Regulation of Transcription Factor E2-2 in Human Plasmacytoid Dendritic Cells by Monocyte-Derived TNFα
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of PBMC
2.2. pDC Enrichment & Monocyte Depletion
2.3. Viruses
2.4. Antibodies
2.5. Flow Cytometry
2.6. Cell Culture
2.7. qRT-PCR
2.8. Enzyme-Linked Immunosorbent Assay
2.9. Statistical Analysis
3. Results
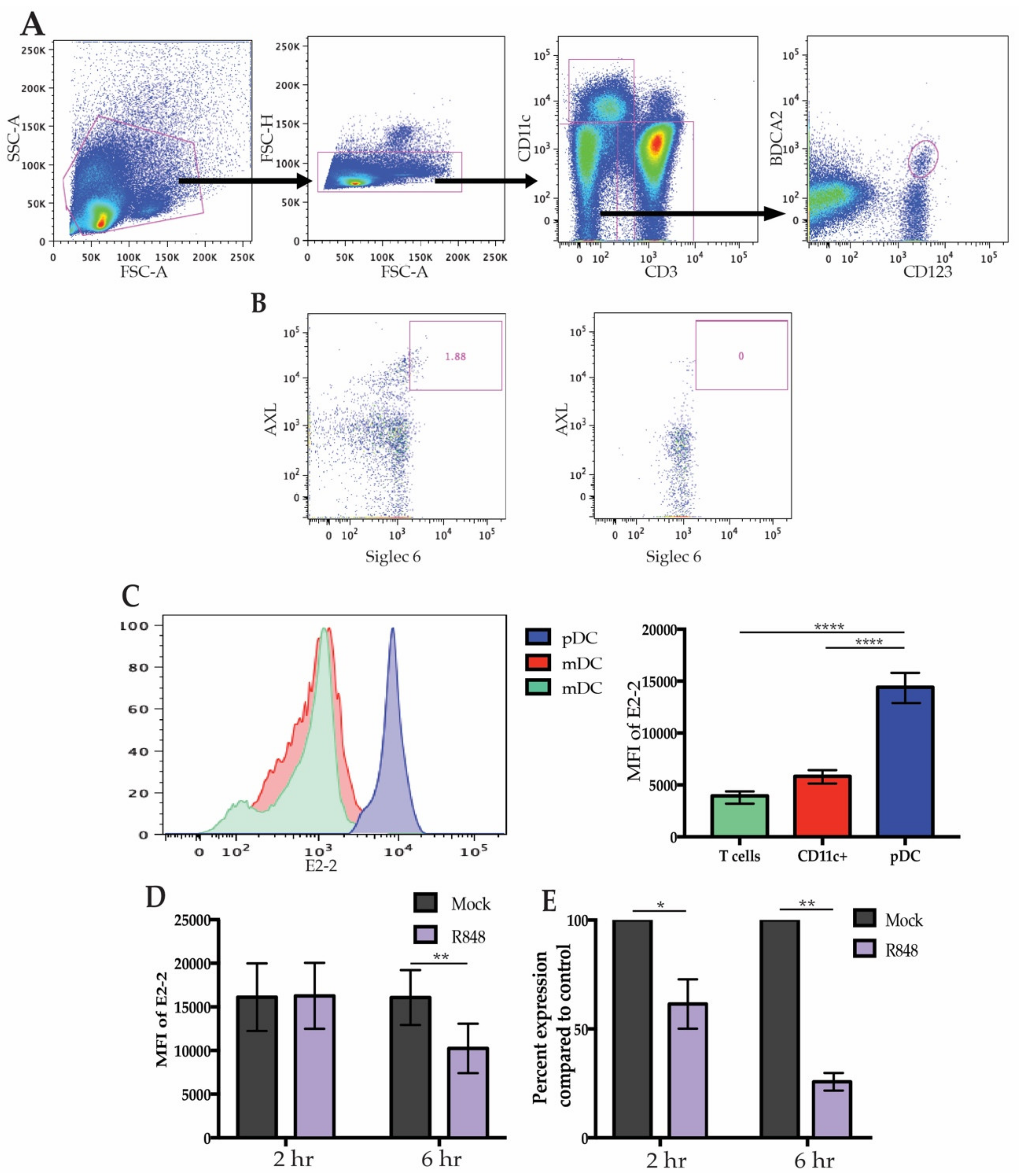
3.1. High E2-2 Expression Is Distinctive to pDCs and Is Downregulated after Stimulation
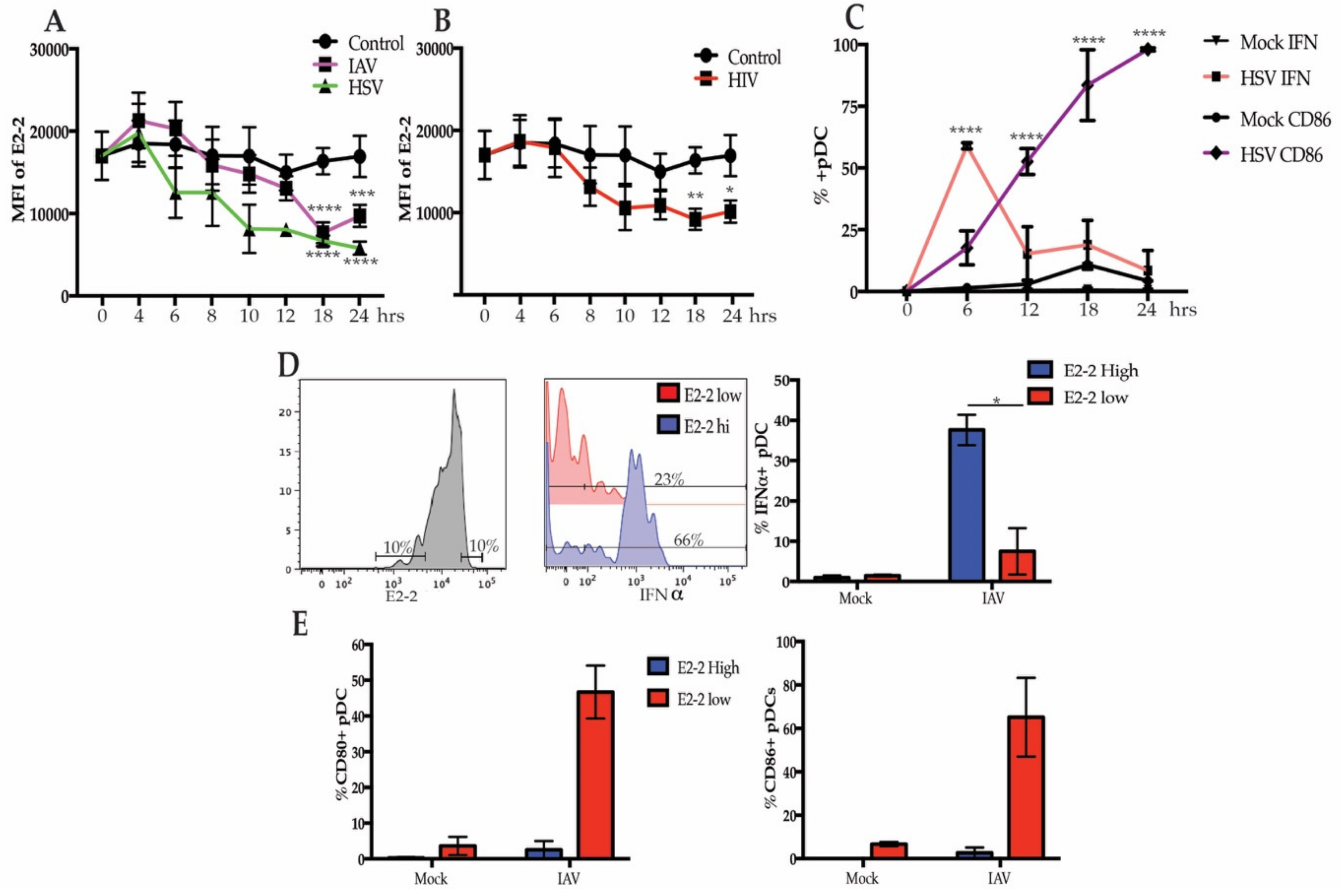
3.2. Differential Expression of E2-2 is Associated with Functional and Phenotypic Differences
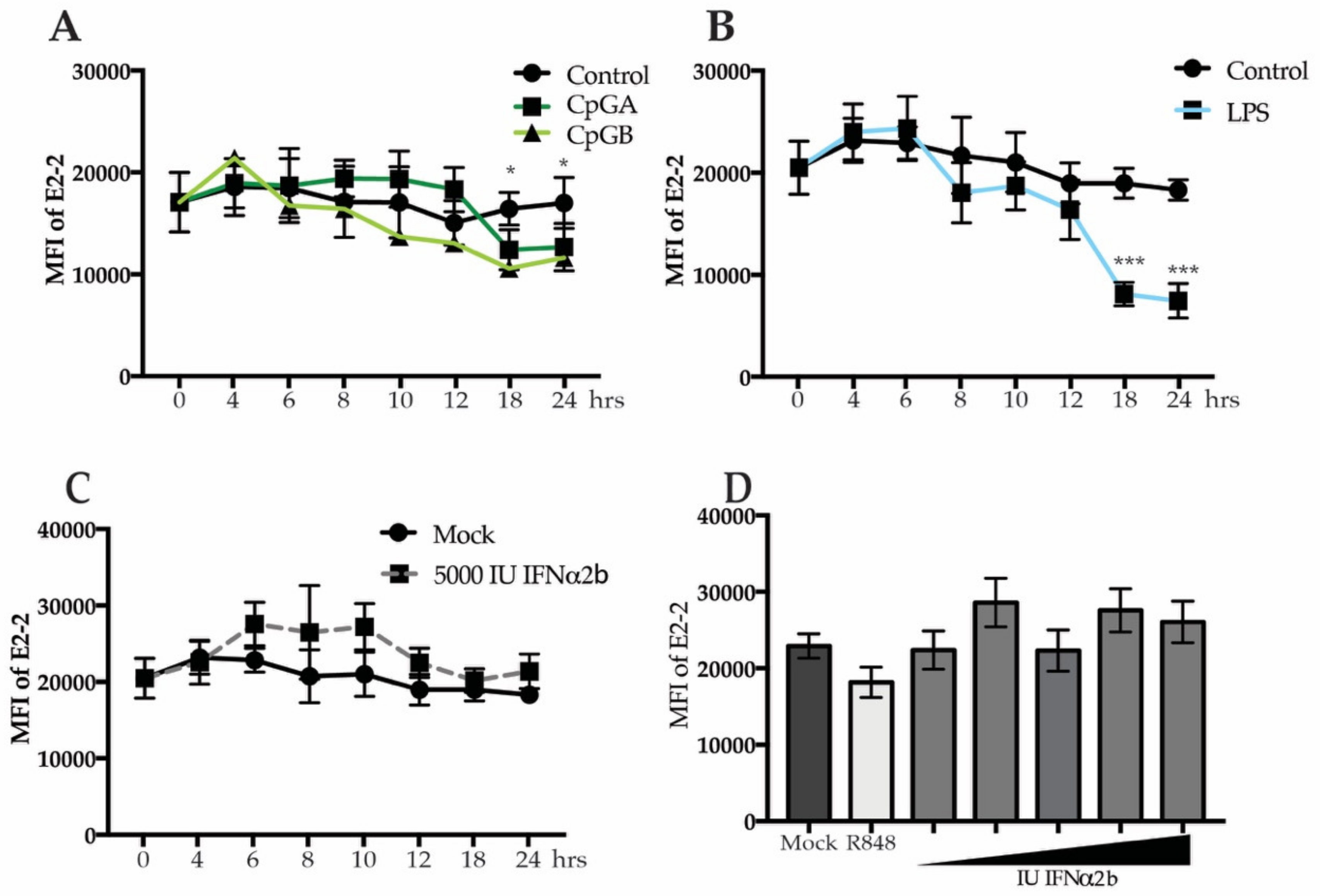
3.3. Decreased E2-2 Expression Is Not Induced by IFNα Receptor Signaling
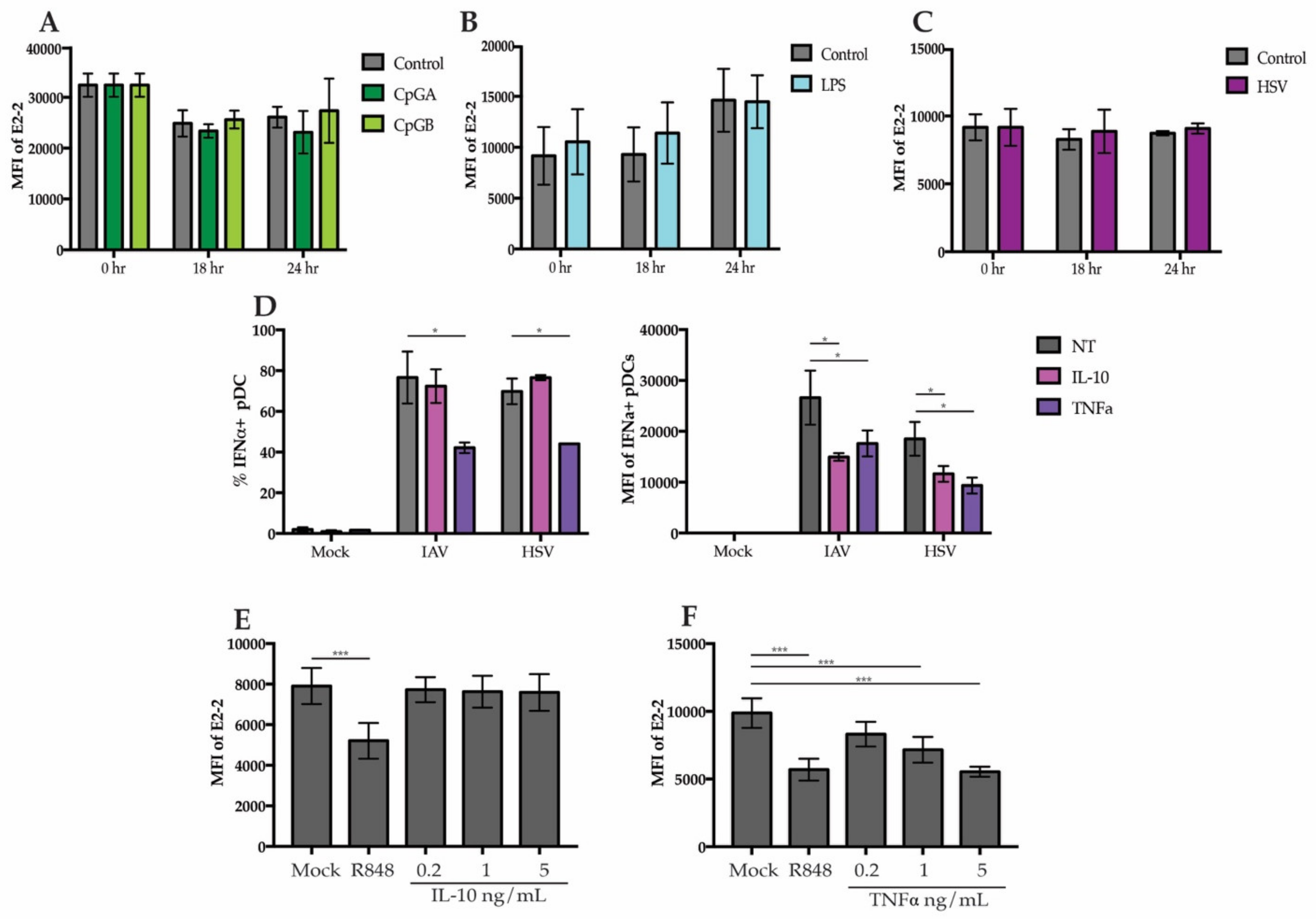
3.4. Secreted Cytokines from Neighboring Cells Lead to Downregulation of E2-2
3.5. Regulation of E2-2 Is Mediated by TNFα Produced by Monocytes
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Fitzgerald-Bocarsly, P.; Dai, J.; Singh, S. Plasmacytoid dendritic cells and type i ifn: 50 years of convergent history. Cytokine Growth Factor Rev. 2008, 19, 3–19. [Google Scholar] [CrossRef]
- Reizis, B. Plasmacytoid dendritic cells: Development, regulation, and function. Immunity 2019, 50, 37–50. [Google Scholar] [CrossRef]
- Fitzgerald-Bocarsly, P. Human natural interferon-alpha producing cells. Pharmac. Ther. 1993, 60, 39–63. [Google Scholar] [CrossRef]
- Swiecki, M.; Colonna, M. The multifaceted biology of plasmacytoid dendritic cells. Nat. Rev. Immunol. 2015, 15, 471–485. [Google Scholar] [CrossRef] [PubMed]
- Fitzgerald-Bocarsly, P.; Feng, D. The role of type I interferon production by dendritic cells in host defense. Biochimie 2007, 89, 843–855. [Google Scholar] [CrossRef] [PubMed]
- Guiducci, C.; Coffman, R.L.; Barrat, F.J. Signalling pathways leading to ifn-alpha production in human plasmacytoid dendritic cell and the possible use of agonists or antagonists of TLR7 and TLR9 in clinical indications. J. Intern. Med. 2009, 265, 43–57. [Google Scholar] [CrossRef] [PubMed]
- Dai, J.; Megjugorac, N.J.; Amrute, S.B.; Fitzgerald-Bocarsly, P. Regulation of IFN regulatory factor-7 and IFN-a production by enveloped virus and lipopolysaccharide in human plasmacytoid dendritic cells. J. Immunol. 2004, 173, 1535–1548. [Google Scholar] [CrossRef]
