Survey on Non-Human Primates and Mosquitoes Does not Provide Evidences of Spillover/Spillback between the Urban and Sylvatic Cycles of Yellow Fever and Zika Viruses Following Severe Outbreaks in Southeast Brazil
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethic Issues
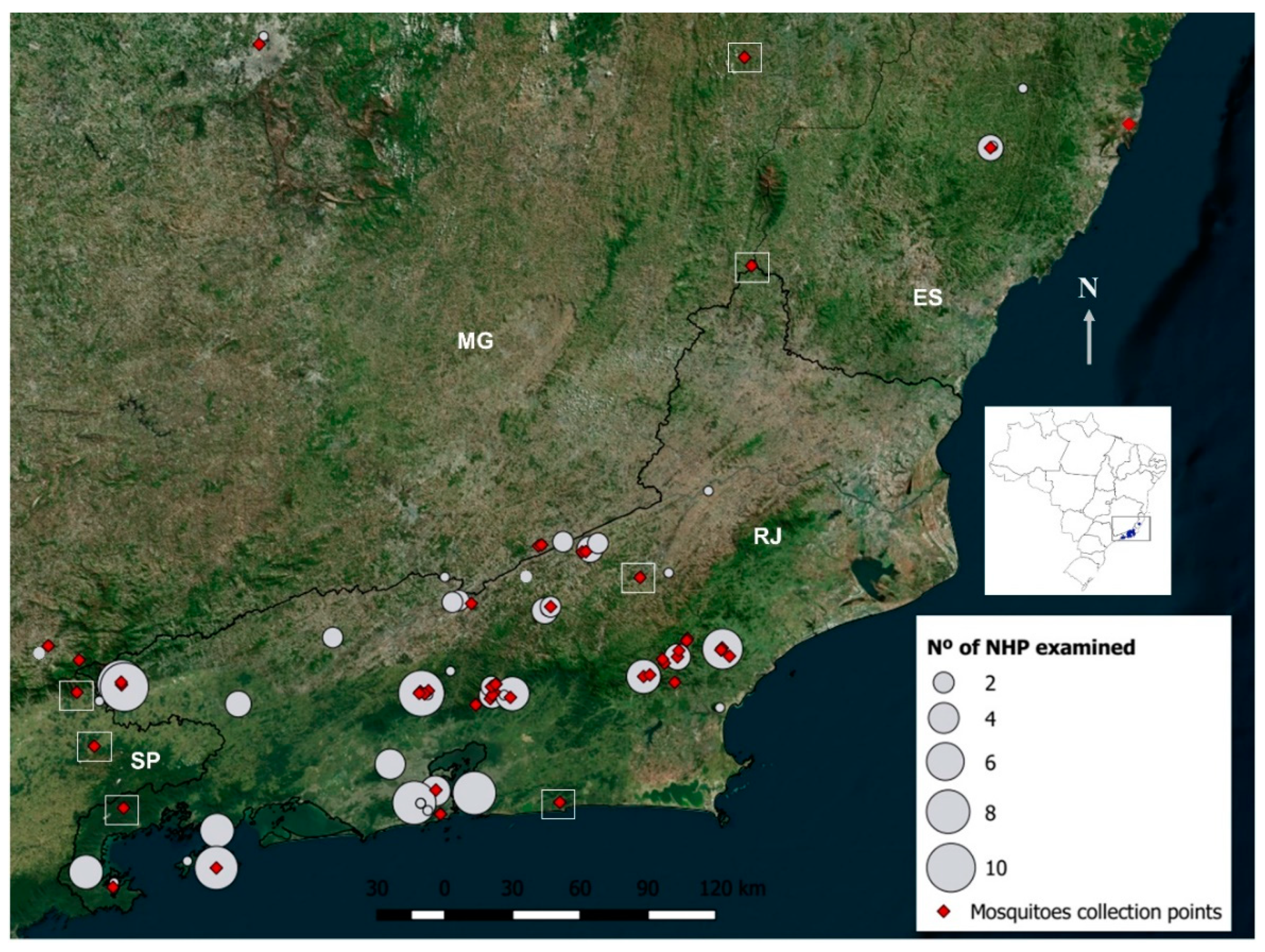
2.2. Field Expeditions
2.3. Non-Human Primate Samplings
2.4. Mosquito Collections
2.5. Molecular Analyses
2.6. Immunological Assays
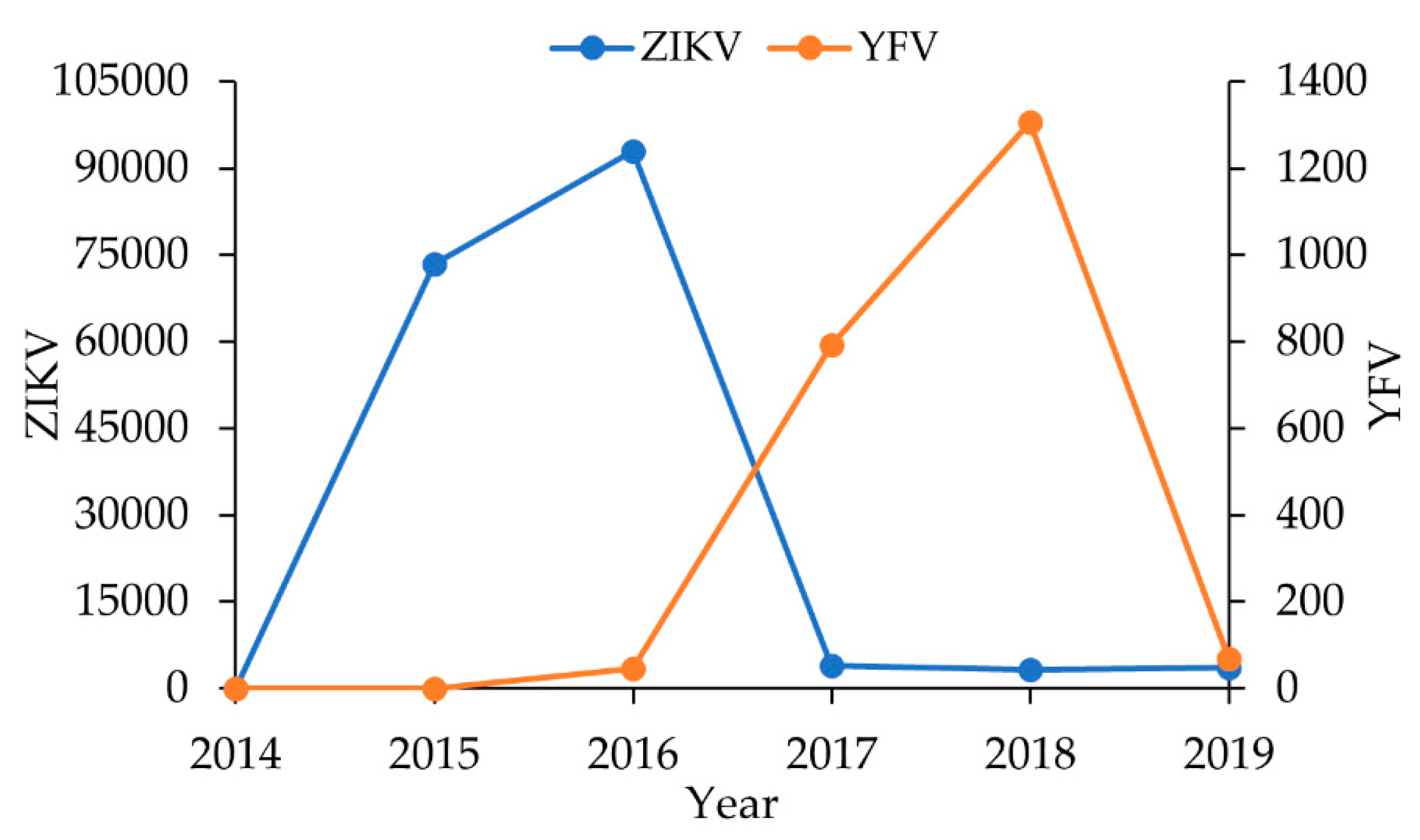
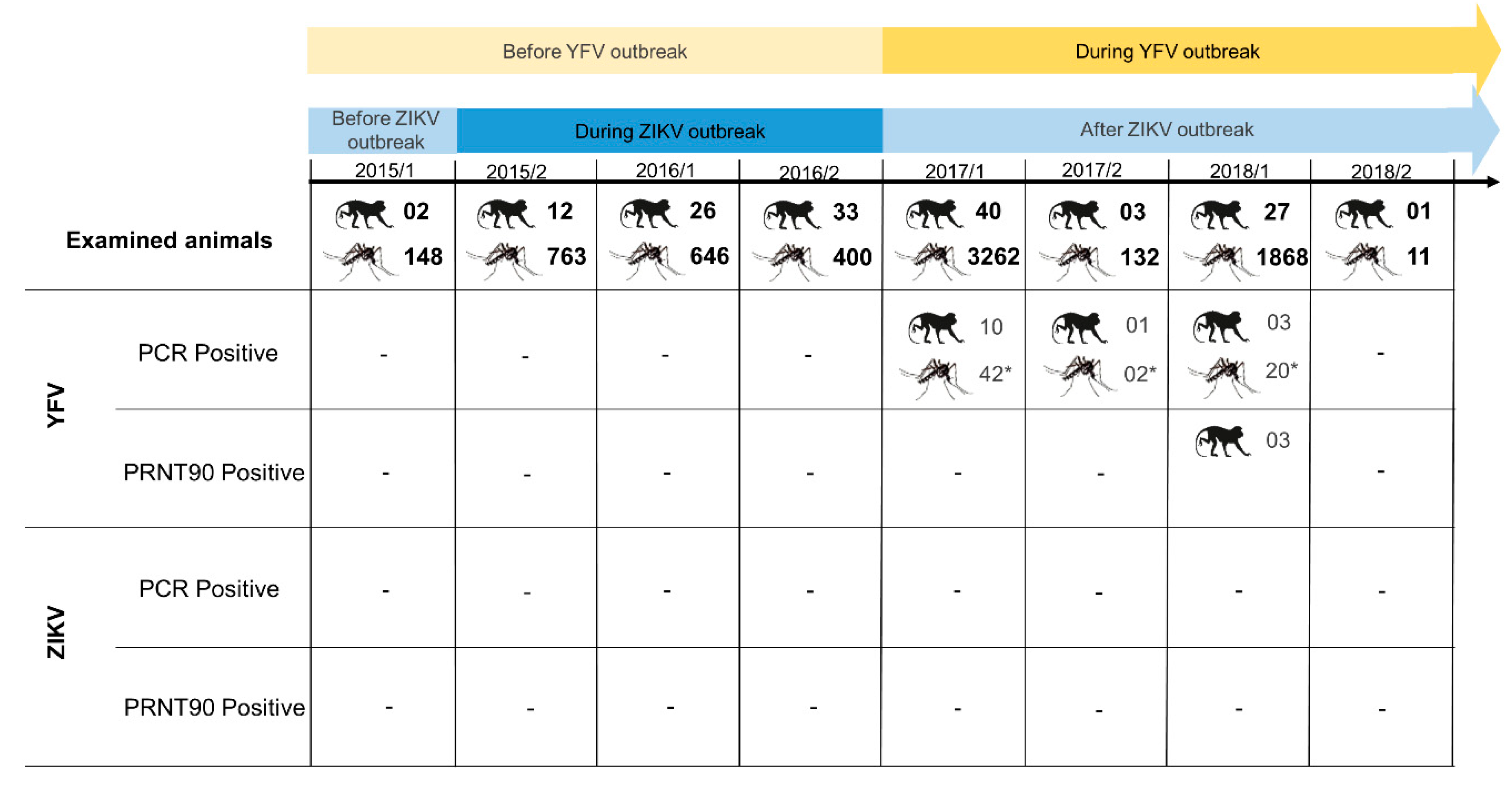
3. Results
3.1. Molecular Findings
3.1.1. Non-Human Primates
3.1.2. Mosquitoes
3.2. Immunological Findings
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- de Oliveira Garcia, M.H. Zika: The continuing threat. Bull. World Health Organ. 2019, 97, 6–7. [Google Scholar]
- Brasil Monitoramento do Período Sazonal da Febre Amarela. Available online: http://portalarquivos2.saude.gov.br/images/pdf/2018/fevereiro/21/Informe-n14-FA-20fev18-c.pdf (accessed on 14 October 2019).
- Possas, C.; Lourenço-de-oliveira, R.; Tauil, P.L.; Pinheiro, F.D.P.; Pissinatti, A.; Venâncio, R.; Freire, M.; Martins, R.M.; Homma, A. Yellow fever outbreak in Brazil: The puzzle of rapid viral spread and challenges for immunisation. Mem. Inst. Oswaldo Cruz 2018, 113, e180278. [Google Scholar] [CrossRef] [PubMed]
- Althouse, B.M.; Vasilakis, N.; Sall, A.A.; Diallo, M.; Weaver, S.C.; Hanley, K.A. Potential for Zika Virus to Establish a Sylvatic Transmission Cycle in the Americas. PLoS Negl. Trop. Dis. 2016, 10, e0005055. [Google Scholar] [CrossRef] [PubMed]
- Couto-Lima, D.; Madec, Y.; Bersot, M.I.; Campos, S.S.; Motta, M.; de Albuquerque Motta, M.; Dos Santos, F.B.; Vazeille, M.; da Costa Vasconcelos, P.F.; Lourenço-de-Oliveira, R.; et al. Potential risk of re-emergence of urban transmission of Yellow Fever virus in Brazil facilitated by competent Aedes populations. Sci. Rep. 2017, 7, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Weaver, S.C. Urbanization and geographic expansion of zoonotic arboviral diseases: Mechanisms and potential strategies for prevention. Trends Microbiol. 2013, 21, 360–363. [Google Scholar] [CrossRef]
