The VP1u of Human Parvovirus B19: A Multifunctional Capsid Protein with Biotechnological Applications
Abstract
1. Introduction
2. Human Erythroparvovirus B19 (B19V)
3. B19V Capsid
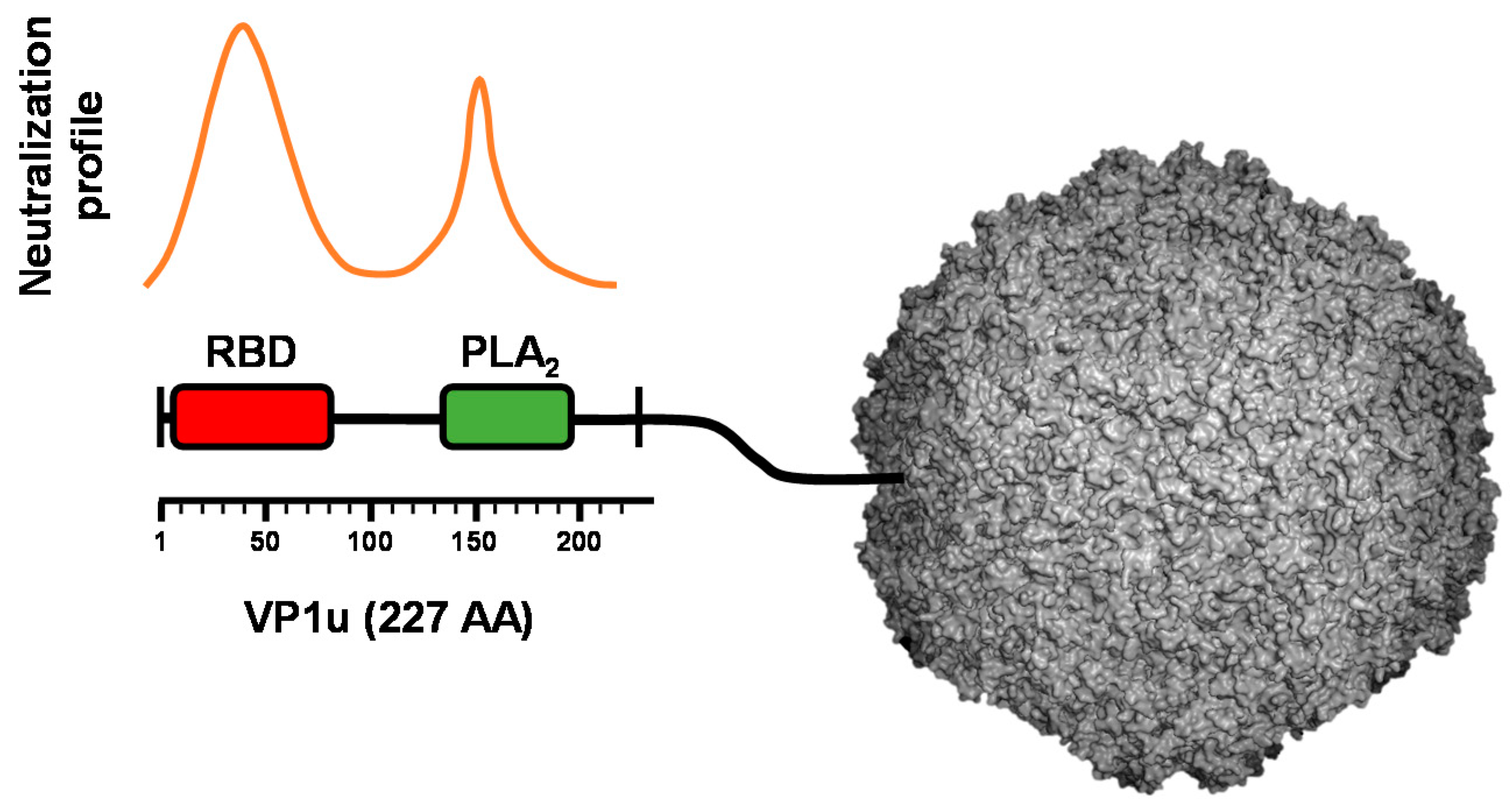
4. VP1u Is the Immunodominant Region of the Capsid but It Is Not Accessible in Native Virions
5. Role of VP1u in the Restricted Tropism of B19V
5.1. VP1u Contains a Receptor-Binding Domain That Is Essential for Virus Entry into Permissive Cells
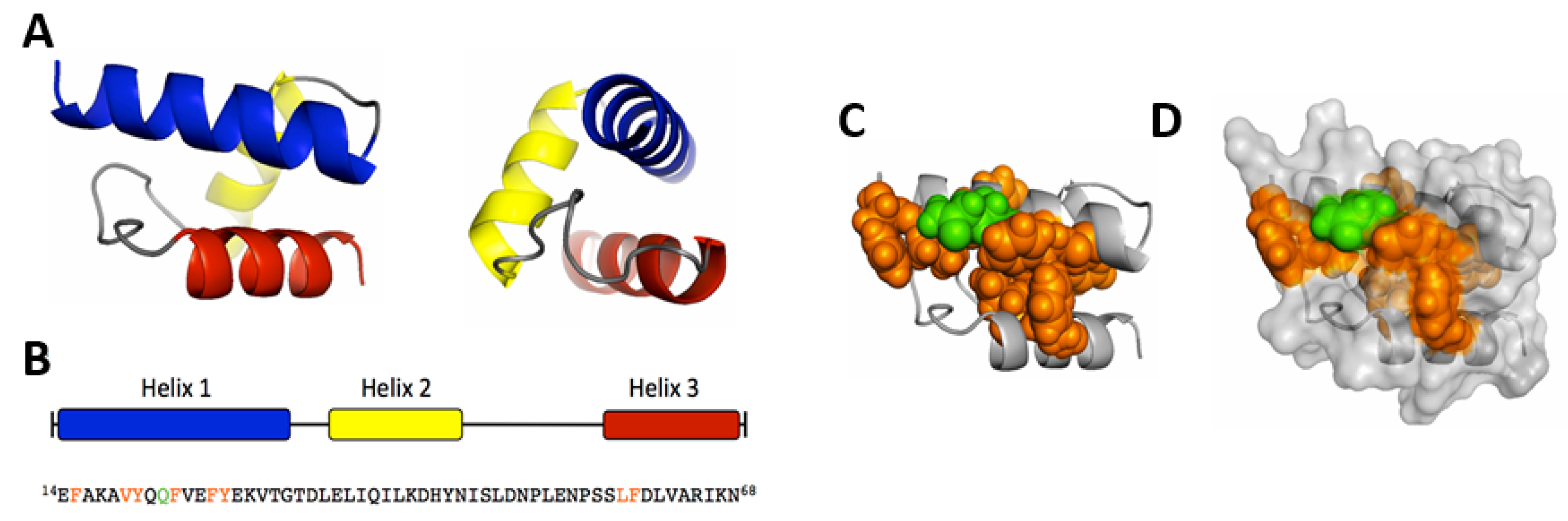
5.2. Mapping and Structural Characterization of the Receptor-Binding Domain in the VP1u
5.3. VP1u Cognate Receptor Facilitates B19V Targeting and Uptake Exclusively into Permissive Cells
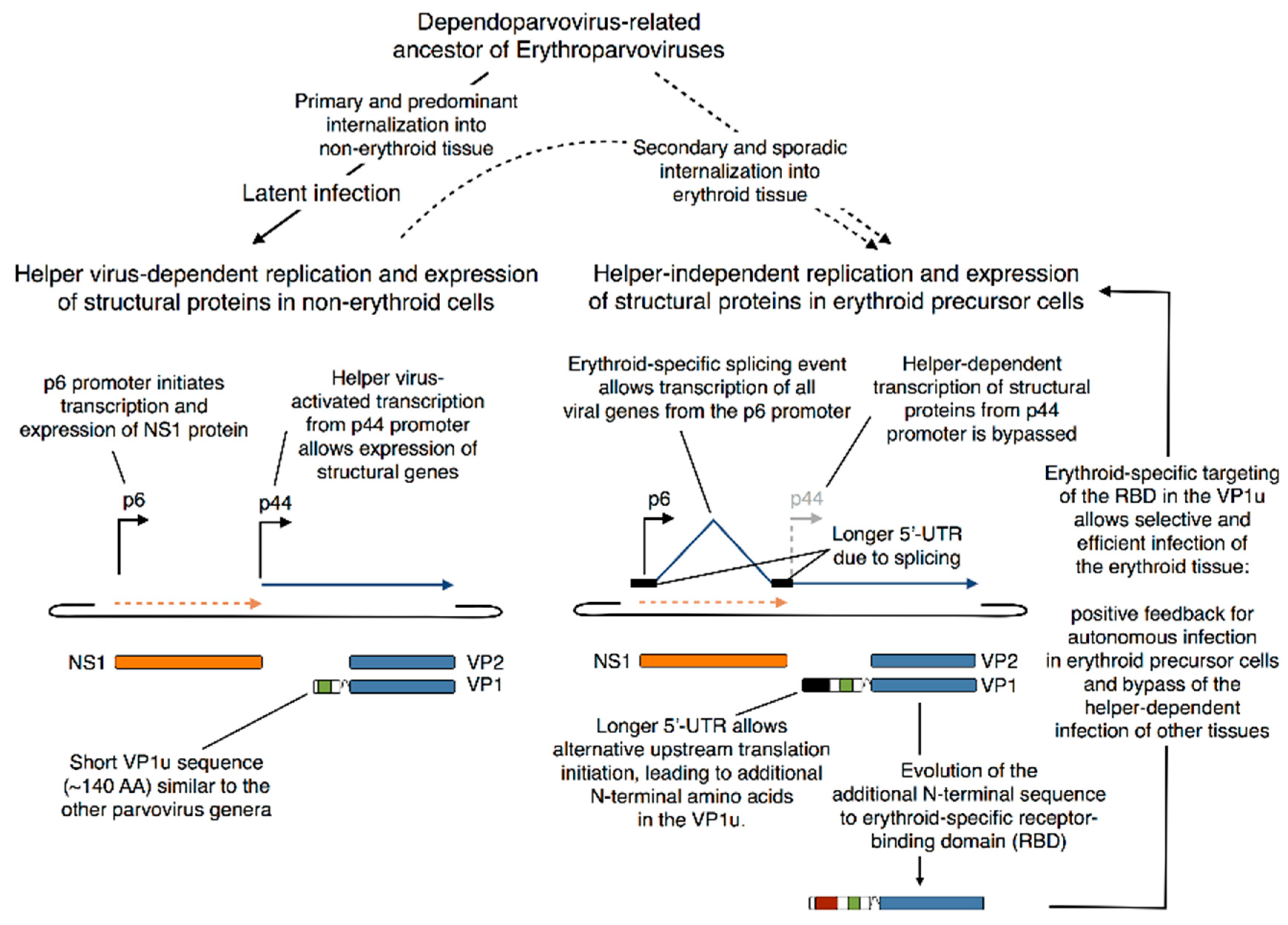
5.4. Evolutionary Aspects of B19V Restricted Tropism and the Origin of the RBD in the VP1u
6. Role of VP1u in the Subcellular Trafficking of Incoming B19V
6.1. The Phospholipase A2 (PLA2) Domain
6.2. Nuclear Localization Signals (NLSs)
7. Biotechnological Applications of the VP1u of B19V
7.1. Specific Biomarker for EPO-Dependent Erythroid Differentiation Stages
7.2. Specific Drug Delivery and Chemotherapy
7.2.1. β-Hemoglobin Disorders
7.2.2. Erythroleukemia
8. Concluding Remarks
Author Contributions
Funding
Conflicts of Interest
References
- Cotmore, S.F.; Tattersall, P. Parvoviruses: Small does not mean simple. Annu. Rev. Virol. 2014, 1, 517–537. [Google Scholar] [CrossRef] [PubMed]
- Kailasan, S.; Agbandje-Mckenna, M.; Parrish, C.R. Parvovirus Family Conundrum: What Makes a Killer? Annu. Rev. Virol. 2015, 2, 425–450. [Google Scholar] [CrossRef] [PubMed]
- François, S.; Filloux, D.; Roumagnac, P.; Bigot, D.; Gayral, P.; Martin, D.P.; Froissart, R.; Ogliastro, M. Discovery of parvovirus-related sequences in an unexpected broad range of animals. Sci. Rep. 2016, 6, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Cotmore, S.F.; Agbandje-McKenna, M.; Canuti, M.; Chiorini, J.A.; Eis-Hubinger, A.M.; Hughes, J.; Mietzsch, M.; Modha, S.; Ogliastro, M.; Pénzes, J.J.; et al. ICTV virus taxonomy profile: Parvoviridae. J. Gen. Virol. 2019, 100, 367–368. [Google Scholar] [CrossRef] [PubMed]
- Samulski, R.J.; Muzyczka, N. AAV-mediated gene therapy for research and therapeutic purposes. Annu. Rev. Virol. 2014, 1, 427–451. [Google Scholar] [CrossRef] [PubMed]
- Cotmore, S.F.; Tattersall, P. Characterization and molecular cloning of a human parvovirus genome. Science 1984, 226, 1161–1165. [Google Scholar] [CrossRef]
- Cotmore, S.F.; Tattersall, P. DNA replication in the autonomous parvoviruses. Semin. Virol. 1995, 6, 271–281. [Google Scholar] [CrossRef]
- Tattersall, P.; Cawte, P.J.; Shatkin, A.J.; Ward, D.C. Three structural polypeptides coded for by minite virus of mice, a parvovirus. J. Virol. 1976, 20, 273–289. [Google Scholar] [CrossRef]
- Becerra, S.P.; Koczot, F.; Fabisch, P.; Rose, J.A. Synthesis of adeno-associated virus structural proteins requires both alternative mRNA splicing and alternative initiations from a single transcript. J. Virol. 1988, 62, 2745–2754. [Google Scholar] [CrossRef]