- See, P.; Dutertre, C.A.; Chen, J.; Gunther, P.; McGovern, N.; Irac, S.E.; Gunawan, M.; Beyer, M.; Handler, K.; Duan, K.; et al. Mapping the human DC lineage through the integration of high-dimensional techniques. Science 2017, 356, eaag3009. [Google Scholar] [CrossRef]
- Reizis, B.; Bunin, A.; Ghosh, H.S.; Lewis, K.L.; Sisirak, V. Plasmacytoid dendritic cells: Recent progress and open questions. Annu Rev. Immunol. 2011, 29, 163–183. [Google Scholar] [CrossRef]
- Nagasawa, M.; Schmidlin, H.; Hazekamp, M.G.; Schotte, R.; Blom, B. Development of human plasmacytoid dendritic cells depends on the combined action of the basic helix-loop-helix factor E2-2 and the ETS factor Spi-b. Eur. J. Immunol. 2008, 38, 2389–2400. [Google Scholar] [CrossRef]
- Li, H.S.; Yang, C.Y.; Nallaparaju, K.C.; Zhang, H.; Liu, Y.J.; Goldrath, A.W.; Watowich, S.S. The signal transducers STAT5 and STAT3 control expression of ID2 and E2-2 during dendritic cell development. Blood 2012, 120, 4363–4373. [Google Scholar] [CrossRef]
- Mastio, J.; Simand, C.; Cova, G.; Kastner, P.; Chan, S.; Kirstetter, P. Ikaros cooperates with notch activation and antagonizes TGFbeta signaling to promote pDC development. PLoS Genet. 2018, 14, e1007485. [Google Scholar] [CrossRef]
- Allman, D.; Dalod, M.; Asselin-Paturel, C.; Delale, T.; Robbins, S.H.; Trinchieri, G.; Biron, C.A.; Kastner, P.; Chan, S. Ikaros is required for plasmacytoid dendritic cell differentiation. Blood 2006, 108, 4025–4034. [Google Scholar] [CrossRef] [PubMed]
- Villani, A.; Satija, R.; Reynolds, G.; Sarkizova, S.; Shekhar, K.; Fletcher, J.; Griesbeck, M.; Butler, A.; Zheng, S.; Lazo, S.; et al. Single-cell RNA-seq reveals new types of human blood dendritic cells, monocytes, and progenitors. Science 2017, 356, eaah4573. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, H.S.; Cisse, B.; Bunin, A.; Lewis, K.L.; Reizis, B. Continuous expression of the transcription factor e2-2 maintains the cell fate of mature plasmacytoid dendritic cells. Immunity 2010, 33, 905–916. [Google Scholar] [CrossRef] [PubMed]
