Transcriptomic Analysis Suggests the M1 Polarization and Launch of Diverse Programmed Cell Death Pathways in Japanese Encephalitis Virus-Infected Macrophages
Abstract
1. Introduction
2. Materials and Methods
2.1. Cells and Virus
2.2. Mice and in Situ Infection of Peritoneal Macrophages
2.3. Isolation of Peritoneal Macrophages
2.4. Smear Preparations of Peritoneal Macrophages
2.5. Viral Load Detection in Peritoneal Lavages and Sera
2.6. Total RNA Extraction and Quality Control
2.7. New England Biolabs (NEB) General Library Building
2.8. Clustering and Sequencing
2.9. Sequencing Data Processing
2.10. Immunofluorescent (IF) Staining
2.11. TUNEL Staining
2.12. Ethical Statement
3. Results
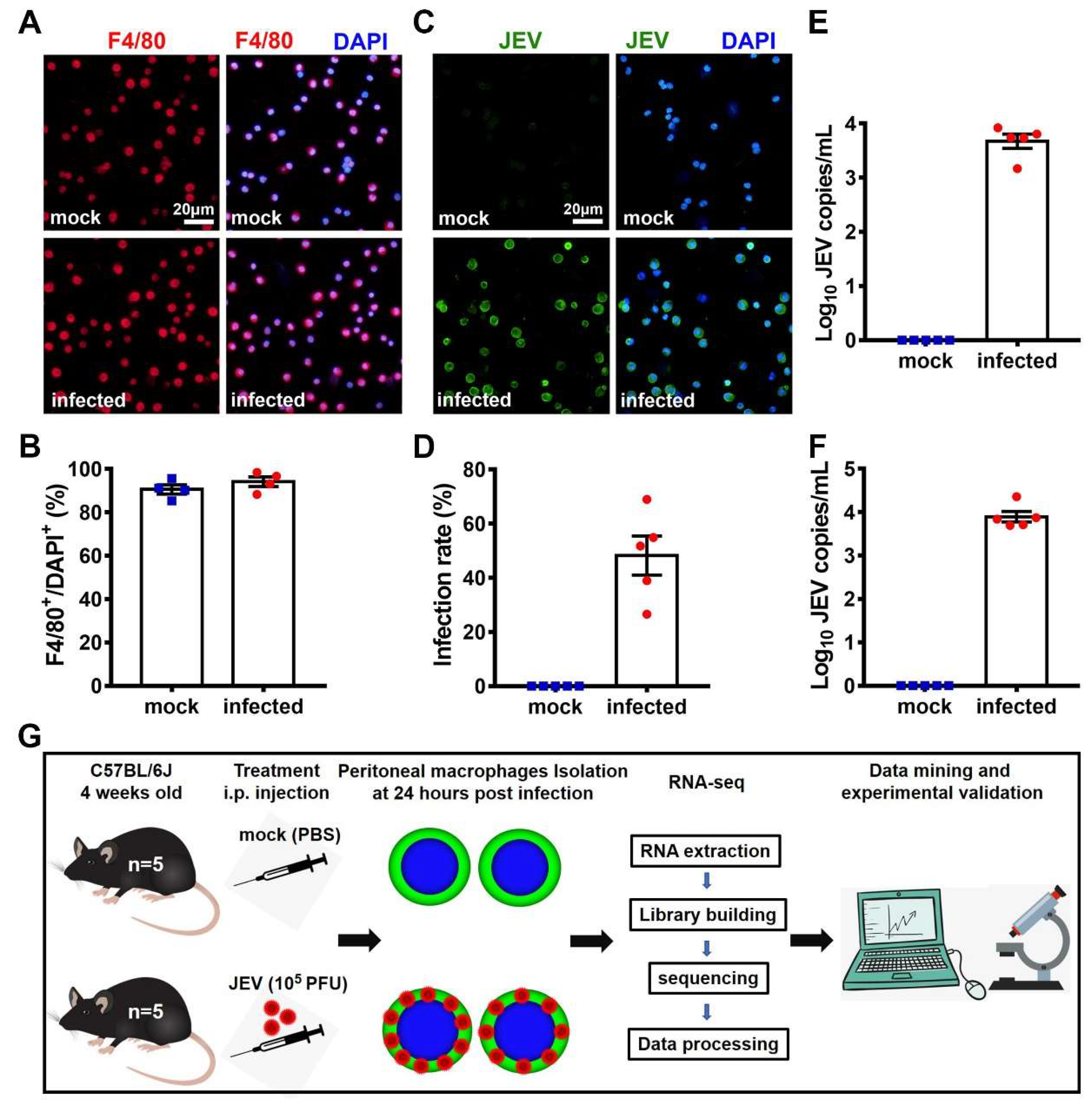
3.1. In Situ JEV Infection Model of the Macrophages and Transcriptomic Research Design.
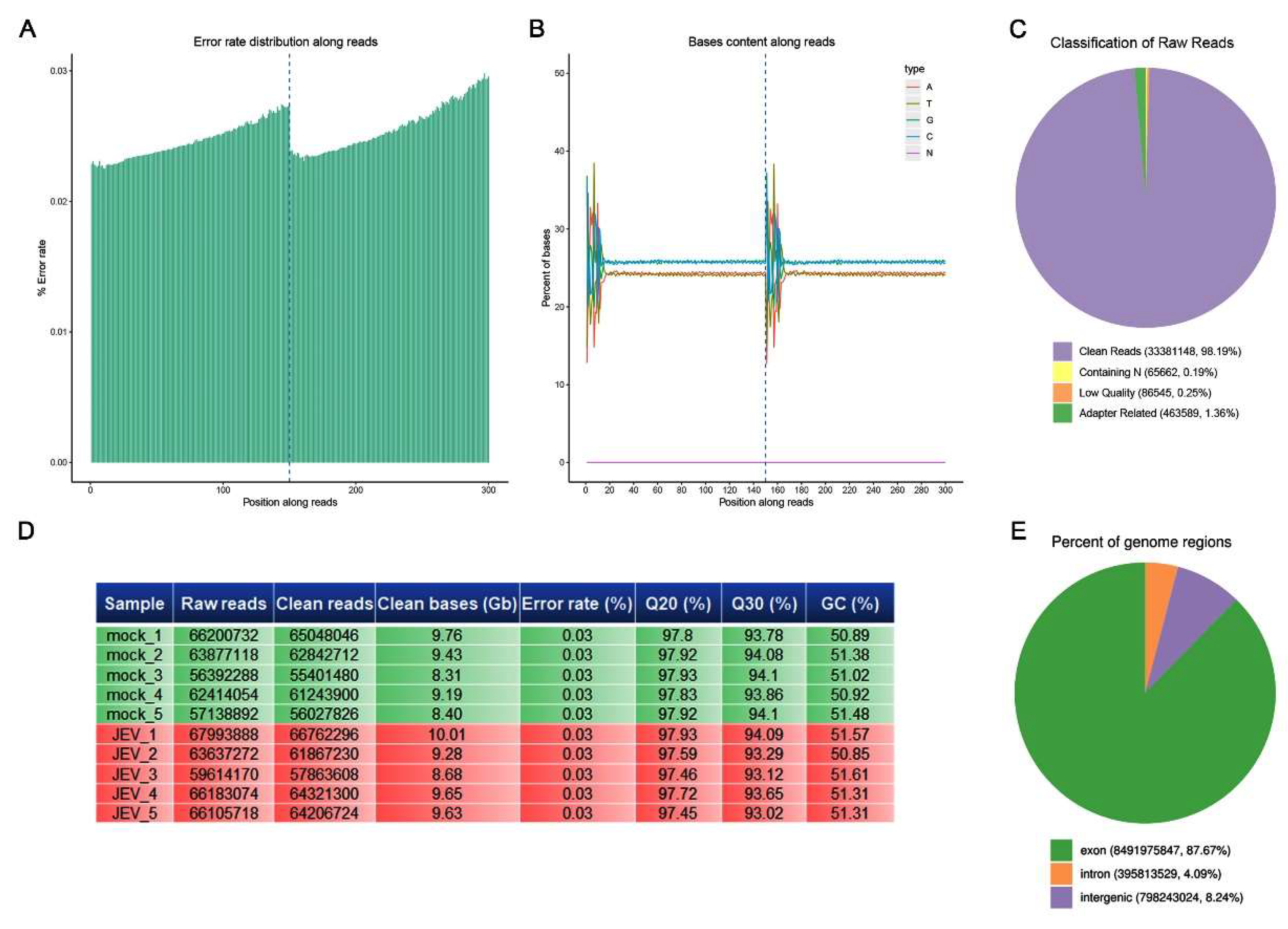
3.2. Quality Control of Sequencing Data
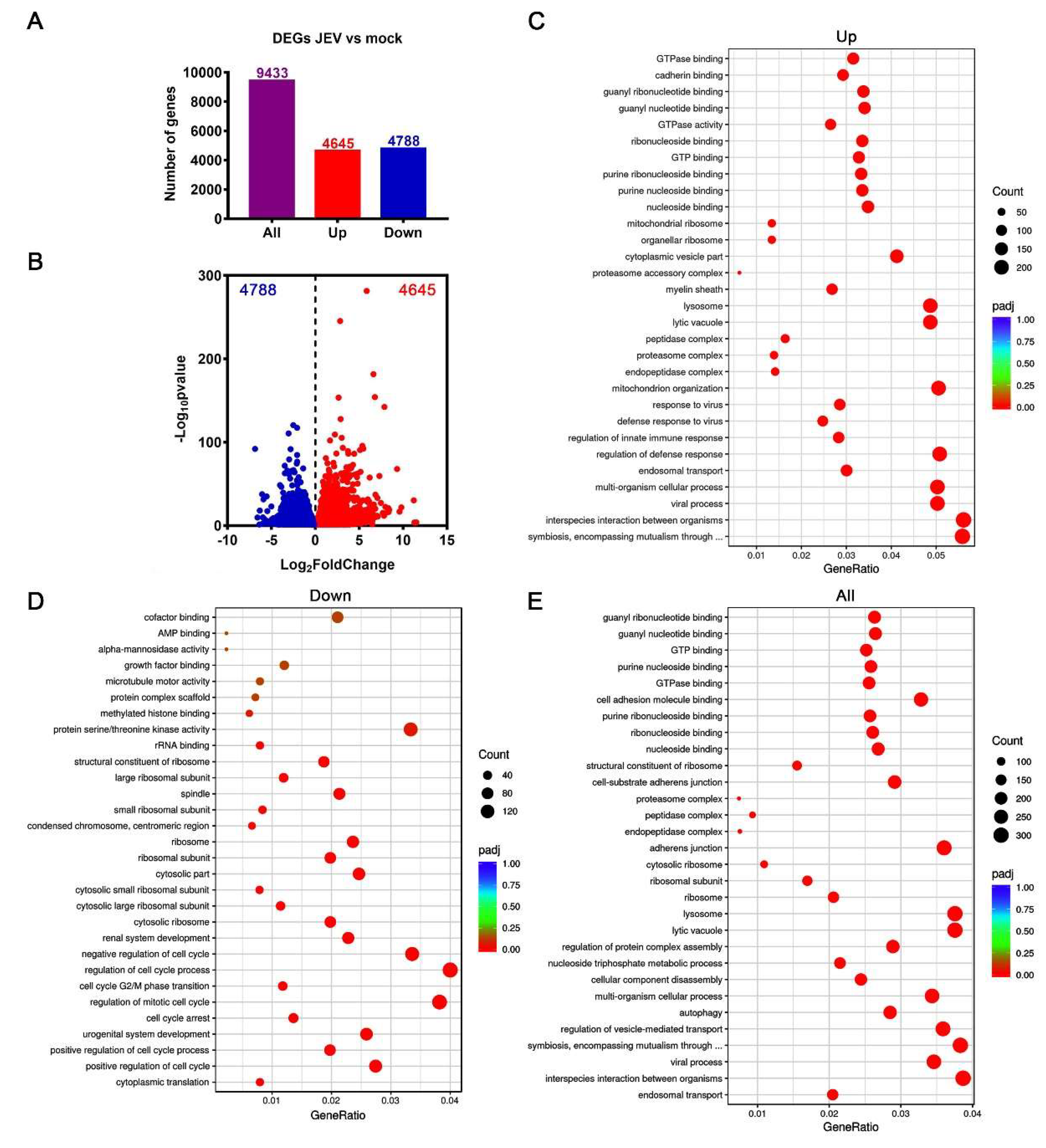
3.3. Transcriptomic Profile of JEV-Infected Macrophages
3.4. M1 Polarization of JEV-Infected Macrophages
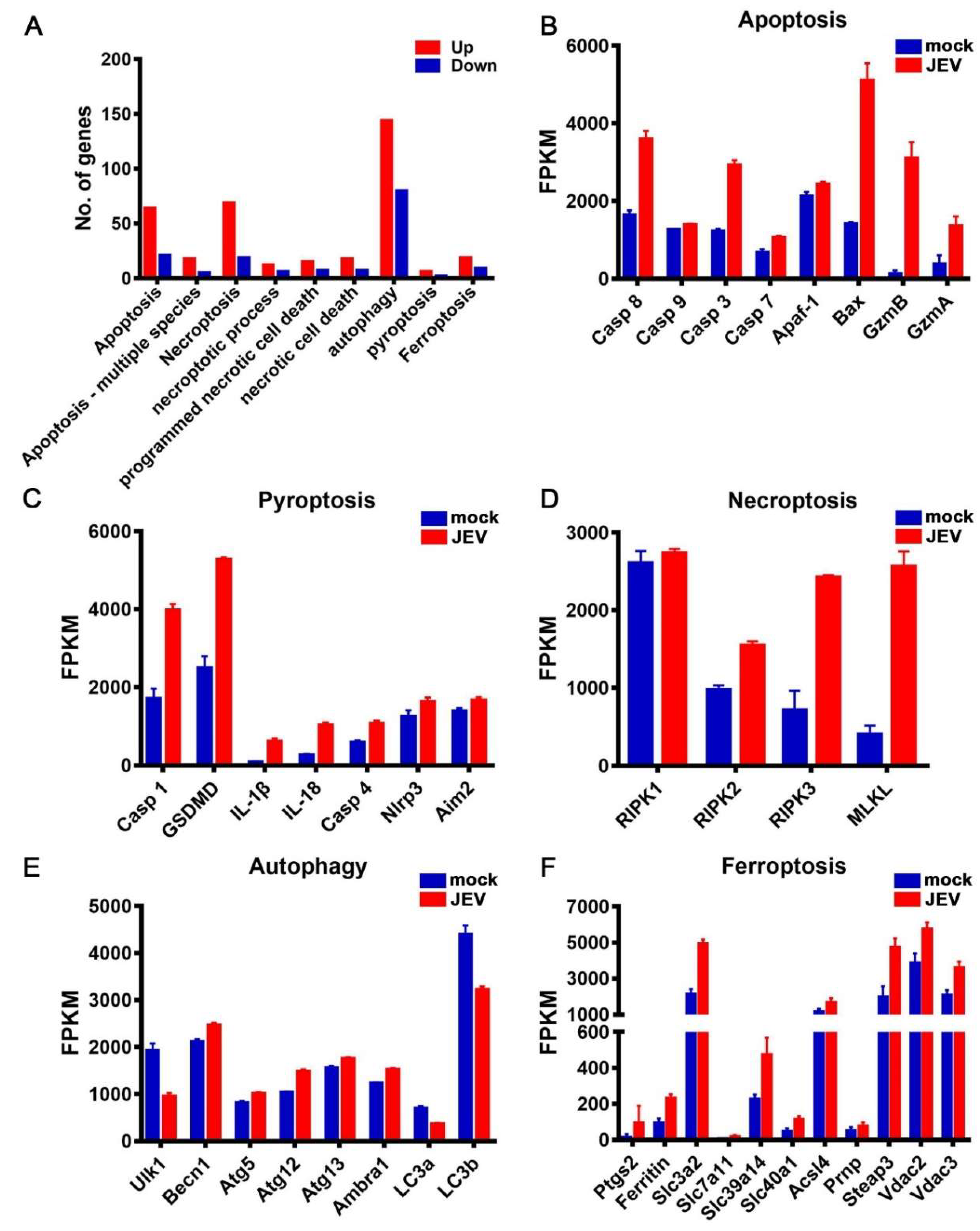
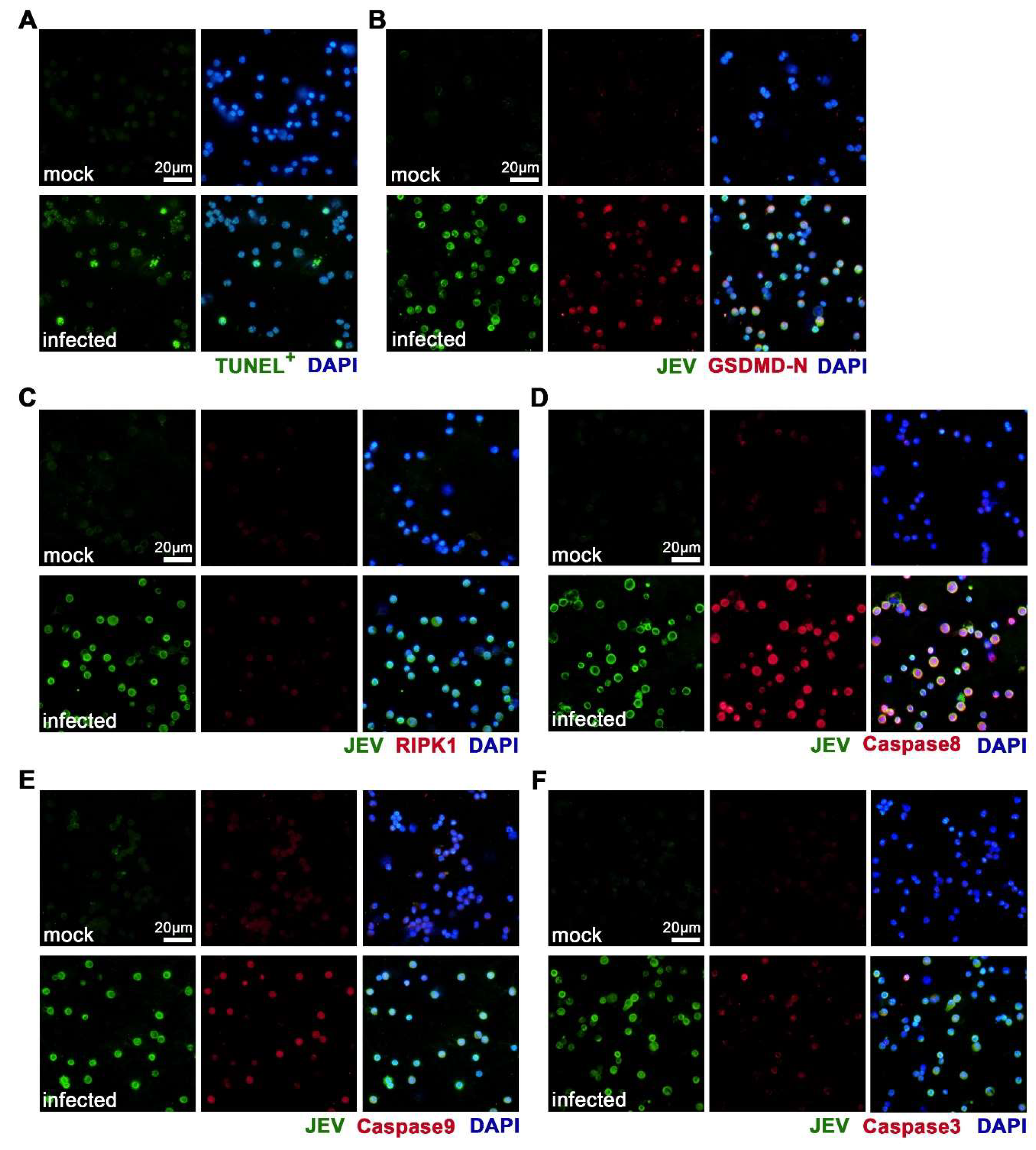
3.5. JEV Infection Activated Diverse Programmed Cell Death Pathways
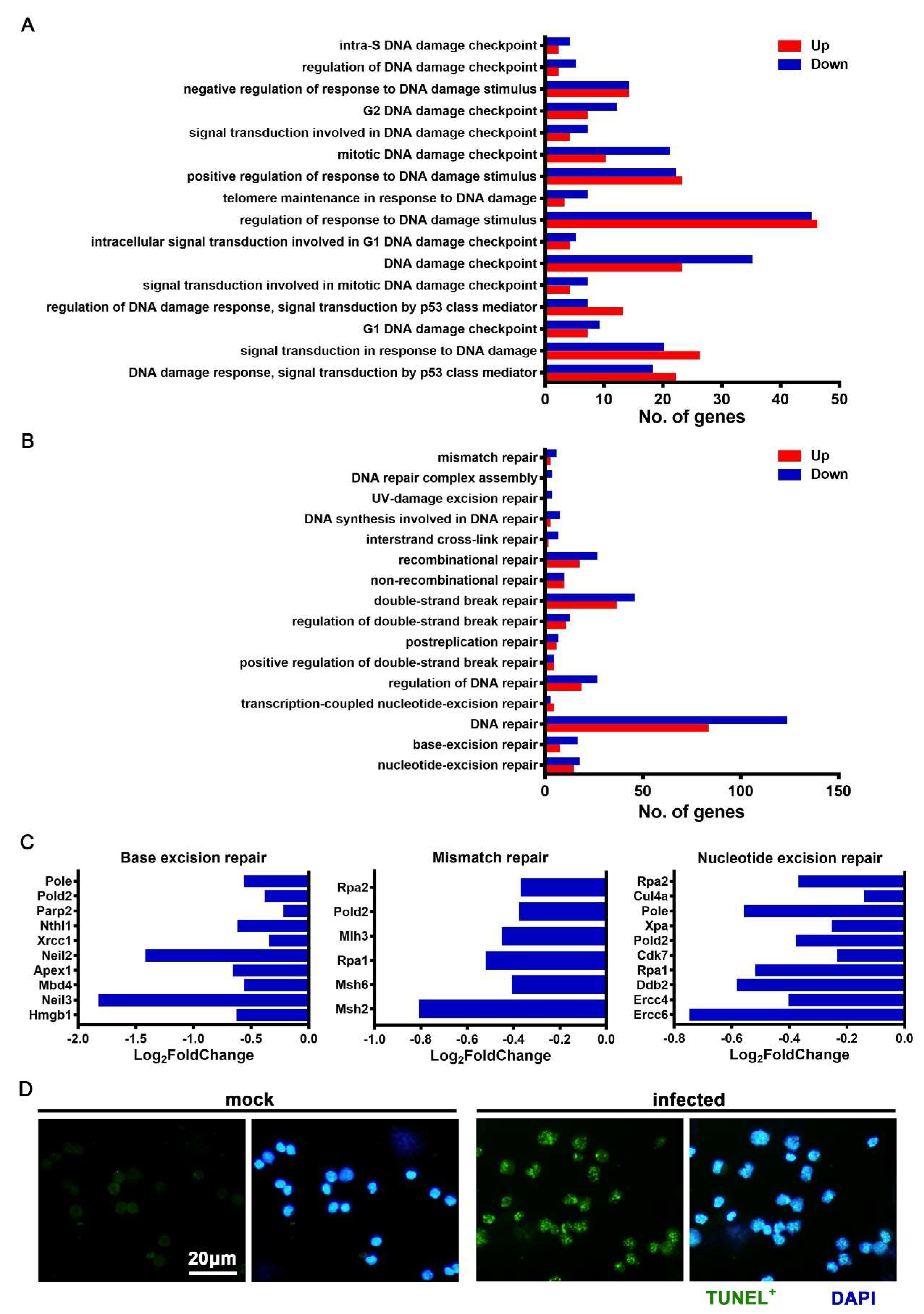
3.6. JEV Infection Elicited DNA Damage and Repair Dysfunction