- Mani, B.; Baltzer, C.; Valle, N.; Almendral, J.M.; Kempf, C.; Ros, C. Low pH-dependent endosomal processing of the incoming parvovirus minute virus of mice virion leads to externalization of the VP1 N-terminal sequence (N-VP1), N-VP2 cleavage, and uncoating of the full-length genome. J. Virol. 2006, 80, 1015–1024. [Google Scholar] [CrossRef]
- Mietzsch, M.; Pénzes, J.J.; Agbandje-Mckenna, M. Twenty-five years of structural parvovirology. Viruses 2019, 11, 362. [Google Scholar] [CrossRef] [PubMed]
- Vihinen-Ranta, M.; Kakkola, L.; Kalela, A.; Vilja, P.; Vuento, M. Characterization of a nuclear localization signal of canine parvovirus capsid proteins. Eur. J. Biochem. 1997, 250, 389–394. [Google Scholar] [CrossRef] [PubMed]
- Lombardo, E.; Ramírez, J.C.; Garcia, J.; Almendral, J.M. Complementary Roles of Multiple Nuclear Targeting Signals in the Capsid Proteins of the Parvovirus Minute Virus of Mice during Assembly and Onset of Infection. J. Virol. 2002, 76, 7049–7059. [Google Scholar] [CrossRef] [PubMed]
- Vihinen-Ranta, M.; Wang, D.; Weichert, W.S.; Parrish, C.R. The VP1 N-Terminal Sequence of Canine Parvovirus Affects Nuclear Transport of Capsids and Efficient Cell Infection. J. Virol. 2002, 76, 1884–1891. [Google Scholar] [CrossRef] [PubMed]
- Grieger, J.C.; Snowdy, S.; Samulski, R.J. Separate Basic Region Motifs within the Adeno-Associated Virus Capsid Proteins Are Essential for Infectivity and Assembly. J. Virol. 2006, 80, 5199–5210. [Google Scholar] [CrossRef] [PubMed]
- Sonntag, F.; Bleker, S.; Leuchs, B.; Fischer, R.; Kleinschmidt, J.A. Adeno-Associated Virus Type 2 Capsids with Externalized VP1/VP2 Trafficking Domains Are Generated prior to Passage through the Cytoplasm and Are Maintained until Uncoating Occurs in the Nucleus. J. Virol. 2006, 80, 11040–11054. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Zhang, Z.; Zheng, Z.; Ke, X.; Luo, H.; Hu, Q.; Wang, H. Identification and characterization of complex dual nuclear localization signals in human bocavirus NP1. J. Gen. Virol. 2013, 94, 1335–1342. [Google Scholar] [CrossRef][Green Version]
- Boisvert, M.; Bouchard-Levesque, V.; Fernandes, S.; Tijssen, P. Classic Nuclear Localization Signals and a Novel Nuclear Localization Motif Are Required for Nuclear Transport of Porcine Parvovirus Capsid Proteins. J. Virol. 2014, 88, 11748–11759. [Google Scholar] [CrossRef]
- Liu, P.; Chen, S.; Wang, M.; Cheng, A. The role of nuclear localization signal in parvovirus life cycle. Virol. J. 2017, 14, 17–22. [Google Scholar] [CrossRef]
- Li, Y.; Zádori, Z.; Bando, H.; Dubuc, R.; Fédière, G.; Szelei, J.; Tijssen, P. Genome organization of the densovirus from Bombyx mori (BmDNV-1) and enzyme activity of its capsid. J. Gen. Virol. 2001, 82, 2821–2825. [Google Scholar] [CrossRef]
- Zádori, Z.; Szelei, J.; Lacoste, M.C.; Li, Y.; Gariépy, S.; Raymond, P.; Allaire, M.; Nabi, I.R.; Tijssen, P. A Viral Phospholipase A2 Is Required for Parvovirus Infectivity. Dev. Cell 2001, 1, 291–302. [Google Scholar] [CrossRef]