- Cisse, B.; Caton, M.L.; Lehner, M.; Maeda, T.; Scheu, S.; Locksley, R.; Holmberg, D.; Zweier, C.; den Hollander, N.S. Transcription factor E2-2 is an essential and specific regulator of plasmacytoid dendritic cell development. Cell 2008, 135, 37–48. [Google Scholar] [CrossRef] [PubMed]
- Cheng, M.; Zhang, X.; Yu, H.; Du, P.; Plumas, J.; Chaperot, L.; Su, L.; Zhang, L. Characterization of species-specific genes regulated by E2-2 in human plasmacytoid dendritic cells. Sci. Rep. 2015, 5, 10752. [Google Scholar] [CrossRef]
- Grajkowska, L.T.; Ceribelli, M.; Lau, C.M.; Warren, M.E.; Tiniakou, I.; Nakandakari Higa, S.; Bunin, A.; Haecker, H.; Mirny, L.A.; Staudt, L.M.; et al. Isoform-specific expression and feedback regulation of E protein TCF4 control dendritic cell lineage specification. Immunity 2017, 46, 65–77. [Google Scholar] [CrossRef]
- Belnoue, E.; Fontannaz, P.; Rochat, A.; Tougne, C.; Bergthaler, A.; Lambert, P.; Pinschewer, D.D.; Siegrist, C. Functional limitations of plasmacytoid dendritic cells limit type I interferon, T cell responses and virus control in early life. PLoS ONE 2013, 8, 1–12. [Google Scholar] [CrossRef]
- Carmona-Sáez, P.; Varela, N.; Luque, M.J.; Toro-Domínguez, D.; Martorell-Marugan, J.; Alarcóln-Riquelme, M.E.; Marañón, C.; Berger, B. Metagene projection characterizes Gen2.2 and Cal-1 as relevant human plasmacytoid dendritic cell models. Bioinformatics 2017, 33, 3691–3695. [Google Scholar] [CrossRef]
- Maeda, T.; Murata, K.; Fukushima, T.; Sugahara, K.; Tsuruda, K.; Anami, M.; Onimaru, Y.; Tsukasaki, K.; Tomonaga, M.; Moriuchi, R.; et al. A novel plasmacytoid dendritic cell line, Cal-1, established from a patient with blastic natural killer cell lymphoma. Int. J. Hematol. 2005, 81, 148–154. [Google Scholar] [CrossRef] [PubMed]
- Chaperot, L.; Blum, A.; Manches, O.; Lui, G.; Angel, J.; Molens, J.P.; Plumas, J. Virus or TLR agonists induce trail-mediated cytotoxic activity of plasmacytoid dendritic cells. J. Immunol. 2006, 176, 248–255. [Google Scholar] [CrossRef]
- Narita, M.; Watanabe, N.; Yamahira, A.; Hashimoto, S.; Tochiki, N.; Saitoh, A.; Kaji, M.; Nakamura, T.; Furukawa, T.; Toba, K.; et al. A leukemic plasmacytoid dendritic cell line, PMDC05, with the ability to secrete IFN-alpha by stimulation via toll-like receptors and present antigens to naive T cells. Leuk. Res. 2009, 33, 1224–1232. [Google Scholar] [CrossRef] [PubMed]