3.7. JEV Infection Caused Oxidative Stress in Macrophages
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Turtle, L.; Solomon, T. Japanese encephalitis—The prospects for new treatments. Nat. Rev. Neurol. 2018, 14, 298–313. [Google Scholar] [CrossRef]
- Gao, X.; Liu, H.; Li, X.; Fu, S.; Cao, L.; Shao, N.; Zhang, W.; Wang, Q.; Lu, Z.; Lei, W.; et al. Changing Geographic Distribution of Japanese Encephalitis Virus Genotypes, 1935–2017. Vector-Borne Zoonotic Dis. 2019, 19, 35–44. [Google Scholar] [CrossRef]
- Murray, P.J.; Wynn, T.A. Protective and pathogenic functions of macrophage subsets. Nat. Rev. Immunol. 2011, 11, 723–737. [Google Scholar] [CrossRef]
- Nikitina, E.; Larionova, I.; Choinzonov, E.; Kzhyshkowska, J. Monocytes and Macrophages as Viral Targets and Reservoirs. Int. J. Mol. Sci. 2018, 19, 2821. [Google Scholar] [CrossRef]
- Zimmerman, M.G.; Quicke, K.; O’Neal, J.T.; Arora, N.; Machiah, D.; Priyamvada, L.; Kauffman, R.C.; Register, E.; Adekunle, O.; Swieboda, D.; et al. Cross-Reactive Dengue Virus Antibodies Augment Zika Virus Infection of Human Placental Macrophages. Cell Host Microbe 2018, 24, 731–742.e6. [Google Scholar] [CrossRef]
- Wu, M.-F.; Chen, S.-T.; Yang, A.-H.; Lin, W.-W.; Lin, Y.-L.; Chen, N.-J.; Tsai, I.-S.; Li, L.; Hsieh, S.-L. CLEC5A is critical for dengue virus–induced inflammasome activation in human macrophages. Blood 2013, 121, 95–106. [Google Scholar] [CrossRef]