- Klitting, R.; Gould, E.A.; Paupy, C. What does the future hold for yellow fever virus? Genes 2018, 9, 291. [Google Scholar] [CrossRef]
- Franco, O. História da Febre Amarela no Brasil. Ministério Saúde 1969, 1, 210. [Google Scholar]
- Bryant, J.E.; Holmes, E.C.; Barrett, A.D.T. Out of Africa: A Molecular Perspective on the Introduction of Yellow Fever Virus into the Americas. PLoS Pathog. 2007, 3, e75. [Google Scholar] [CrossRef]
- Soper, F.L.; Penna, H.A.; Cardoso, E.; Serafim, J., Jr.; Frobisher, M., Jr.; Pinheiro, J. Yellow Fever without Aedes aegypti. Study of a rural epidemic in the Valle do Chanaan, Espirito Santo, Brazil, 1932. Am. J. Hyg. 1933, 18, 555–587. [Google Scholar] [CrossRef]
- Soper, F.L. Jungle Yellow Fever. A New Epidemiological Entity in South America. Rev. Hig. Saude Publica 1937, 10, 104–144. [Google Scholar]
- Davis, N.C. Susceptibility of Capuchin (Cebus) monkeys to yellow fever virus. Am. J. Epidemiol. 1930, 11, 321–334. [Google Scholar] [CrossRef]
- Haddow, A.J.; Smithburn, K.C.; Dick, G.W.A.; Kitchen, S.F.; Lumsden, W.H.R. Implication of the Mosquito aëdes (Stegomyia) Africanus Theobald in the Forest Cycle of Yellow Fever in Uganda. Ann. Trop. Med. Parasitol. 1948, 42, 218–223. [Google Scholar] [CrossRef] [PubMed]
- Vasconcelos, P.F.D.C. Yellow fever in Brazil: Thoughts and hypotheses on the emergence in previously free areas. Rev. Saude Publica 2010, 44, 1144–1149. [Google Scholar] [CrossRef] [PubMed]
- Monath, T.P.; Vasconcelos, P.F.C. Yellow fever. J. Clin. Virol. 2015, 64, 160–173. [Google Scholar] [CrossRef]
- Brasil Aspectos Epidemiológicos da Febre amarela Silvestre e a Vigilância Intensificada Durante Período de Monitoramento, Brasil, 2012/2013. Available online: https://portalarquivos2.saude.gov.br/images/pdf/2014/maio/27/BE-V45-n---07-FebreAmarela.pdf (accessed on 10 December 2019).