- Bar-On, L.; Birnberg, T.; Lewis, K.L.; Edelson, B.T.; Bruder, D.; Hildner, K.; Buer, J.; Murphy, K.M.; Reizis, B.; Jung, S. CX3CR1+ CD8alpha+ dendritic cells are a steady-state population related to plasmacytoid dendritic cells. Proc. Natl. Acad. Sci. USA 2010, 107, 14745–14750. [Google Scholar] [CrossRef] [PubMed]
- Lindstedt, M.; Lundberg, K.; Borrebaeck, C.A.K. Gene family clustering identifies functionally associated subsets of human in vivo blood and tonsillar dendritic cells. J. Immunol. 2005, 175, 4839–4846. [Google Scholar] [CrossRef] [PubMed]
- Li, H.S.; Gelbard, A.; Martinez, G.J.; Esashi, E.; Zhang, H.; Nguyen-Jackson, H.; Liu, Y.J.; Overwijk, W.W.; Watowich, S.S. Cell-intrinsic role for IFN-alpha-STAT1 signals in regulating murine peyer patch plasmacytoid dendritic cells and conditioning an inflammatory response. Blood 2011, 118, 3879–3889. [Google Scholar] [CrossRef] [PubMed]
- Macal, M.; Jo, Y.; Dallari, S.; Chang, A.Y.; Dai, J.; Swaminathan, S.; Wehrens, E.J.; Fitzgerald-Bocarsly, P.; Zúñiga, E.I. Self-renewal and toll-like receptor signaling sustain exhausted plasmacytoid dendritic cells during chronic viral infection. Immunity 2018, 48, 730–744. [Google Scholar] [CrossRef]
- Fitzgerald-Bocarsly, P.; Jacobs, E.S. Plasmacytoid dendritic cells in hiv infection: Striking a delicate balance. J. Leukoc. Biol. 2010, 87, 609–620. [Google Scholar] [CrossRef]
- Di Domizio, J.; Cao, W. Fueling autoimmunity: Type I interferon in autoimmune diseases. Expert Rev. Clin. Immunol. 2013, 9, 201–210. [Google Scholar] [CrossRef]
- Sozzani, S.; Del Prete, A.; Bosisio, D. Dendritic cell recruitment and activation in autoimmunity. J. Autoimmun. 2017, 85, 126–140. [Google Scholar] [CrossRef]
- Feldman, S.; Stein, D.; Amrute, S.; Denny, T.; Garcia, Z.; Kloser, P.; Sun, Y.; Megjugorac, N.; Fitzgerald-Bocarsly, P. Decreased interferon-a production in HIV-infected patients correlates with numerical and functional deficiencies in circulating type 2 dendritic cell precursors. Clin. Immunol. 2001, 101, 201–210. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, M.; Manches, O.; Bhardwaj, N. Plasmacytoid dendritic cells in HIV infection. Adv. Exp. Med. Biol. 2013, 762, 71–107. [Google Scholar] [CrossRef] [PubMed]