- Sheng, Z.; Gao, N.; Cui, X.; Fan, D.; Chen, H.; Wu, N.; Wei, J.; An, J. Electroporation enhances protective immune response of a DNA vaccine against Japanese encephalitis in mice and pigs. Vaccine 2016, 34, 5751–5757. [Google Scholar] [CrossRef]
- Huang, J.-L.; Lin, H.-T.; Wang, Y.-M.; Weng, M.-H.; Ji, D.-D.; Kuo, M.-D.; Liu, H.-W.; Lin, C.-S. Sensitive and specific detection of strains of Japanese encephalitis virus using a one-step TaqMan RT-PCR technique. J. Med. Virol. 2004, 74, 589–596. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 002832. [Google Scholar] [CrossRef]
- Wang, N.; Liang, H.; Zen, K. Molecular Mechanisms That Influence the Macrophage M1–M2 Polarization Balance. Front. Immunol. 2014, 5, 614. [Google Scholar] [CrossRef]
- Verreck, F.A.; De Boer, T.; Langenberg, D.M.L.; Hoeve, M.A.; Kramer, M.; Vaisberg, E.; Kastelein, R.; Kolk, A.; Malefyt, R.D.W.; Ottenhoff, T.H.M. Human IL-23-producing type 1 macrophages promote but IL-10-producing type 2 macrophages subvert immunity to (myco)bacteria. Proc. Natl. Acad. Sci. USA 2004, 101, 4560–4565. [Google Scholar] [CrossRef]
- Martinez, F.O.; Helming, L.; Gordon, S. Alternative Activation of Macrophages: An Immunologic Functional Perspective. Annu. Rev. Immunol. 2009, 27, 451–483. [Google Scholar] [CrossRef] [PubMed]