- Bonaldo, M.C.; Gómez, M.M.; dos Santos, A.A.; Abreu, F.V.S.; Ferreira-de-Brito, A.; Miranda, R.M.; de Castro, M.G.; Lourenço-de-Oliveira, R. Genome analysis of yellow fever virus of the ongoing outbreak in Brazil reveals polymorphisms. Mem. Inst. Oswaldo Cruz 2017, 112, 447–451. [Google Scholar] [CrossRef]
- Mayer, S.V.; Tesh, R.B.; Vasilakis, N. The emergence of arthropod-borne viral diseases: A global prospective on dengue, chikungunya and zika fevers. Acta Trop. 2017, 166, 155–163. [Google Scholar] [CrossRef]
- Zanluca, C.; de Melo, V.C.A.; Mosimann, A.L.P.; dos Santos, G.I.V.; dos Santos, C.N.D.; Luz, K. First report of autochthonous transmission of Zika virus in Brazil. Mem. Inst. Oswaldo Cruz 2015, 110, 569–572. [Google Scholar] [CrossRef]
- de Araújo, T.V.B.; Rodrigues, L.C.; de Alencar Ximenes, R.A.; de Barros Miranda-Filho, D.; Montarroyos, U.R.; de Melo, A.P.L.; Valongueiro, S.; de Albuquerque, M.; Souza, W.V.; Braga, C.; et al. Association between Zika virus infection and microcephaly in Brazil, January to May, 2016: Preliminary report of a case-control study. Lancet Infect. Dis. 2016, 16, 1356–1363. [Google Scholar] [CrossRef]
- Heukelbach, J.; Alencar, C.H.; Kelvin, A.A.; de Oliveira, W.K.; de Góes Cavalcanti, L.P. Zika virus outbreak in Brazil. J. Infect. Dev. Ctries 2016, 10, 116–120. [Google Scholar] [CrossRef]
- Ferreira-de-Brito, A.; Ribeiro, I.P.; de Miranda, R.M.; Fernandes, R.S.; Campos, S.S.; da Silva, K.A.B.; de Castro, M.G.; Bonaldo, M.C.; Brasil, P.; Lourenço-de-Oliveira, R. First detection of natural infection of Aedes aegypti with Zika virus in Brazil and throughout South America. Mem. Inst. Oswaldo Cruz 2016, 111, 655–658. [Google Scholar] [CrossRef]
- Terzian, A.C.B.; Zini, N.; Sacchetto, L.; Rocha, R.F.; Parra, M.C.P.; Del Sarto, J.L.; Dias, A.C.F.; Coutinho, F.; Rayra, J.; da Silva, R.A.; et al. Evidence of natural Zika virus infection in neotropical non-human primates in Brazil. Sci. Rep. 2018, 8, 16034. [Google Scholar] [CrossRef]
- Favoretto, S.R.; Araujo, D.B.; Duarte, N.F.H.; Oliveira, D.B.L.; da Crus, N.G.; Mesquita, F.; Leal, F.; Machado, R.R.G.; Gaio, F.; Oliveira, W.F.; et al. Zika Virus in Peridomestic Neotropical Primates, Northeast Brazil. Ecohealth 2019, 16, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Berry, N.; Ferguson, D.; Ham, C.; Hall, J.; Jenkins, A.; Giles, E.; Devshi, D.; Kempster, S.; Rose, N.; Dowall, S.; et al. High susceptibility, viral dynamics and persistence of South American Zika virus in New World monkey species. Sci. Rep. 2019, 9, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Travassos-da-Rosa, A.P.D.A. The history of Arbovirology at Instituto Evandro Chagas, Belém, Pará, Brazil, from 1954 to 1998. Rev. Pan-Amaz. Saúde 2016, 7, 61–70. [Google Scholar] [CrossRef][Green Version]
- Laemmert, H.W.; Ferreira, L.D.C.; Taylor, R.M. Part II—Investigations of vertebrate hosts and arthropod vectors. Am. J. Trop. Med. Hyg. 1946, 1, 23–69. [Google Scholar] [CrossRef]
- Catenacci, L.S.; Ferreira, M.; Martins, L.C.; De Vleeschouwer, K.M.; Cassano, C.R.; Oliveira, L.C.; Canale, G.; Deem, S.L.; Tello, J.S.; Parker, P.; et al. Surveillance of Arboviruses in Primates and Sloths in the Atlantic Forest, Bahia, Brazil. Ecohealth 2018, 4, 777–791. [Google Scholar] [CrossRef] [PubMed]
- Lopes, O.D.S.; Coimbra, T.L.M.; Sachetta, L.D.A.; Calisher, C.H. Emergence of a new arbovirus disease in Brazil. Am. J. Epidemiol. 1978, 107, 444–449. [Google Scholar] [CrossRef]
- Iversson, L.B.; Coimbra, T.L.M. Encefalite na região do Vale do Ribeira, São Paulo, Brasil, no período pós-epidêmico de 1978 a 1983: Situação do diagnóstico etiológico e características epidemiológicas. Rev. Saude Publica 1984, 18, 323–332. [Google Scholar] [CrossRef]
- Brasil Guia de Vigilância de Epizootias em Primatas não Humanos e Entomologia Aplicada à Vigilância da Febre Amarela, 2nd ed.; Ministério da Saúde: Brasília, Brazil, 2017; ISBN 9788533421028.