- Ceribelli, M.; Hou, Z.E.; Kelly, P.N.; Huang, D.W.; Wright, G.; Ganapathi, K.; Evbuomwan, M.O.; Pittaluga, S.; Shaffer, A.L.; Marcucci, G.; et al. A druggable TCF4- and Brd4-dependent transcriptional network sustains malignancy in blastic plasmacytoid dendritic cell neoplasm. Cancer Cell 2016, 30, 764–778. [Google Scholar] [CrossRef] [PubMed]
- Alcantara-Hernandez, M.; Leylek, R.; Wagar, L.E.; Engleman, E.G.; Keler, T.; Marinkovich, M.P.; Davis, M.M.; Nolan, G.P.; Idoyaga, J. High-dimensional phenotypic mapping of human dendritic cells reveals interindividual cariation and tissue specialization. Immunity 2017, 47, 1037–1050. [Google Scholar] [CrossRef] [PubMed]
- Gibson, S.J.; Lindh, J.M.; Riter, T.R.; Gleason, R.M.; Rogers, L.M.; Fuller, A.E.; Oesterich, J.L.; Gorden, K.B.; Qiu, X.; McKane, S.W.; et al. Plasmacytoid dendritic cells produce cytokines and mature in response to the TLR7 agonists, imiquimod and resiquimod. Cell Immunol. 2002, 218, 74–86. [Google Scholar] [CrossRef]
- Beignon, A.S.; McKenna, K.; Skoberne, M.; Manches, O.; DaSilva, I.; Kavanagh, D.G.; Larsson, M.; Gorelick, R.J.; Lifson, J.D.; Bhardwaj, N. Endocytosis of HIV-1 activates plasmacytoid dendritic cells via toll-like receptor-viral RNA interactions. J. Clin. Invest. 2005, 115, 3265–3275. [Google Scholar] [CrossRef]
- Di Domizio, J.; Blum, A.; Gallagher-Gambarelli, M.; Molens, J.; Chaperot, L.; Plumas, J. Tlr7 stimulation in human plasmacytoid dendritic cells leads to the induction of early IFN-inducible genes in the absence of type I IFN. Blood 2009, 114, 1794–1802. [Google Scholar] [CrossRef]
- Coccia, E.M.; Severa, M.; Giacomini, E.; Monneron, D.; Remoli, M.E.; Julkunen, I.; Cella, M.; Lande, R.; Uze, G. Viral infection and toll-like receptor agonists induce a differential expression of type I and lambda interferons in human plasmacytoid and monocyte-derived dendritic cells. Eur. J. Immunol. 2004, 34, 796–805. [Google Scholar] [CrossRef]
- Kerkmann, M.; Rothenfusser, S.; Hornung, V.; Towarowski, A.; Wagner, M.; Sarris, A.; Giese, T.; Endres, S.; Hartmann, G. Activation with CpG-a and CpG-b oligonucleotides reveals two distinct regulatory pathways of type I IFN synthesis in human plasmacytoid dendritic cells. J. Immunol. 2003, 170, 4465–4474. [Google Scholar] [CrossRef]