- Hsu, H.; Viney, J.L. The tale of TL1A in inflammation. Mucosal Immunol. 2011, 4, 368–370. [Google Scholar] [CrossRef] [PubMed]
- Migone, T.S.; Zhang, J.; Luo, X.; Zhuang, L.; Chen, C.; Hu, B.; Hong, J.S.; Perry, J.W.; Chen, S.F.; Zhou, J.X.; et al. TL1A is a TNF-like ligand for DR3 and TR6/DcR3 and functions as a T cell costimulator. Immunity 2002, 16, 479–492. [Google Scholar] [CrossRef]
- Moore, B.B.; Murray, L.; Das, A.; Wilke, C.A.; Herrygers, A.B.; Toews, G.B. The Role of CCL12 in the Recruitment of Fibrocytes and Lung Fibrosis. Am. J. Respir. Cell Mol. Boil. 2006, 35, 175–181. [Google Scholar] [CrossRef]
- Kwiatkowski, K.; Popiolek-Barczyk, K.; Piotrowska, A.; Rojewska, E.; Ciapała, K.; Makuch, W.; Mika, J. Chemokines CCL2 and CCL7, but not CCL12, play a significant role in the development of pain-related behavior and opioid-induced analgesia. Cytokine 2019, 119, 202–213. [Google Scholar] [CrossRef]
- McMillan, T.R.; Moore, B.B.; Weinberg, J.B.; Vannella, K.M.; Fields, W.B.; Christensen, P.J.; Van Dyk, L.; Toews, G.B. Exacerbation of Established Pulmonary Fibrosis in a Murine Model by Gammaherpesvirus. Am. J. Respir. Crit. Care Med. 2008, 177, 771–780. [Google Scholar] [CrossRef]