- Abreu, F.V.S.; dos Santos, E.; Gomes, M.Q.; Vargas, W.P.; Oliveira Passos, P.H.; Nunes e Silva, C.; Araújo, P.C.; Pires, J.R.; Romano, A.P.M.; Teixeira, D.S.; et al. Capture of Alouatta guariba clamitans for the surveillance of sylvatic yellow fever and zoonotic malaria: Which is the best strategy in the tropical Atlantic Forest? Am. J. Primatol. 2019, 81, e23000. [Google Scholar] [CrossRef]
- Santos, T.P. Potential of Aedes albopictus as a bridge vector for enzootic pathogens at the urban-forest interfacein Brazil—Approved with minor corrections. Emerg. Microbes Infect. 2018, 7, 1–8. [Google Scholar] [CrossRef]
- Abreu, F.V.S.D.; Ribeiro, I.P.; Ferreira-de-Brito, A.; Santos, A.A.C.D.; Miranda, R.M.D.; Bonelly, I.D.S.; Neves, M.S.A.S.; Bersot, M.I.; Santos, T.P.D.; Gomes, M.Q.; et al. Haemagogus leucocelaenus and Haemagogus janthinomys are the primary vectors in the major yellow fever outbreak in Brazil, 2016–2018. Emerg. Microbes Infect. 2019, 8, 218–231. [Google Scholar] [CrossRef] [PubMed]
- Moutailler, S.; Yousfi, L.; Mousson, L.; Devillers, E.; Vazeille, M.; Vega-Rúa, A.; Perrin, Y.; Jourdain, F.; Chandre, F.; Cannet, A.; et al. A New High-Throughput Tool to Screen Mosquito-Borne Viruses in Zika Virus Endemic/Epidemic Areas. Viruses 2019, 11, 904. [Google Scholar] [CrossRef] [PubMed]
- Bonaldo, M.C.; Ribeiro, I.P.; Lima, N.S.; dos Santos, A.A.C.; Menezes, L.S.R.; da Cruz, S.O.D.; de Mello, I.S.; Furtado, N.D.; de Moura, E.E.; Damasceno, L.; et al. Isolation of Infective Zika Virus from Urine and Saliva of Patients in Brazil. PLoS Negl. Trop. Dis. 2016, 10, e0004816. [Google Scholar] [CrossRef]
- WHO Guidelines for Plaque Reduction Neutralization Testing of Human Antibodies to Dengue Viruses; WHO: Geneva, Switzerland, 2003.
- Moreira-Soto, A.; Carneiro, I.D.O.; Fischer, C.; Feldmann, M.; Kümmerer, B.M.; Silva, N.S.; Santos, U.G.; Souza, B.F.D.C.D.; Liborio, F.D.A.; Valença-Montenegro, M.M.; et al. Limited Evidence for Infection of Urban and Peri-urban Nonhuman Primates with Zika and Chikungunya Viruses in Brazil. mSphere 2018, 3, e00523-17. [Google Scholar] [CrossRef]
- Miagostovich, M.P.; Nogueira, R.M.; dos Santos, F.B.; Schatzmayr, H.G.; Araújo, E.S.; Vorndam, V. Evaluation of an IgG enzyme-linked immunosorbent assay for dengue diagnosis. J. Clin. Virol. 1999, 14, 183–189. [Google Scholar] [CrossRef]
- Steinhagen, K.; Probst, C.; Radzimski, C.; Schmidt-Chanasit, J.; Emmerich, P.; van Esbroeck, M.; Schinkel, J.; Grobusch, M.P.; Goorhuis, A.; Warnecke, J.M.; et al. Serodiagnosis of Zika virus (ZIKV) infections by a novel NS1-based ELISA devoid of cross-reactivity with dengue virus antibodies: A multicohort study of assay performance, 2015 to 2016. Euro Surveill. 2016, 21, 30426. [Google Scholar] [CrossRef] [PubMed]
- Gómez, M.M.; de Abreu, F.V.S.; Dos Santos, A.A.C.; de Mello, I.S.; Santos, M.P.; Ribeiro, I.P.; Ferreira-de-Brito, A.; de Miranda, R.M.; de Castro, M.G.; Ribeiro, M.S.; et al. Genomic and structural features of the yellow fever virus from the 2016-2017 Brazilian outbreak. J. Gen. Virol. 2018, 99, 536–548. [Google Scholar] [CrossRef]
- Pinheiro, G.G.; Rocha, M.N.; de Oliveira, M.A.; Moreira, L.A.; Andrade Filho, J.D. Detection of Yellow Fever Virus in Sylvatic Mosquitoes during Disease Outbreaks of 2017–2018 in Minas Gerais State, Brazil. Insects 2019, 10, 136. [Google Scholar] [CrossRef]
- Faria, N.R.; Kraemer, M.U.G.; Hill, S.C.; Jesus, J.G.D.; Aguiar, R.S.; Iani, F.C.M.; Xavier, J.; Quick, J.; Plessis, L.D.; Dellicour, S.; et al. Genomic and epidemiological monitoring of yellow fever virus transmission potential. Science 2018, 361, 894–899. [Google Scholar] [CrossRef]
- Moreira-Soto, A.; Torres, M.C.; Lima de Mendonça, M.C.; Mares-Guia, M.A.; dos Santos Rodrigues, C.D.; Fabri, A.A.; dos Santos, C.C.; Machado Araújo, E.S.; Fischer, C.; Ribeiro Nogueira, R.M.; et al. Evidence for multiple sylvatic transmission cycles during the 2016–2017 yellow fever virus outbreak, Brazil. Clin. Microbiol. Infect. 2018, 24, 1019.e1–1019.e4. [Google Scholar] [CrossRef]
- Delatorre, E.; Abreu, F.V.S.D.; Ribeiro, I.P.; Gómez, M.M.; dos Santos, A.A.C.; Ferreira-de-Brito, A.; Neves, M.S.A.S.; Bonelly, I.D.S.; Miranda, R.M.D.; Furtado, N.D.; et al. Distinct YFV Lineages Co-circulated in the Central-Western and Southeastern Brazilian Regions from 2015 to 2018. Front. Microbiol. 2019, 10, 1079. [Google Scholar] [CrossRef] [PubMed]
- Moussallem, T.M.; Gava, C.; Ardisson, K.S.; Marques, C.S.; Graceli, G.C.; Valadares Koski, A.D.P.; Almada, G.L.; Silva, A.R.D.; Jesus, F.A.A.D.; Rodrigues, G.A.P.; et al. Yellow fever outbreak in a rural-urban mixed community of Espírito Santo, Brazil: Epidemiological aspects. Rev. Panam. Salud Pública 2019, 43, e29. [Google Scholar] [CrossRef] [PubMed]
- Brasil, P.; Zalis, M.G.; de Pina-Costa, A.; Siqueira, A.M.; Júnior, C.B.; Silva, S.; Areas, A.L.L.; Pelajo-Machado, M.; de Alvarenga, D.A.M.; da Silva Santelli, A.C.F.; et al. Outbreak of human malaria caused by Plasmodium simium in the Atlantic Forest in Rio de Janeiro: A molecular epidemiological investigation. Lancet Glob. Health 2017, 5, e1038–e1046. [Google Scholar] [CrossRef]
- Cunha, M.D.P.; Duarte-Neto, A.N.; Pour, S.Z.; Ortiz-Baez, A.S.; Černý, J.; Pereira, B.B.D.S.; Braconi, C.T.; Ho, Y.L.; Perondi, B.; Sztajnbok, J.; et al. Origin of the São Paulo Yellow Fever epidemic of 2017–2018 revealed through molecular epidemiological analysis of fatal cases. Sci. Rep. 2019, 9, 1–10. [Google Scholar] [CrossRef]
- Ávila-Pires, F.D.D. The spread of jungle Yellow fever. Rev. Patol. Trop. J. Trop. Pathol. 2018, 47, 1. [Google Scholar] [CrossRef]
- Brasil Não há Registro Confirmado de Febre Amarela Urbana no Brasil. Available online: http://www.saude.gov.br/noticias/agencia-saude/42486-nao-ha-registro-confirmado-de-febre-amarela-urbana-no-brasil (accessed on 6 June 2019).