- Kornbluth, R.S.; Edgington, T.S. Tumor necrosis factor production by human monocytes is a regulated event: Induction of TNF-alpha-mediated cellular cytotoxicity by endotoxin. J. Immunol. 1986, 137, 2585–2591. [Google Scholar]
- Shi, B.; Ren, G.; Hu, Y.; Wang, S.; Zhang, Z.; Yuan, Z. Hbsag inhibits IFN-a production in plasmacytoid dendritic cells through TNF-a and IL-10 induction in monocytes. PLoS ONE 2012, 7, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Gupta, M.; Mahanty, S.; Ahmed, R.; Rollin, P.E. Monocyte-derived human macrophages and peripheral blood mononuclear cells infected with ebola virus secrete mip-1α and TNF-α and inhibit poly-ic-induced ifn-α in vitro. Virology 2001, 284, 20–25. [Google Scholar] [CrossRef] [PubMed]
- Payvandi, F.; Amrute, S.; Fitzgerald-Bocarsly, P. Exogenous and endogenous IL-10 regulate IFN- α production by peripheral blood mononuclear cells in response to viral stimulation. J. Immunol. 1998, 160, 5861–5868. [Google Scholar] [PubMed]
- Megjugorac, N.J.; Young, H.A.; Amrute, S.B.; Olshalsky, S.L.; Fitzgerald-Bocarsly, P. Virally stimulated plasmacytoid dendritic cells produce chemokines and induce migration of T and NK cells. J. Leukoc. Biol. 2004, 75, 504–514. [Google Scholar] [CrossRef] [PubMed]
- Alculumbre, S.G.; Saint-Andre, V.; Di Domizio, J.; Vargas, P.; Sirven, P.; Bost, P.; Maurin, M.; Maiuri, P.; Wery, M.; Roman, M.S.; et al. Diversification of human plasmacytoid predendritic cells in response to a single stimulus. Nat. Immunol. 2018, 19, 63–75. [Google Scholar] [CrossRef] [PubMed]
- Saadeh, D.; Kurban, M.; Abbas, O. Update on the role of plasmacytoid dendritic cells in inflammatory/autoimmune skin diseases. Exp. Dermatol. 2016, 25, 415–421. [Google Scholar] [CrossRef] [PubMed]
- Picard, C.; Belot, A. Does type-I interferon drive systemic autoimmunity? Autoimmun. Rev. 2017, 16, 897–902. [Google Scholar] [CrossRef]
- Raieli, S.; Trichot, C.; Korniotis, S.; Pattarini, L.; Soumelis, V. TLR1/2 orchestrate human plasmacytoid pre-dendritic cell response to gram+ bacteria. PLoS Biol. 2019, 17. [Google Scholar] [CrossRef]