- Hennenberg, E.M.; Eyking, A.; Reis, H.; Cario, E. MDR1A deficiency restrains tumor growth in murine colitis-associated carcinogenesis. PLoS ONE 2017, 12, e0180834. [Google Scholar] [CrossRef]
- Garcia-Tapia, D.; Hassett, D.E.; Mitchell, W.J.; Johnson, G.C.; Kleiboeker, S.B. West Nile virus encephalitis: Sequential histopathological and immunological events in a murine model of infection. J. NeuroVirol. 2007, 13, 130–138. [Google Scholar] [CrossRef]
- Yang, T.-C.; Lai, C.-C.; Shiu, S.-L.; Chuang, P.-H.; Tzou, B.-C.; Lin, Y.-Y.; Tsai, F.-J.; Lin, C.-W. Japanese encephalitis virus down-regulates thioredoxin and induces ROS-mediated ASK1-ERK/p38 MAPK activation in human promonocyte cells. Microbes Infect. 2010, 12, 643–651. [Google Scholar] [CrossRef]
- Suzuki, T.; Okamoto, T.; Katoh, H.; Sugiyama, Y.; Kusakabe, S.; Tokunaga, M.; Hirano, J.; Miyata, Y.; Fukuhara, T.; Ikawa, M.; et al. Infection with flaviviruses requires BCLXL for cell survival. PLOS Pathog. 2018, 14, e1007299. [Google Scholar] [CrossRef] [PubMed]
- Guo, F.; Yu, X.; Xu, A.; Xu, J.; Wang, Q.; Guo, Y.; Wu, X.; Tang, Y.; Ding, Z.; Zhang, Y.; et al. Japanese encephalitis virus induces apoptosis by inhibiting Foxo signaling pathway. Veter Microbiol. 2018, 220, 73–82. [Google Scholar] [CrossRef]
