxic activity of tat from human immunodeficiency virus type 1. J. Virol. 1991, 65, 961–967. [Google Scholar] [CrossRef]
- Johnson, T.P.; Patel, K.; Johnson, K.R.; Maric, D.; Calabresi, P.A.; Hasbun, R.; Nath, A. Induction of IL-17 and nonclassical T-cell activation by HIV-Tat protein. Proc. Natl. Acad. Sci. USA 2013, 110, 13588–13593. [Google Scholar] [CrossRef]
- Haughey, N.J.; Holden, C.P.; Nath, A.; Geiger, J.D. Involvement of Inositol 1,4,5-Trisphosphate-Regulated Stores of Intracellular Calcium in Calcium Dysregulation and Neuron Cell Death Caused by HIV-1 Protein Tat. J. Neurochem. 1999, 73, 1363–1374. [Google Scholar] [CrossRef] [PubMed]
- Haughey, N.J.; Mattson, M.P. Calcium dysregulation and neuronal apoptosis by the HIV-1 proteins Tat and gp120. J. Acquir. Immune. Defic. Syndr. 2002, 31 (Suppl. 2), S55-61. [Google Scholar] [CrossRef] [PubMed]
- Deme, P.; Rojas, C.; Slusher, B.S.; Rais, R.; Afghah, Z.; Geiger, J.D.; Haughey, N.J. Bioenergetic adaptations to HIV infection. Could modulation of energy substrate utilization improve brain health in people living with HIV-1? Exp. Neurol. 2020, 327, 113181. [Google Scholar] [CrossRef] [PubMed]
- Tyagi, M.; Rusnati, M.; Presta, M.; Giacca, M. Internalization of HIV-1 Tat Requires Cell Surface Heparan Sulfate Proteoglycans. J. Biol. Chem. 2001, 276, 3254–3261. [Google Scholar] [CrossRef] [PubMed]
- Ensoli, B.; Buonaguro, L.; Barillari, G.; Fiorelli, V.; Gendelman, R.; Morgan, R.; Wingfield, P.; Gallo, R. Release, uptake, and effects of extracellular human immunodeficiency virus type 1 Tat protein on cell growth and viral transactivation. J. Virol. 1993, 67, 277–287. [Google Scholar] [CrossRef] [PubMed]
- Weihofen, W.A.; Liu, J.; Reutter, W.; Saenger, W.; Fan, H. Crystal Structures of HIV-1 Tat-derived Nonapeptides Tat-(1–9) and Trp2-Tat-(1–9) Bound to the Active Site of Dipeptidyl-peptidase IV (CD26). J. Biol. Chem. 2005, 280, 14911–14917. [Google Scholar] [CrossRef]
- Khan, N.; Halcrow, P.W.; Lakpa, K.L.; Afghah, Z.; Miller, N.M.; Dowdy, S.F.; Geiger, J.D.; Chen, X. Two-pore channels regulate Tat endolysosome escape and Tat-mediated HIV-1 LTR transactivation. FASEB J. 2020, 34, 4147–4162. [Google Scholar] [CrossRef]
- Khan, N.; Lakpa, K.L.; Halcrow, P.W.; Afghah, Z.; Miller, N.M.; Geiger, J.D.; Chen, X. BK channels regulate extracellular Tat-mediated HIV-1 LTR transactivation. Sci. Rep. 2019, 9, 12285. [Google Scholar] [CrossRef]
- Khan, N.; Datta, G.; Geiger, J.D.; Chen, X. Apolipoprotein E isoform dependently affects Tat-mediated HIV-1 LTR transactivation. J. Neuroinflamm. 2018, 15, 91. [Google Scholar] [CrossRef]
- Fan, Y.; He, J.J. HIV-1 Tat Promotes Lysosomal Exocytosis in Astrocytes and Contributes to Astrocyte-mediated Tat Neurotoxicity. J. Biol. Chem. 2016, 291, 22830–22840. [Google Scholar] [CrossRef]
- Rahimian, P.; He, J.J. Exosome-associated release, uptake, and neurotoxicity of HIV-1 Tat protein. J. Neurovirol 2016, 22, 774–788. [Google Scholar] [CrossRef]
- Hofman, F.; Wright, A.; Dohadwala, M.; Wong-Staal, F.; Walker, S. Exogenous tat protein activates human endothelial cells. Blood 1993, 82, 2774–2780. [Google Scholar] [CrossRef] [PubMed]
- Westendorp, M.O.; Li-Weber, M.; Frank, R.W.; Krammer, P.H. Human immunodeficiency virus type 1 Tat upregulates interleukin-2 secretion in activated T cells. J. Virol. 1994, 68, 4177–4185. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Li, C.J.; Pardee, A.B. Human immunodeficiency virus type 1 TAT protein activates B lymphocytes. Biochem. Biophys. Res. Commun. 1997, 237, 461–464. [Google Scholar] [CrossRef] [PubMed]