- Almeida, M.; Dos Santos, E.; Da Cruz Cardoso, J.; Da Fonseca, D.F.; Noll, C.A.; Silveira, V.R.; Maeda, A.Y.; De Souza, R.P.; Kanamura, C.; Brasil, R.A. Yellow fever outbreak affecting Alouatta populations in southern Brazil (Rio Grande do Sul State), 2008–2009. Am. J. Primatol. 2012, 74, 68–76. [Google Scholar] [CrossRef]
- Moreno, E.S.; Agostini, I.; Holzmann, I.; Di Bitetti, M.S.; Oklander, L.I.; Kowalewski, M.M.; Beldomenico, P.M.; Goenaga, S.; Martínez, M.; Lestani, E.; et al. Yellow fever impact on brown howler monkeys (Alouatta guariba clamitans) in Argentina: A metamodelling approach based on population viability analysis and epidemiological dynamics. Mem. Inst. Oswaldo Cruz 2015, 110, 865–876. [Google Scholar] [CrossRef]
- de Souza, R.P.; Petrella, S.; Coimbra, T.L.M.; Maeda, A.Y.; Rocco, I.M.; Bisordi, I.; Silveira, V.R.; Pereira, L.E.; Suzuki, A.; Santos Silva, S.J.D.; et al. Isolation of Yellow Fever virus (YFV) from naturally infected Haemagogus (Conopostegus) leucocelaenus (Diptera, Culicidae) in São Paulo state, Brazil, 2009. Rev. Inst. Med. Trop. Sao Paulo 2011, 53, 133–139. [Google Scholar] [CrossRef]
- Bicca-marques, J.C.; Calegaro-Marques, C.; Rylands, A.B.; Karen, B.; Mittermeier, R.A.; Almeida, M.A.B.; Castro, P.H.G.; Chaves, Ó.M.; Ferraz, L.P.; Fortes, V.B.; et al. Yellow fever threatens Atlantic Forest primates Júlio. Sci. Adv. 2017, 1600946, 18–20. [Google Scholar]
- Cunha, M.S.; da Costa, A.C.; de Azevedo Fernandes, N.C.C.; Guerra, J.M.; dos Santos, F.C.P.; Nogueira, J.S.; D’Agostino, L.G.; Komninakis, S.V.; Witkin, S.S.; Ressio, R.A.; et al. Epizootics due to Yellow Fever Virus in São Paulo State, Brazil: Viral dissemination to new areas (2016–2017). Sci. Rep. 2019, 9, 5474. [Google Scholar] [CrossRef]
- Kumm, H.W.; Laemmert, H.W. The geographical distribution of immunity to yellow fever among the primates of Brazil. Am. J. Trop. Med. Hyg. 1950, 30, 733–748. [Google Scholar] [CrossRef] [PubMed]
- Almeida, M.A.B.D.; Santos, E.D.; Cardoso, J.D.C.; Noll, C.A.; Lima, M.D.M.; Silva, F.D.A.E.; Ferreira, M.S.; Martins, L.C.; Vasconcelos, P.F.D.C.; Bicca-Marques, J.C. Detection of antibodies against Icoaraci, Ilhéus, and Saint Louis Encephalitis arboviruses during yellow fever monitoring surveillance in non-human primates ( Alouatta caraya ) in southern Brazil. J. Med. Primatol. 2019, 48, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Abreu, F.V.S.D.; Delatorre, E.; dos Santos, A.A.C.; Ferreira-de-Brito, A.; de Castro, M.G.; Ribeiro, I.P.; Furtado, N.D.; Vargas, W.P.; Ribeiro, M.S.; Meneguete, P.; et al. Combination of surveillance tools reveals that Yellow Fever virus can remain in the same Atlantic Forest area at least for three transmission seasons. Mem. Inst. Oswaldo Cruz 2019, 114, e190076. [Google Scholar] [CrossRef] [PubMed]
- Causey, O.R.; Hughes, T.P.; Laemmert, H.W. The Invasion of Small Forests by Yellow Fever Virus as Indicated by Immunity in Cebus Monkeys 1. Am. J. Trop. Med. Hyg. 1949, 1, 555–565. [Google Scholar]
- Fedigan, L.M. Capuchin Monkeys ( Sapajus and Cebus ). In The International Encyclopedia of Primatology; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2017; pp. 1–2. [Google Scholar]
- de Oliveira-Filho, E.F.; Oliveira, R.A.S.; Ferreira, D.R.A.; Laroque, P.O.; Pena, L.J.; Valença-Montenegro, M.M.; Mota, R.A.; Gil, L.H.V.G. Seroprevalence of selected flaviviruses in free-living and captive capuchin monkeys in the state of Pernambuco, Brazil. Transbound. Emerg. Dis. 2018, 65, 1094–1097. [Google Scholar] [CrossRef]
- Davis, N.C. The susceptibility of Marmosets to Yellow fever virus. J. Exp. Med. 1930, 52, 405–416. [Google Scholar] [CrossRef]
- Araújo, F.A.A.; Ramos, D.G.; Santos, A.L.; Passos, P.H.D.O.; Elkhoury, A.N.S.M.; Costa, Z.G.A.; Leal, S.G.; Romano, A.P.M. Epizootias em primatas não humanos durante reemergência do vírus da febre amarela no Brasil, 2007 a 2009. Epidemiol. Serviços Saúde 2011, 20, 527–536. [Google Scholar] [CrossRef]
- Fernandes, N.C.C.D.A.; Cunha, M.S.; Guerra, J.M.; Réssio, R.A.; Cirqueira, C.D.S.; Iglezias, S.D.; de Carvalho, J.; Araujo, E.L.L.; Catão-Dias, J.L.; Díaz-Delgado, J. Outbreak of Yellow Fever among Nonhuman Primates, Espirito Santo, Brazil, 2017. Emerg. Infect. Dis. 2017, 23, 2038–2041. [Google Scholar] [CrossRef]
- Kuno, G.; Chang, G.J.; Tsuchiya, K.R.; Karabatsos, N.; Cropp, C.B. Phylogeny of the genus Flavivirus. J. Virol. 1998, 72, 73–83. [Google Scholar] [CrossRef]
- Shan, C.; Ortiz, D.A.; Yang, Y.; Wong, S.J.; Kramer, L.D.; Shi, P.-Y.; Loeffelholz, M.J.; Ren, P. Evaluation of a Novel Reporter Virus Neutralization Test for Serological Diagnosis of Zika and Dengue Virus Infection. J. Clin. Microbiol. 2017, 55, 3028–3036. [Google Scholar] [CrossRef]
- Karna, A.K.; Azar, S.R.; Plante, J.A.; Yun, R.; Vasilakis, N.; Weaver, S.C.; Hansen, I.A.; Hanley, A.A. Colonized Sabethes cyaneus, a Sylvatic New World Mosquito Species, Shows a Low Vector Competence for Zika Virus Relative to Aedes aegypti. Viruses 2018, 10, 434. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, R.S.; Bersot, M.I.; Castro, M.G.; Telleria, E.L.; Ferreira-de-Brito, A.; Raphael, L.M.; Bonaldo, M.C.; Lourenço-de-Oliveira, R. Low vector competence in sylvatic mosquitoes limits Zika virus to initiate an enzootic cycle in South America. Sci. Rep. 2019, 9, 20151. [Google Scholar] [CrossRef] [PubMed]