- Dorsch, S.; Liebisch, G.; Kaufmann, B.; von Landenberg, P.; Hoffmann, J.H.; Drobnik, W.; Modrow, S. The VP1 Unique Region of Parvovirus B19 and Its Constituent Phospholipase A2-Like Activity. J. Virol. 2002, 76, 2014–2018. [Google Scholar] [CrossRef] [PubMed]
- Girod, A.; Wobus, C.E.; Zádori, Z.; Ried, M.; Leike, K.; Tijssen, P.; Kleinschmidt, J.A.; Hallek, M. The VP1 capsid protein of adeno-associated virus type 2 is carrying a phospholipase A2 domain required for virus infectivity. J. Gen. Virol. 2002, 83, 973–978. [Google Scholar] [CrossRef] [PubMed]
- Farr, G.A.; Zhang, L.G.; Tattersall, P. Parvoviral virions deploy a capsid-tethered lipolytic enzyme to breach the endosomal membrane during cell entry. Proc. Natl. Acad. Sci. USA 2005, 102, 17148–17153. [Google Scholar] [CrossRef] [PubMed]
- Qu, X.W.; Liu, W.P.; Qi, Z.Y.; Duan, Z.J.; Zheng, L.S.; Kuang, Z.Z.; Zhang, W.J.; Hou, Y. De Phospholipase A2-like activity of human bocavirus VP1 unique region. Biochem. Biophys. Res. Commun. 2008, 365, 158–163. [Google Scholar] [CrossRef] [PubMed]
- Stahnke, S.; Lux, K.; Uhrig, S.; Kreppel, F.; Hösel, M.; Coutelle, O.; Ogris, M.; Hallek, M.; Büning, H. Intrinsic phospholipase A2 activity of adeno-associated virus is involved in endosomal escape of incoming particles. Virology 2011, 409, 77–83. [Google Scholar] [CrossRef]
- Popa-Wagner, R.; Porwal, M.; Kann, M.; Reuss, M.; Weimer, M.; Florin, L.; Kleinschmidt, J.A. Impact of VP1-Specific Protein Sequence Motifs on Adeno-Associated Virus Type 2 Intracellular Trafficking and Nuclear Entry. J. Virol. 2012, 86, 9163–9174. [Google Scholar] [CrossRef]
- Leisi, R.; Di Tommaso, C.; Kempf, C.; Ros, C. The receptor-binding domain in the VP1u region of parvovirus B19. Viruses 2016, 8, 61. [Google Scholar] [CrossRef]
- Harbison, C.E.; Chiorini, J.A.; Parrish, C.R. The parvovirus capsid odyssey: From the cell surface to the nucleus. Trends Microbiol. 2008, 16, 208–214. [Google Scholar] [CrossRef]
- Ros, C.; Bayat, N.; Wolfisberg, R.; Almendral, J.M. Protoparvovirus cell entry. Viruses 2017, 9, 313. [Google Scholar] [CrossRef]
- Kronenberg, S.; Böttcher, B.; von der Lieth, C.W.; Bleker, S.; Kleinschmidt, J.A. A Conformational Change in the Adeno-Associated Virus Type 2 Capsid Leads to the Exposure of Hidden VP1 N Termini. J. Virol. 2005, 79, 5296–5303. [Google Scholar] [CrossRef] [PubMed]
- Bönsch, C.; Zuercher, C.; Lieby, P.; Kempf, C.; Ros, C. The Globoside Receptor Triggers Structural Changes in the B19 Virus Capsid That Facilitate Virus Internalization. J. Virol. 2010, 84, 11737–11746. [Google Scholar] [CrossRef] [PubMed]
- Tsao, J.; Chapman, M.S.; Agbandje, M.; Keller, W.; Smith, K.; Wu, H.; Luo, M.; Smith, T.J.; Rossmann, M.G.; Compans, R.W.; et al. The three-dimensional structure of canine parvovirus and its functional implications. Science 1991, 251, 1456–1464. [Google Scholar] [CrossRef] [PubMed]