- Huang, M.; Xu, A.; Wu, X.; Zhang, Y.; Guo, Y.; Guo, F.; Pan, Z.; Kong, L. Japanese encephalitis virus induces apoptosis by the IRE1/JNK pathway of ER stress response in BHK-21 cells. Arch. Virol. 2016, 161, 699–703. [Google Scholar] [CrossRef]
- Mukherjee, S.; Singh, N.; Sengupta, N.; Fatima, M.; Seth, P.; Mahadevan, A.; Shankar, S.K.; Bhattacharyya, A.; Basu, A. Japanese encephalitis virus induces human neural stem/progenitor cell death by elevating GRP78, PHB and hnRNPC through ER stress. Cell Death Dis. 2017, 8, e2556. [Google Scholar] [CrossRef]
- Bian, P.; Zheng, X.; Wei, L.; Ye, C.; Fan, H.; Cai, Y.; Zhang, Y.; Zhang, F.; Jia, Z.; Lei, Y. MLKL Mediated Necroptosis Accelerates JEV-Induced Neuroinflammation in Mice. Front. Microbiol. 2017, 8, 304. [Google Scholar] [CrossRef]
- Galluzzi, L.; Kepp, O.; Chan, F.K.L.; Kroemer, G. Necroptosis: Mechanisms and Relevance to Disease. Annu. Rev. Pathol. Mech. Dis. 2016, 12, 103–130. [Google Scholar] [CrossRef]
- Hartlova, A.; Erttmann, S.F.; Raffi, F.A.; Schmalz, A.M.; Resch, U.; Anugula, S.; Lienenklaus, S.; Nilsson, J.A.; Kröger, A.; Nilsson, J.A.; et al. DNA Damage Primes the Type I Interferon System via the Cytosolic DNA Sensor STING to Promote Anti-Microbial Innate Immunity. Immunity 2015, 42, 332–343. [Google Scholar] [CrossRef]