- Chiu, C.Y.; Sánchez-San Martín, C.; Bouquet, J.; Li, T.; Yagi, S.; Tamhankar, M.; Hodara, V.L.; Parodi, L.M.; Somasekar, S.; Yu, G.; et al. Experimental Zika Virus Inoculation in a New World Monkey Model Reproduces Key Features of the Human Infection. Sci. Rep. 2017, 7, 17126. [Google Scholar] [CrossRef] [PubMed]
- Vanchiere, J.A.; Ruiz, J.C.; Brady, A.G.; Kuehl, T.J.; Williams, L.E.; Baze, W.B.; Wilkerson, G.K.; Nehete, P.N.; Mcclure, G.B.; Rogers, D.L.; et al. Experimental Zika Virus Infection of Neotropical Primates. J. Trop. Med. Hyg. 2017, 98, 173–177. [Google Scholar] [CrossRef]
| Species Per Year / Semester | Number | Date | Counties (State) | Result PCRa |
|---|---|---|---|---|
| Aedes albopictus | 4 | 2015/1 | Macaé (RJ) | Neg. |
| Aedes scapularis | 12 | 2015/1 | Neg. | |
| Culex sp. | 1 | 2015/1 | Neg. | |
| Haemagogus leucocelaenus | 17 | 2015/1 | Neg. | |
| Limatus durhamii | 2 | 2015/1 | Neg. | |
| Psorophora ferox | 10 | 2015/1 | Neg. | |
| Psorophora sp. | 5 | 2015/1 | Neg. | |
| Runchomyia humboldti | 1 | 2015/1 | Neg. | |
| Runchomyia reversa / theobaldi | 1 | 2015/1 | Neg. | |
| Sabethes albiprivus | 1 | 2015/1 | Neg. | |
| Wyeomyia aporonoma/staminifera | 1 | 2015/1 | Neg. | |
| Wyeomyia (Pho.) sp. | 89 | 2015/1 | Neg. | |
| Wyeomyia sp. | 4 | 2015/1 | Neg. | |
| 2015/1 | 148 | Neg. | ||
| Aedes albopictus | 14 | 2015/2 | Guapimirim, Macaé, Magé, Miguel Pereira, Teresópolis (RJ) | Neg. |
| Aedes fulvithorax | 1 | 2015/2 | Neg. | |
| Aedes scapularis | 94 | 2015/2 | Neg. | |
| Aedes serratus | 1 | 2015/2 | Neg. | |
| Aedes terrens | 25 | 2015/2 | Neg. | |
| Anopheles cruzii | 1 | 2015/2 | Neg. | |
| Anopheles sp. | 3 | 2015/2 | Neg. | |
| Culex sp. | 9 | 2015/2 | Neg. | |
| Culex nigripalpus | 10 | 2015/2 | Neg. | |
| Haemagogus janthinomys | 60 | 2015/2 | Neg. | |
| Haemagogus leucocelaenus | 87 | 2015/2 | Neg. | |
| Limatus durhamii | 26 | 2015/2 | Neg. | |
| Limatus pseudomethisticus | 2 | 2015/2 | Neg. | |
| Onirion personatum | 41 | 2015/2 | Neg. | |
| Psorophora ferox | 21 | 2015/2 | Neg. | |
| Psorophora sp. | 3 | 2015/2 | Neg. | |
| Runchomyia cerqueirai | 15 | 2015/2 | Neg. | |
| Runchomyia frontosa | 8 | 2015/2 | Neg. | |
| Runchomyia humboldti | 48 | 2015/2 | Neg. | |
| Runchomyia reversa / theobaldi | 1 | 2015/2 | Neg. | |
| Runchomyia sp. | 20 | 2015/2 | Neg. | |
| Sabethes albiprivus | 2 | 2015/2 | Neg. | |
| Sabethes aurescens | 19 | 2015/2 | Neg. | |
| Sabethes chloropterus | 1 | 2015/2 | Neg. | |
| Sabethes chloropterus’ | 5 | 2015/2 | Neg. | |
| Sabethes fabricii’ | 2 | 2015/2 | Neg. | |
| Sabethes identicus | 1 | 2015/2 | Neg. | |
| Sabethes intermedius | 2 | 2015/2 | Neg. | |
| Sabethes melanonymphe | 7 | 2015/2 | Neg. | |
| Sabethes xyphides | 2 | 2015/2 | Neg. | |
| Sabethes sp. | 8 | 2015/2 | Neg. | |
| Sh. fluviatilis | 49 | 2015/2 | Neg. | |
| Shanonniana sp | 28 | 2015/2 | Neg. | |
| Trichoprosopon digitatum | 24 | 2015/2 | Neg. | |
| Trichoprosopon pallidiventer | 5 | 2015/2 | Neg. | |
| Trichoprosopon sp. | 1 | 2015/2 | Neg. | |
| Wyeomyia aporonoma/staminifera | 5 | 2015/2 | Neg. | |
| Wyeomyia bonnei/deanei | 1 | 2015/2 | Neg. | |
| Wyeomyia davisi | 11 | 2015/2 | Neg. | |
| Wyeomyia mystes | 5 | 2015/2 | Neg. | |
| Wyeomyia pilicauda | 42 | 2015/2 | Neg. | |
| Wyeomyia theobaldi | 4 | 2015/2 | Neg. | |
| Wyeomyia (Pho.) sp. | 43 | 2015/2 | Neg. | |
| Wyeomyia sp. | 6 | 2015/2 | Neg. | |
| 2015/2 | 763 | Neg. | ||
| Aedes albopictus | 1 | 2016/1 | Guapimirim, Macaé, Miguel Pereira, Nova Friburgo (RJ) | Neg. |
| Aedes scapularis | 56 | 2016/1 | Neg. | |
| Aedes serratus | 2 | 2016/1 | Neg. | |
| Aedes terrens | 44 | 2016/1 | Neg. | |
| Anopheles bellator | 2 | 2016/1 | Neg. | |
| Anopheles cruzii | 12 | 2016/1 | Neg. | |
| An. hominales | 1 | 2016/1 | Neg. | |
| Anopheles lutzi | 1 | 2016/1 | Neg. | |
| Anopheles sp. | 14 | 2016/1 | Neg. | |
| Culex sp. | 44 | 2016/1 | Neg. | |
| Haemagogus janthinomys | 7 | 2016/1 | Neg. | |
| Haemagogus leucocelaenus | 10 | 2016/1 | Neg. | |
| Limatus durhamii | 31 | 2016/1 | Neg. | |
| Limatus pseudomethisticus | 12 | 2016/1 | Neg. | |
| Onirion personatum | 30 | 2016/1 | Neg. | |
| Psorophora ferox | 3 | 2016/1 | Neg. | |
| Runchomyia cerqueirai | 1 | 2016/1 | Neg. | |
| Runchomyia frontosa | 22 | 2016/1 | Neg. | |
| Runchomyia humboldti | 25 | 2016/1 | Neg. | |
| Runchomyia sp. | 29 | 2016/1 | Neg. | |
| Sabethes aurescens | 4 | 2016/1 | Neg. | |
| Sabethes identicus | 3 | 2016/1 | Neg. | |
| Sabethes melanonymphe | 6 | 2016/1 | Neg. | |
| Sabethes sp. | 19 | 2016/1 | Neg. | |
| Sabethini | 5 | 2016/1 | Neg. | |
| Shannoniana fluviatilis | 84 | 2016/1 | Neg. | |
| Trichoprosopon digitatum | 4 | 2016/1 | Neg. | |
| Trichoprosopon pallidiventer | 18 | 2016/1 | Neg. | |
| Wyeomyia aporonoma/staminifera | 5 | 2016/1 | Neg. | |
| Wyeomyia bonnei/deanei | 4 | 2016/1 | Neg. | |
| Wyeomyia cerqueirai | 1 | 2016/1 | Neg. | |
| Wyeomyia confusa | 1 | 2016/1 | Neg. | |
| Wyeomyia davisi | 1 | 2016/1 | Neg. | |
| Wyeomyia pallidoventer | 6 | 2016/1 | Neg. | |