- Chapman, M.S.; Rossmann, M.G. Structure, sequence, and function correlations among parvoviruses. Virology 1993, 194, 491–508. [Google Scholar] [CrossRef]
- Llamas-Saiz, A.L.; Agbandje-McKenna, M.; Wikoff, W.R.; Bratton, J.; Tattersall, P.; Rossmann, M.G. Structure determination of minute virus of mice. Acta Crystallogr. Sect. D Biol. Crystallogr. 1997, 53, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Kaufmann, B.; Simpson, A.A.; Rossmann, M.G. The structure of human parvovirus B19. Proc. Natl. Acad. Sci. USA 2004, 101, 11628–11633. [Google Scholar] [CrossRef]
- Agbandje-McKenna, M.; Llamas-Saiz, A.L.; Wang, F.; Tattersall, P.; Rossmann, M.G. Functional implications of the structure of the murine parvovirus, minute virus of mice. Structure 1998, 6, 1369–1381. [Google Scholar] [CrossRef]
- Bleker, S.; Sonntag, F.; Kleinschmidt, J.A. Mutational Analysis of Narrow Pores at the Fivefold Symmetry Axes of Adeno-Associated Virus Type 2 Capsids Reveals a Dual Role in Genome Packaging and Activation of Phospholipase A2 Activity. J. Virol. 2005, 79, 2528–2540. [Google Scholar] [CrossRef]
- Farr, G.A.; Cotmore, S.F.; Tattersall, P. VP2 Cleavage and the Leucine Ring at the Base of the Fivefold Cylinder Control pH-Dependent Externalization of both the VP1 N Terminus and the Genome of Minute Virus of Mice. J. Virol. 2006, 80, 161–171. [Google Scholar] [CrossRef]
- Plevka, P.; Hafenstein, S.; Li, L.; D’Abrgamo, A.; Cotmore, S.F.; Rossmann, M.G.; Tattersall, P. Structure of a Packaging-Defective Mutant of Minute Virus of Mice Indicates that the Genome Is Packaged via a Pore at a 5-Fold Axis. J. Virol. 2011, 85, 4822–4827. [Google Scholar] [CrossRef]
- Castellanos, M.; Pérez, R.; Rodríguez-Huete, A.; Grueso, E.; Almendral, J.M.; Mateu, M.G. A slender tract of glycine residues is required for translocation of the VP2 protein N-terminal domain through the parvovirus MVM capsid channel to initiate infection. Biochem. J. 2013, 455, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Farr, G.A.; Tattersall, P. A conserved leucine that constricts the pore through the capsid fivefold cylinder plays a central role in parvoviral infection. Virology 2004, 323, 243–256. [Google Scholar] [CrossRef] [PubMed]
- Cotmore, S.F.; Tattersall, P. Mutations at the Base of the Icosahedral Five-Fold Cylinders of Minute Virus of Mice Induce 3′-to-5′ Genome Uncoating and Critically Impair Entry Functions. J. Virol. 2012, 86, 69–80. [Google Scholar] [CrossRef] [PubMed]
- Ros, C.; Gerber, M.; Kempf, C. Conformational changes in the VP1-unique region of native human parvovirus B19 lead to exposure of internal sequences that play a role in virus neutralization and infectivity. J. Virol. 2006, 80, 12017–12024. [Google Scholar] [CrossRef] [PubMed]
- Qiu, J.; Söderlund-Venermo, M.; Young, N.S. Human Parvoviruses. Clin. Microbiol. Rev. 2017, 30, 43–113. [Google Scholar] [CrossRef] [PubMed]
- Vassilopoulos, D.; Calabrese, L.H. Virally associated arthritis 2008: Clinical, epidemiologic, and pathophysiologic considerations. Arthritis Res. Ther. 2008, 10, 1–8. [Google Scholar] [CrossRef]