- Srivastava, S.; Khanna, N.; Saxena, S.K.; Singh, A.; Mathur, A.; Dhole, T.N. Degradation of Japanese encephalitis virus by neutrophils. Int. J. Exp. Pathol. 1999, 80, 17–24. [Google Scholar] [CrossRef]
- Raung, S.-L.; Kuo, M.-D.; Wang, Y.-M.; Chen, C.-J. Role of reactive oxygen intermediates in Japanese encephalitis virus infection in murine neuroblastoma cells. Neurosci. Lett. 2001, 315, 9–12. [Google Scholar] [CrossRef]
- Mishra, M.; Kumawat, K.L.; Basu, A. Japanese encephalitis virus differentially modulates the induction of multiple pro-inflammatory mediators in human astrocytoma and astroglioma cell-lines. Cell Boil. Int. 2008, 32, 1506–1513. [Google Scholar] [CrossRef]
- Brüne, B.; Dehne, N.; Grossmann, N.; Jung, M.; Namgaladze, D.; Schmid, T.; Von Knethen, A.; Weigert, A. Redox Control of Inflammation in Macrophages. Antioxid. Redox Signal. 2013, 19, 595–637. [Google Scholar] [CrossRef] [PubMed]
- De Jager, T.L.; Cockrell, A.E.; Du Plessis, S.S. Ultraviolet Light Induced Generation of Reactive Oxygen Species. Adv. Exp. Med. Biol. 2017, 996, 15–23. [Google Scholar] [PubMed]
- Schieber, M.; Chandel, N.S. ROS function in redox signaling and oxidative stress. Curr. Boil. 2014, 24, R453–462. [Google Scholar] [CrossRef]
- Mishra, M.; Ghosh, D.; Duseja, R.; Basu, A. Antioxidant potential of Minocycline in Japanese Encephalitis Virus infection in murine neuroblastoma cells: Correlation with membrane fluidity and cell death. Neurochem. Int. 2009, 54, 464–470. [Google Scholar] [CrossRef]



© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Z.-Y.; Zhen, Z.-D.; Fan, D.-Y.; Wang, P.-G.; An, J. Transcriptomic Analysis Suggests the M1 Polarization and Launch of Diverse Programmed Cell Death Pathways in Japanese Encephalitis Virus-Infected Macrophages. Viruses 2020, 12, 356. https://doi.org/10.3390/v12030356
Wang Z-Y, Zhen Z-D, Fan D-Y, Wang P-G, An J. Transcriptomic Analysis Suggests the M1 Polarization and Launch of Diverse Programmed Cell Death Pathways in Japanese Encephalitis Virus-Infected Macrophages. Viruses. 2020; 12(3):356. https://doi.org/10.3390/v12030356
Chicago/Turabian StyleWang, Zhao-Yang, Zi-Da Zhen, Dong-Ying Fan, Pei-Gang Wang, and Jing An. 2020. "Transcriptomic Analysis Suggests the M1 Polarization and Launch of Diverse Programmed Cell Death Pathways in Japanese Encephalitis Virus-Infected Macrophages" Viruses 12, no. 3: 356. https://doi.org/10.3390/v12030356
APA StyleWang, Z.-Y., Zhen, Z.-D., Fan, D.-Y., Wang, P.-G., & An, J. (2020). Transcriptomic Analysis Suggests the M1 Polarization and Launch of Diverse Programmed Cell Death Pathways in Japanese Encephalitis Virus-Infected Macrophages. Viruses, 12(3), 356. https://doi.org/10.3390/v12030356