- Pierog, P.L.; Zhao, Y.; Singh, S.; Dai, J.; Yap, G.S.; Fitzgerald-Bocarsly, P. Toxoplasma gondii inactivates human plasmacytoid dendritic cells by functional mimicry of IL-10. J. Immunol. 2018, 200, 186–195. [Google Scholar] [CrossRef]
- Soumelis, V.; Liu, Y.J. From plasmacytoid to dendritic cell: Morphological and functional switches during plasmacytoid pre-dendritic cell differentiation. Eur. J. Immunol. 2006, 36, 2286–2292. [Google Scholar] [CrossRef]
- Salio, M.; Palmowski, M.J.; Atzberger, A.; Hermans, I.F.; Cerundolo, V. CpG-matured murine plasmacytoid dendritic cells are capable of in vivo priming of functional CD8 T cell responses to endogenous but not exogenous antigens. J. Exp. Med. 2004, 199, 567–579. [Google Scholar] [CrossRef] [PubMed]
- Fonteneau, J.F.; Gilliet, M.; Larsson, M.; Dasilva, I.; Munz, C.; Liu, Y.J.; Bhardwaj, N. Activation of influenza virus-specific CD4+ and CD8+ T cells: A new role for plasmacytoid dendritic cells in adaptive immunity. Blood 2003, 101, 3520–3526. [Google Scholar] [CrossRef] [PubMed]
- Grouard, G.; Rissoan, M.C.; Filgueira, L.; Durand, I.; Banchereau, J.; Liu, Y.J. The enigmatic plasmacytoid T cells develop into dendritic cells with interleukin (IL)-3 and CD40-ligand. J. Exp. Med. 1997, 185, 1101–1112. [Google Scholar] [CrossRef] [PubMed]
- Zuniga, E.I.; McGavern, D.B.; Pruneda-Paz, J.L.; Teng, C.; Oldstone, M.B.A. Bone marrow plasmacytoid dendritic cells can differentiate into myeloid dendritic cells upon virus infection. Nat. Immunol. 2004, 5, 1227–1234. [Google Scholar] [CrossRef]
- Hoeffel, G.; Ripoche, A.C.; Matheoud, D.; Nascimbeni, M.; Escriou, N.; Lebon, P.; Heshmati, F.; Guillet, J.G.; Gannage, M.; Caillat-Zucman, S.; et al. Antigen crosspresentation by human plasmacytoid dendritic cells. Immunity 2007, 27, 481–492. [Google Scholar] [CrossRef]
- Fanning, S.L.; George, T.C.; Feng, D.; Feldman, S.B.; Megjugorac, N.J.; Izaguirre, A.G.; Fitzgerald-Bocarsly, P. Receptor cross-linking on human plasmacytoid dendritic cells leads to the regulation of IFN-α production. J. Immunol. 2006, 177, 5829–5839. [Google Scholar] [CrossRef]
- Riboldi, E.; Daniele, R.; Cassatella, M.A.; Sozzani, S.; Bosisio, D. Engagement of BDCA-2 blocks trail-mediated cytotoxic activity of plasmacytoid dendritic cells. Immunobiology 2009, 214, 868–876. [Google Scholar] [CrossRef]