- Heegaard, E.D.; Brown, K.E. Human Parvovirus B19. Clin. Microbiol. Rev. 2002, 15, 485–505. [Google Scholar] [CrossRef]
- Bonvicini, F.; Bua, G.; Gallinella, G. Parvovirus B19 infection in pregnancy—Awareness and opportunities. Curr. Opin. Virol. 2017, 27, 8–14. [Google Scholar] [CrossRef]
- Cossart, Y.E.; Cant, B.; Field, A.M.; Widdows, D. Parvovirus-Like Particles in Human Sera. Lancet 1975, 305, 72–73. [Google Scholar] [CrossRef]
- Christensen, A.; Kesti, O.; Elenius, V.; Eskola, A.L.; Døllner, H.; Altunbulakli, C.; Akdis, C.A.; Söderlund-Venermo, M.; Jartti, T. Human bocaviruses and paediatric infections. Lancet Child Adolesc. Health 2019, 3, 418–426. [Google Scholar] [CrossRef]
- Söderlund-Venermo, M. Emerging Human Parvoviruses: The Rocky Road to Fame. Annu. Rev. Virol. 2019, 6, 71–91. [Google Scholar] [CrossRef] [PubMed]
- Ozawa, K.; Kurtzman, G.; Young, N. Replication of the B19 parvovirus in human bone marrow cell cultures. Science 1986, 233, 883–886. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, T.; Ozawa, K.; Takahashi, K.; Asano, S.; Takaku, F. Susceptibility of human erythropoietic cells to B19 parvovirus in vitro increases with differentiation. Blood 1990, 75, 603–610. [Google Scholar] [CrossRef] [PubMed]
- Leisi, R.; Ruprecht, N.; Kempf, C.; Ros, C. Parvovirus B19 uptake is a highly selective process controlled by VP1u, a novel determinant of viral tropism. J. Virol. 2013, 87, 13161–13167. [Google Scholar] [CrossRef]
- Guan, W.; Cheng, F.; Yoto, Y.; Kleiboeker, S.; Wong, S.; Zhi, N.; Pintel, D.J.; Qiu, J. Block to the Production of Full-Length B19 Virus Transcripts by Internal Polyadenylation Is Overcome by Replication of the Viral Genome. J. Virol. 2008, 82, 9951–9963. [Google Scholar] [CrossRef]
- Wong, S.; Zhi, N.; Filippone, C.; Keyvanfar, K.; Kajigaya, S.; Brown, K.E.; Young, N.S. Ex Vivo-Generated CD36+ Erythroid Progenitors Are Highly Permissive to Human Parvovirus B19 Replication. J. Virol. 2008, 82, 2470–2476. [Google Scholar] [CrossRef]
- Chen, A.Y.; Guan, W.; Lou, S.; Liu, Z.; Kleiboeker, S.; Qiu, J. Role of Erythropoietin Receptor Signaling in Parvovirus B19 Replication in Human Erythroid Progenitor Cells. J. Virol. 2010, 84, 12385–12396. [Google Scholar] [CrossRef]
- Wolfisberg, R.; Ruprecht, N.; Kempf, C.; Ros, C. Impaired genome encapsidation restricts the in vitro propagation of human parvovirus B19. J. Virol. Methods 2013, 193, 215–225. [Google Scholar] [CrossRef]
- Leisi, R.; Nordheim, M.V.; Ros, C.; Kempf, C. The VP1u receptor restricts parvovirus B19 uptake to permissive erythroid cells. Viruses 2016, 8, 265. [Google Scholar] [CrossRef]
- Gallinella, G.; Manaresi, E.; Zuffi, E.; Venturoli, S.; Bonsi, L.; Bagnara, G.P.; Musiani, M.; Zerbini, M. Different patterns of restriction to B19 parvovirus replication in human blast cell lines. Virology 2000, 278, 361–367. [Google Scholar] [CrossRef]