| Wyeomyia palmata/galvoi | 3 | 2016/1 | Neg. | |
| Wyeomyia pilicauda | 8 | 2016/1 | Neg. | |
| Wyeomyia (Pho.) sp. | 118 | 2016/1 | Neg. | |
| Wyeomyia sp. | 9 | 2016/1 | Neg. | |
| 2016/1 | 646 | Neg. | ||
| Aedes aegypti | 9 | 2016/2 | Itamonte (MG); Queluz (SP); Itatiaia, Miguel Pereira, Rio de Janeiro, Sumidouro, Teresópolis (RJ) | Neg. |
| Aedes fluviatilis | 1 | 2016/2 | Neg. | |
| Aedes scapularis | 11 | 2016/2 | Neg. | |
| Aedes terrens | 4 | 2016/2 | Neg. | |
| Anopheles cruzii | 4 | 2016/2 | Neg. | |
| Anopheles sp. | 13 | 2016/2 | Neg. | |
| Culex sp. | 1 | 2016/2 | Neg. | |
| Haemagogus janthinomys | 8 | 2016/2 | Neg. | |
| Haemagogus leucocelaenus | 22 | 2016/2 | Neg. | |
| Limatus durhamii | 34 | 2016/2 | Neg. | |
| Limatus pseudomethisticus | 42 | 2016/2 | Neg. | |
| Onirion personatum | 8 | 2016/2 | Neg. | |
| Psorophora ferox | 1 | 2016/2 | Neg. | |
| Runchomyia frontosa | 7 | 2016/2 | Neg. | |
| Runchomyia humboldti | 3 | 2016/2 | Neg. | |
| Runchomyia sp. | 13 | 2016/2 | Neg. | |
| Sabethes albiprivus | 4 | 2016/2 | Neg. | |
| Sabethes aurescens | 3 | 2016/2 | Neg. | |
| Sabethes auresces | 2 | 2016/2 | Neg. | |
| Sabethes intermedius | 3 | 2016/2 | Neg. | |
| Sabethes melanonymphe | 3 | 2016/2 | Neg. | |
| Sabethes sp. | 26 | 2016/2 | Neg. | |
| Shannoniana fluviatilis | 18 | 2016/2 | Neg. | |
| Trichoprosopon castroi/similis | 9 | 2016/2 | Neg. | |
| Trichoprosopon digitatum | 6 | 2016/2 | Neg. | |
| Trichoprosopon pallidiventer | 18 | 2016/2 | Neg. | |
| Wyeomyia antunesi | 3 | 2016/2 | Neg. | |
| Wyeomyia aporonoma/staminifera | 1 | 2016/2 | Neg. | |
| Wyeomyia confusa | 60 | 2016/2 | Neg. | |
| Wyeomyia davisi | 5 | 2016/2 | Neg. | |
| Wyeomyia exallos | 1 | 2016/2 | Neg. | |
| Wyeomyia incaudata | 2 | 2016/2 | Neg. | |
| Wyeomyia longirostris | 2 | 2016/2 | Neg. | |
| Wyeomyia lutzi | 4 | 2016/2 | Neg. | |
| Wyeomyia palmata/galvoi | 6 | 2016/2 | Neg. | |
| Wyeomyia pilicauda | 11 | 2016/2 | Neg. | |
| Wyeomyia (Pho.) sp. | 23 | 2016/2 | Neg. | |
| Wyeomyia sp. | 9 | 2016/2 | Neg. | |
| 2016/2 | 400 | Neg. | ||
| Aedes aegypti | 94 | 2017/1 | Belo Horizonte, Simonésia (MG); Domingos Martins, Serra (ES); Angra dos Reis, Casimiro de Abreu, Macaé, Maricá, Petrópolis, Rio de Janeiro (RJ) | Neg |
| Aedes albopictus | 1 | 2017/1 | Neg. | |
| Aedes argyrothorax | 2 | 2017/1 | Neg. | |
| Aedes fulvithorax | 2 | 2017/1 | Neg. | |
| Aedes rhyacophilus | 1 | 2017/1 | Neg. | |
| Aedes scapularis | 876 | 2017/1 | YFV | |
| Aedes serratus | 9 | 2017/1 | Neg. | |
| Aedes taeniorhynchus | 892 | 2017/1 | YFV | |
| Aedes terrens | 7 | 2017/1 | Neg. | |
| Aedes sp. | 22 | 2017/1 | Neg. | |
| Anopheles sp. | 4 | 2017/1 | Neg. | |
| Coquillettidia sp. | 72 | 2017/1 | Neg. | |
| Coquillettidia albicosta | 1 | 2017/1 | Neg. | |
| Coquillettidia hermanoi | 1 | 2017/1 | Neg. | |
| Coquillettidia justamansonia | 16 | 2017/1 | Neg. | |
| Coquillettidia nigricans | 6 | 2017/1 | Neg. | |
| Coquillettidia shannoni | 1 | 2017/1 | Neg. | |
| Coquillettidia venezuelensis | 2 | 2017/1 | Neg. | |
| Culex grupo Coronata | 1 | 2017/1 | Neg. | |
| Culex sp. | 56 | 2017/1 | Neg. | |
| Culex declarator | 4 | 2017/1 | Neg. | |
| Culex nigripalpus | 103 | 2017/1 | Neg. | |
| Haemagogus janthinomys | 8 | 2017/1 | YFV | |
| Haemagogus leucocelaenus | 199 | 2017/1 | YFV | |
| Limatus durhamii | 189 | 2017/1 | Neg. | |
| Limatus pseudomethisticus | 2 | 2017/1 | Neg. | |
| Limatus sp. | 12 | 2017/1 | Neg. | |
| Mansonia indubitans | 75 | 2017/1 | Neg. | |
| Mansonia titillans | 14 | 2017/1 | Neg. | |
| Mansonia sp. | 10 | 2017/1 | Neg. | |
| Psorophora ferox | 21 | 2017/1 | Neg. | |
| Psorophora lutzii/amazonica | 4 | 2017/1 | Neg. | |
| Psorophora sp. | 10 | 2017/1 | Neg. | |
| Runchomyia frontosa | 13 | 2017/1 | Neg. | |
| Runchomyia humboldti | 12 | 2017/1 | Neg. | |
| Runchomyia sp. | 10 | 2017/1 | Neg. | |
| Sabethes petrocchiae | 60 | 2017/1 | Neg. | |
| Sabethes albiprivus | 194 | 2017/1 | Neg. | |
| Sabethes aurescens | 2 | 2017/1 | Neg. | |
| Sabethes chloropterus | 4 | 2017/1 | YFV | |
| Sabethes fabricii’ | 3 | 2017/1 | Neg. | |
| Sabethes quasicyaneus | 1 | 2017/1 | Neg. | |
| Sabethes whitmani | 1 | 2017/1 | Neg. | |
| Sabethes sp. | 47 | 2017/1 | Neg. | |
| Shannoniana fluviatilis | 2 | 2017/1 | Neg. | |
| Trichoprosopon digitatum | 1 | 2017/1 | Neg. | |
| Trichoprosopon pallidiventer | 7 | 2017/1 | Neg. | |
| Trichoprosopon sp. | 3 | 2017/1 | Neg. | |
| Wyeomyia aporonoma/staminifera | 7 | 2017/1 | Neg. | |
| Wyeomyia bourrouli/ forcipenis | 11 | 2017/1 | Neg. | |
| Wyeomyia confusa | 40 | 2017/1 | Neg. | |
| Wyeomyia edwardsi | 9 | 2017/1 | Neg. | |
| Wyeomyia incaudata | 7 | 2017/1 | Neg. | |
| Wyeomyia medioalbipes | 14 | 2017/1 | Neg. | |
| Wyeomyia melanocephala’ | 1 | 2017/1 | Neg. | |
| Wyeomyia mystes | 9 | 2017/1 | Neg. | |
| Wyeomyia palmata/galvoi | 17 | 2017/1 | Neg. | |
| Wyeomyia pilicauda | 4 | 2017/1 | Neg. | |
| Wyeomyia (Mia.) sp. | 1 | 2017/1 | Neg. | |
| Wyeomyia (Pho.) sp. | 20 | 2017/1 | Neg. | |
| Wyeomyia sp. | 45 | 2017/1 | Neg. | |
| 2017/1 | 3262 | |||
| TOTAL | 5219 | 2015–2017 | 19 | YFV |
 Mosquitoes captured before ZIKV and YFV outbreaks.