- Dzionek, A.; Sohma, Y.; Nagafune, J.; Cella, M.; Colonna, M.; Facchetti, F.; Gunther, G.; Johnston, I.; Lanzavecchia, A.; Nagasaka, T.; et al. BDCA-2, a novel plasmacytoid dendritic cell-specific type II c-type lectin, mediates antigen capture and is a potent inhibitor of interferon alpha/beta induction. J. Exp. Med. 2001, 194, 1823–1834. [Google Scholar] [CrossRef]
- Cao, W.; Bover, L.; Cho, M.; Wen, X.; Hanabuchi, S.; Bao, M.; Rosen, D.B.; Wang, Y.H.; Shaw, J.L.; Du, Q.; et al. Regulation of TLR7/9 responses in plasmacytoid dendritic cells by Bst2 and ILT7 receptor interaction. J. Exp. Med. 2009, 206, 1603–1614. [Google Scholar] [CrossRef]
- Cho, M.; Ishida, K.; Chen, J.; Ohkawa, J.; Chen, W.; Namiki, S.; Kotaki, A.; Arai, N.; Arai, K.; Kamogawa-Schifter, Y. Sage library screening reveals ILT7 as a specific plasmacytoid dendritic cell marker that regulates type Ii IFN production. Int. Immunol. 2008, 20, 155–164. [Google Scholar] [CrossRef]
- Cao, W.; Rosen, D.B.; Ito, T.; Bover, L.; Bao, M.; Watanabe, G.; Yao, Z.; Zhang, L.; Lanier, L.L.; Liu, Y.J. Plasmacytoid dendritic cell-specific receptor ILT7-fc epsilonri gamma inhibits toll-like receptor-induced interferon production. J. Exp. Med. 2006, 203, 1399–1405. [Google Scholar] [CrossRef] [PubMed]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dewald, H.K.; Hurley, H.J.; Fitzgerald-Bocarsly, P. Regulation of Transcription Factor E2-2 in Human Plasmacytoid Dendritic Cells by Monocyte-Derived TNFα. Viruses 2020, 12, 162. https://doi.org/10.3390/v12020162
Dewald HK, Hurley HJ, Fitzgerald-Bocarsly P. Regulation of Transcription Factor E2-2 in Human Plasmacytoid Dendritic Cells by Monocyte-Derived TNFα. Viruses. 2020; 12(2):162. https://doi.org/10.3390/v12020162
Chicago/Turabian StyleDewald, Hannah K., Harry J. Hurley, and Patricia Fitzgerald-Bocarsly. 2020. "Regulation of Transcription Factor E2-2 in Human Plasmacytoid Dendritic Cells by Monocyte-Derived TNFα" Viruses 12, no. 2: 162. https://doi.org/10.3390/v12020162
APA StyleDewald, H. K., Hurley, H. J., & Fitzgerald-Bocarsly, P. (2020). Regulation of Transcription Factor E2-2 in Human Plasmacytoid Dendritic Cells by Monocyte-Derived TNFα. Viruses, 12(2), 162. https://doi.org/10.3390/v12020162