- Ganaie, S.S.; Qiu, J. Recent advances in replication and infection of human parvovirus B19. Front. Cell. Infect. Microbiol. 2018, 8, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Qiu, J. Human parvovirus B19: A mechanistic overview of infection and DNA replication. Future Virol. 2015, 10, 155–167. [Google Scholar] [CrossRef] [PubMed]
- Stramer, S.L.; Dodd, R.Y. Transfusion-transmitted emerging infectious diseases: 30 years of challenges and progress. Transfusion 2013, 53, 2375–2383. [Google Scholar] [CrossRef] [PubMed]
- Deiss, V.; Tratschin, J.D.; Weitz, M.; Siegl, G. Cloning of the human parvovirus B19 genome and structural analysis of its palindromic termini. Virology 1990, 175, 247–254. [Google Scholar] [CrossRef]
- Cotmore, S.F.; McKie, V.C.; Anderson, L.J.; Astell, C.R.; Tattersall, P. Identification of the major structural and nonstructural proteins encoded by human parvovirus B19 and mapping of their genes by procaryotic expression of isolated genomic fragments. J. Virol. 1986, 60, 548–557. [Google Scholar] [CrossRef] [PubMed]
- Kaufmann, B.; Chipman, P.R.; Kostyuchenko, V.A.; Modrow, S.; Rossmann, M.G. Visualization of the Externalized VP2 N Termini of Infectious Human Parvovirus B19. J. Virol. 2008, 82, 7306–7312. [Google Scholar] [CrossRef]
- Saikawa, T.; Anderson, S.; Momoeda, M.; Kajigaya, S.; Young, N.S. Neutralizing linear epitopes of B19 parvovirus cluster in the VP1 unique and VP1-VP2 junction regions. J. Virol. 1993, 67, 3004–3009. [Google Scholar] [CrossRef]
- Anderson, S.; Momoeda, M.; Kawase, M.; Kajigaya, S.; Young, N.S. Peptides derived from the unique region of B19 parvovirus minor capsid protein elicitneutralizing antibodies in rabbits. Virology 1995, 206, 626–632. [Google Scholar] [CrossRef]
- Klasse, P.J.; Sattentau, Q.J. Occupancy and mechanism in antibody-mediated neutralization of animal viruses. J. Gen. Virol. 2002, 83, 2091–2108. [Google Scholar] [CrossRef]
- Klasse, P.J. Neutralization of Virus Infectivity by Antibodies: Old Problems in New Perspectives. Adv. Biol. 2014, 2014, 157895. [Google Scholar] [CrossRef]
- Burton, D.R. Antibodies, viruses and vaccines. Nat. Rev. Immunol. 2002, 2, 706–713. [Google Scholar] [CrossRef] [PubMed]
- Modrow, S.; Dorsch, S. Antibody responses in parvovirus B19 infected patients. Pathol. Biol. 2002, 50, 326–331. [Google Scholar] [CrossRef]
- Kurtzman, G.J.; Cohen, B.J.; Field, A.M.; Oseas, R.; Blaese, R.M.; Young, N.S. Immune response to B19 parvovirus and an antibody defect in persistent viral infection. J. Clin. Investig. 1989, 84, 1114–1123. [Google Scholar] [CrossRef] [PubMed]
- Kurtzman, G.; Frickhofen, N.; Kimball, J.; Jenkins, D.W.; Nienhuis, A.W.; Young, N.S. Pure Red-Cell Aplasia of 10 Years’ Duration Due to Persistent Parvovirus B19 Infection and Its Cure with Immunoglobulin Therapy. N. Engl. J. Med. 1989, 321, 519–523. [Google Scholar] [CrossRef] [PubMed]