- Gibellini, D.; Bassini, A.; Pierpaoli, S.; Bertolaso, L.; Milani, D.; Capitani, S.; La Placa, M.; Zauli, G. Extracellular HIV-1 Tat protein induces the rapid Ser133 phosphorylation and activation of CREB transcription factor in both Jurkat lymphoblastoid T cells and primary peripheral blood mononuclear cells. J. Immunol. 1998, 160, 3891–3898. [Google Scholar] [PubMed]
- Kumar, A.; Manna, S.K.; Dhawan, S.; Aggarwal, B.B. HIV-Tat protein activates c-Jun N-terminal kinase and activator protein-1. J. Immunol. 1998, 161, 776–781. [Google Scholar] [PubMed]
- Ott, M.; Lovett, J.L.; Mueller, L.; Verdin, E. Superinduction of IL-8 in T cells by HIV-1 Tat protein is mediated through NF-κB factors. J. Immunol. 1998, 160, 2872–2880. [Google Scholar]
- Hui, L.; Chen, X.; Haughey, N.J.; Geiger, J.D. Role of endolysosomes in HIV-1 Tat-induced neurotoxicity. ASN Neuro 2012, 4, 243–252. [Google Scholar] [CrossRef]
- El-Hage, N.; Rodriguez, M.; Dever, S.M.; Masvekar, R.R.; Gewirtz, D.A.; Shacka, J.J. HIV-1 and Morphine Regulation of Autophagy in Microglia: Limited Interactions in the Context of HIV-1 Infection and Opioid Abuse. J. Virol. 2015, 89, 1024–1035. [Google Scholar] [CrossRef]
- Frankel, A.D.; Chen, L.; Cotter, R.J.; Pabo, C.O. Dimerization of the tat protein from human immunodeficiency virus: A cysteine-rich peptide mimics the normal metal-linked dimer interface. Proc. Natl. Acad. Sci. USA 1988, 85, 6297–6300. [Google Scholar] [CrossRef]
- Pierleoni, R.; Menotta, M.; Antonelli, A.; Sfara, C.; Serafini, G.; Dominici, S.; Laguardia, M.E.; Salis, A.; Damonte, G.; Banci, L.; et al. Effect of the redox state on HIV-1 tat protein multimerization and cell internalization and trafficking. Mol. Cell Biochem. 2010, 345, 105–118. [Google Scholar] [CrossRef] [PubMed]
- Zecca, L.; Youdim, M.B.H.; Riederer, P.; Connor, J.R.; Crichton, R.R. Iron, brain ageing and neurodegenerative disorders. Nat. Rev. Neurosci. 2004, 5, 863–873. [Google Scholar] [CrossRef] [PubMed]
- Ward, R.J.; Zucca, F.A.; Duyn, J.H.; Crichton, R.R.; Zecca, L. The role of iron in brain ageing and neurodegenerative disorders. Lancet Neurol. 2014, 13, 1045–1060. [Google Scholar] [CrossRef]
- Liu, J.-L.; Fan, Y.-G.; Yang, Z.-S.; Wang, Z.-Y.; Guo, C. Iron and Alzheimer’s Disease: From Pathogenesis to Therapeutic Implications. Front. Neurosci. 2018, 12, 632. [Google Scholar] [CrossRef]
- Drakesmith, H.; Prentice, A.M. Hepcidin and the Iron-Infection Axis. Science 2012, 338, 768–772. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khan, N.; Chen, X.; Geiger, J.D. Role of Divalent Cations in HIV-1 Replication and Pathogenicity. Viruses 2020, 12, 471. https://doi.org/10.3390/v12040471
Khan N, Chen X, Geiger JD. Role of Divalent Cations in HIV-1 Replication and Pathogenicity. Viruses. 2020; 12(4):471. https://doi.org/10.3390/v12040471
Chicago/Turabian StyleKhan, Nabab, Xuesong Chen, and Jonathan D. Geiger. 2020. "Role of Divalent Cations in HIV-1 Replication and Pathogenicity" Viruses 12, no. 4: 471. https://doi.org/10.3390/v12040471
APA StyleKhan, N., Chen, X., & Geiger, J. D. (2020). Role of Divalent Cations in HIV-1 Replication and Pathogenicity. Viruses, 12(4), 471. https://doi.org/10.3390/v12040471