Mosquitoes captured before ZIKV and YFV outbreaks.  Mosquitoes examined during the ZIKV and before YFV outbreaks.
Mosquitoes examined during the ZIKV and before YFV outbreaks.  Mosquitoes examined after ZIKV and during YFV outbreaks
Mosquitoes examined after ZIKV and during YFV outbreaks| Non-Human Primate Data | Molecular Results | PRNT Results | ELISA Results | Conclusions | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Code | Species | State | Health state | rt-PCR | CT | YFV 90% | ZIKV 90% | DENV1 90% | DENV2 90% | DENV3 90% | DENV 4 90% | Serum Quality | YFV | ZIKV | DENV | Imunological | Molecular |
| RJ10 | Callithrix jacchus* | RJ | Healthy | Neg. | _ | < 5 | 168.3 | 28.9 | 23.9 | 48.0 | < 10 | Hemoly zed++ | Neg. | Neg. | Neg. | Negative | Negative |
| RJ18 | Callithrix jacchus* | RJ | Healthy | Neg. | _ | < 5 | 17.2 | <20 | < 10 | < 10 | < 10 | Hemoly zed++ | Neg. | Neg. | Neg. | Negative | Negative |
| RJ46 | Alouatta g. clamitans | MG | Dying | Neg. | _ | 29.9 | 341.8 | 180.0 | <10 | < 10 | < 10 | Hemoly zed+++ | Neg. | Neg. | Neg. | Negative | Negative |
| RJ60 | Leontopithecus rosalia | RJ | Healthy | Neg. | _ | <10 | 10.8 | - | < 10 | < 10 | < 10 | Hemoly zed++ | Neg. | Neg. | Neg. | Negative | Negative |
| RJ62 | Leontopithecus rosalia | RJ | Healthy | Neg. | _ | < 5 | 41.6 | <100 | < 10 | < 10 | < 10 | Hemoly zed++ | - | - | - | Negative | Negative |
| RJ64 | Leontopithecus rosalia | RJ | Healthy | Neg. | _ | < 5 | 13.1 | <100 | 14.4 | < 10 | < 10 | Hemoly zed++ | - | - | - | Negative | Negative |
| RJ87 | Alouatta g. clamitans | RJ | Dead | YFV | Conv. | <20 | 87.1 | <500 | < 10 | 29.5 | < 10 | Hemoly zed+++ | Neg. | Neg. | Neg. | Negative | YFV |
| RJ91B | Callithrix jacchus* | RJ | Healthy | Neg. | _ | < 5 | 31.26 | <20 | - | < 10 | < 10 | Hemoly zed++ | Neg. | Neg. | Neg. | Negative | Negative |
| RJ95 | Alouatta g. clamitans | RJ | Dead | YFV | 11.7 | < 5 | 65.9 | 30.0 | 10.3 | < 10 | < 10 | Hemoly zed+++ | Neg. | Neg. | Neg. | Negative | YFV |
| RJ96 | Alouatta g. clamitans | RJ | Dead | YFV | Conv. | <40 | 420.3 | - | >250 | < 10 | < 10 | Hemoly zed+++ | Neg. | Neg. | Neg. | Negative | YFV |
| RJ104 | Callithrix jacchus* | RJ | Dead | YFV | 13.7 | < 5 | 11.6 | - | - | - | - | Hemoly zed+ | Neg. | Neg. | Neg. | Negative | YFV |
| AR03 | Alouatta g. clamitans | RJ | Healthy | Neg. | _ | < 5 | 12.7 | - | - | - | - | Hemoly zed++ | Neg. | Neg. | Neg. | Negative | Negative |
| RJ118 | Callithrix jacchus* | RJ | Healthy | Neg. | _ | < 5 | 39.1 | - | - | - | - | Hemoly zed+++ | Neg. | Neg. | Neg. | Negative | Negative |
| ES01 | Brachyteles arachnoides | ES | Healthy | Neg. | _ | < 5 | 14.5 | <10 | < 10 | 17.17 | < 10 | Normal | Neg. | Neg. | Neg. | Negative | Negative |
| ES04 | Alouatta g. clamitans | ES | Dying | YFV | Conv. | 6.9 | 85.4 | - | 24.51 | < 10 | < 10 | Hemoly zed+++ | Neg. | Neg. | Neg. | Negative | YFV |
 NHPs examined during Zika (ZIKV) and before Yellow Fever (YFV) outbreaks.
NHPs examined during Zika (ZIKV) and before Yellow Fever (YFV) outbreaks.  NHPs examined after ZIKV and during YFV outbreaks.
NHPs examined after ZIKV and during YFV outbreaks.© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abreu, F.V.S.d.; Ferreira-de-Brito, A.; Azevedo, A.d.S.; Linhares, J.H.R.; de Oliveira Santos, V.; Hime Miranda, E.; Neves, M.S.A.S.; Yousfi, L.; Ribeiro, I.P.; Santos, A.A.C.d.; et al. Survey on Non-Human Primates and Mosquitoes Does not Provide Evidences of Spillover/Spillback between the Urban and Sylvatic Cycles of Yellow Fever and Zika Viruses Following Severe Outbreaks in Southeast Brazil. Viruses 2020, 12, 364. https://doi.org/10.3390/v12040364
Abreu FVSd, Ferreira-de-Brito A, Azevedo AdS, Linhares JHR, de Oliveira Santos V, Hime Miranda E, Neves MSAS, Yousfi L, Ribeiro IP, Santos AACd, et al. Survey on Non-Human Primates and Mosquitoes Does not Provide Evidences of Spillover/Spillback between the Urban and Sylvatic Cycles of Yellow Fever and Zika Viruses Following Severe Outbreaks in Southeast Brazil. Viruses. 2020; 12(4):364. https://doi.org/10.3390/v12040364
Chicago/Turabian StyleAbreu, Filipe Vieira Santos de, Anielly Ferreira-de-Brito, Adriana de Souza Azevedo, José Henrique Rezende Linhares, Vanessa de Oliveira Santos, Emily Hime Miranda, Maycon Sebastião Alberto Santos Neves, Lena Yousfi, Ieda Pereira Ribeiro, Alexandre Araújo Cunha dos Santos, and et al. 2020. "Survey on Non-Human Primates and Mosquitoes Does not Provide Evidences of Spillover/Spillback between the Urban and Sylvatic Cycles of Yellow Fever and Zika Viruses Following Severe Outbreaks in Southeast Brazil" Viruses 12, no. 4: 364. https://doi.org/10.3390/v12040364
APA StyleAbreu, F. V. S. d., Ferreira-de-Brito, A., Azevedo, A. d. S., Linhares, J. H. R., de Oliveira Santos, V., Hime Miranda, E., Neves, M. S. A. S., Yousfi, L., Ribeiro, I. P., Santos, A. A. C. d., dos Santos, E., Santos, T. P. d., Teixeira, D. S., Gomes, M. Q., Fernandes, C. B., Silva, A. M. V. d., Lima, M. d. R. Q., Paupy, C., Romano, A. P. M., ... Lourenço-de-Oliveira, R. (2020). Survey on Non-Human Primates and Mosquitoes Does not Provide Evidences of Spillover/Spillback between the Urban and Sylvatic Cycles of Yellow Fever and Zika Viruses Following Severe Outbreaks in Southeast Brazil. Viruses, 12(4), 364. https://doi.org/10.3390/v12040364