- Bernstein, D.I.; El Sahly, H.M.; Keitel, W.A.; Wolff, M.; Simone, G.; Segawa, C.; Wong, S.; Shelly, D.; Young, N.S.; Dempsey, W. Safety and immunogenicity of a candidate parvovirus B19 vaccine. Vaccine 2011, 29, 7357–7363. [Google Scholar] [CrossRef]
- Chandramouli, S.; Medina-Selby, A.; Coit, D.; Schaefer, M.; Spencer, T.; Brito, L.A.; Zhang, P.; Otten, G.; Mandl, C.W.; Mason, P.W.; et al. Generation of a parvovirus B19 vaccine candidate. Vaccine 2013, 31, 3872–3878. [Google Scholar] [CrossRef]
- Bua, G.; Manaresi, E.; Bonvicini, F.; Gallinella, G. Parvovirus B19 Replication and Expression in Differentiating Erythroid Progenitor Cells. PLoS ONE 2016, 11, 1–19. [Google Scholar] [CrossRef]
- Brown, K.E.; Anderson, S.M.; Young, N.S. Erythrocyte P antigen: Cellular receptor for B19 parvovirus. Science 1993, 262, 114–117. [Google Scholar] [CrossRef]
- Brown, K.E.; Cohen, B.J. Haemagglutination by parvovirus B19. J. Gen. Virol. 1992, 73, 2147–2149. [Google Scholar] [CrossRef]
- Brown, K.E.; Hibbs, J.R.; Gallinella, G.; Anderson, S.M.; Lehman, E.D.; McCarthy, P.; Young, N.S. Resistance to parvovirus B19 infection due to lack of virus receptor (erythrocyte P antigen). N. Engl. J. Med. 1994, 330, 1192–1196. [Google Scholar] [CrossRef]
- Chipman, P.R.; Agbandje-Mckenna, M.; Kajigaya, S.; Brown, K.E.; Young, N.S.; Baker, T.S.; Rossmann, M.G. Cryo-electron microscopy studies of empty capsids of human parvovirus B19 complexed with its cellular receptor. Proc. Natl. Acad. Sci. USA 1996, 93, 7502–7506. [Google Scholar] [CrossRef] [PubMed]
- Nasir, W.; Nilsson, J.; Olofsson, S.; Bally, M.; Rydell, G.E. Parvovirus B19 VLP recognizes globoside in supported lipid bilayers. Virology 2014, 456–457, 364–369. [Google Scholar] [CrossRef] [PubMed]
- Weigel-Kelley, K.A.; Yoder, M.C.; Srivastava, A. Recombinant Human Parvovirus B19 Vectors: Erythrocyte P Antigen Is Necessary but Not Sufficient for Successful Transduction of Human Hematopoietic Cells. J. Virol. 2001, 75, 4110–4116. [Google Scholar] [CrossRef] [PubMed]
- Bieri, J.; Ros, C. Globoside Is Dispensable for Parvovirus B19 Entry but Essential at a Postentry Step for Productive Infection. J. Virol. 2019, 93, 1–15. [Google Scholar] [CrossRef]
- Weigel-Kelley, K.A.; Yoder, M.C.; Srivastava, A. α5β1 integrin as a cellular coreceptor for human parvovirus B19: Requirement of functional activation of β1 integrin for viral entry. Blood 2003, 102, 3927–3933. [Google Scholar] [CrossRef] [PubMed]
- Munakata, Y.; Saito-Ito, T.; Kumura-Ishii, K.; Huang, J.; Kodera, T.; Ishii, T.; Hirabayashi, Y.; Koyanagi, Y.; Sasaki, T. Ku80 autoantigen as a cellular coreceptor for human parvovirus B19 infection. Blood 2005, 106, 3449–3456. [Google Scholar] [CrossRef]
- Bönsch, C.; Kempf, C.; Ros, C. Interaction of parvovirus B19 with human erythrocytes alters virus structure and cell membrane integrity. J. Virol. 2008, 82, 11784–11791. [Google Scholar] [CrossRef]
- Xu, D.; Zhang, Y. Ab initio protein structure assembly using continuous structure fragments and optimized knowledge-based force field. Proteins Struct. Funct. Bioinform. 2012, 80, 1715–1735. [Google Scholar] [CrossRef]
- Shimomura, S.; Komatsu, N.; Frickhofen, N.; Anderson, S.; Kajigaya, S.; Young, N.S. First continuous propagation of B19 parvovirus in a cell line. Blood 1992, 79, 18–24. [Google Scholar] [CrossRef]
- Miyagawa, E.; Yoshida, T.; Takahashi, H.; Yamaguchi, K.; Nagano, T.; Kiriyama, Y.; Okochi, K.; Sato, H. Infection of the erythroid cell line, KU812Ep6 with human parvovirus B19 and its application to titration of B19 infectivity. J. Virol. Methods 1999, 83, 45–54. [Google Scholar] [CrossRef]
- Koury, M.J.; Bondurant, M.C. Maintenance by erythropoietin of viability and maturation of murine erythroid precursor cells. J. Cell. Physiol. 1988, 137, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Koury, M.J.; Bondurant, M.C. Erythropoietin retards DNA breakdown and prevents programmed death in erythroid progenitor cells. Science 1990, 248, 378–381. [Google Scholar] [CrossRef] [PubMed]
- Pop, R.; Shearstone, J.R.; Shen, Q.; Liu, Y.; Hallstrom, K.; Koulnis, M.; Gribnau, J.; Socolovsky, M. A key commitment step in erythropoiesis is synchronized with the cell cycle clock through mutual inhibition between PU.1 and S-phase progression. PLoS Biol. 2010, 8, e1000484. [Google Scholar] [CrossRef] [PubMed]
- Chen, A.Y.; Kleiboeker, S.; Qiu, J. Productive parvovirus B19 infection of primary human erythroid progenitor cells at hypoxia is regulated by STAT5A and MEK signaling but not HIFα. PLoS Pathog. 2011, 7, e1002088. [Google Scholar] [CrossRef][Green Version]
- Ganaie, S.S.; Zou, W.; Xu, P.; Deng, X.; Kleiboeker, S.; Qiu, J. Phosphorylated STAT5 directly facilitates parvovirus B19 DNA replication in human erythroid progenitors through interaction with the MCM complex. PLoS Pathog. 2017, 13, 1–27. [Google Scholar] [CrossRef]
- An, X.; Schulz, V.P.; Li, J.; Wu, K.; Liu, J.; Xue, F.; Hu, J.; Mohandas, N.; Gallagher, P.G. Global transcriptome analyses of human and murine terminal erythroid differentiation. Blood 2014, 123, 3466–3477. [Google Scholar] [CrossRef]
- Kerr, J.R.; Linden, M.R. Human dependovirus infection. In Parvoviruses; Hodder Arnold, Oxford University Press: London, UK, 2006; pp. 381–384. [Google Scholar]
- Guan, W.; Wong, S.; Zhi, N.; Qiu, J. The Genome of Human Parvovirus B19 Can Replicate in Nonpermissive Cells with the Help of Adenovirus Genes and Produces Infectious Virus. J. Virol. 2009, 83, 9541–9553. [Google Scholar] [CrossRef]
- Winter, K.; von Kietzell, K.; Heilbronn, R.; Pozzuto, T.; Fechner, H.; Weger, S. Roles of E4orf6 and VA I RNA in Adenovirus-Mediated Stimulation of Human Parvovirus B19 DNA Replication and Structural Gene Expression. J. Virol. 2012, 86, 5099–5109. [Google Scholar] [CrossRef]
- Doerig, C.; Hirt, B.; Antonietti, J.P.; Beard, P. Nonstructural protein of parvoviruses B19 and minute virus of mice controls transcription. J. Virol. 1990, 64, 387–396. [Google Scholar] [CrossRef]
- Ponnazhagan, S.; Woody, M.J.; Wang, X.S.; Zhou, S.Z.; Srivastava, A. Transcriptional transactivation of parvovirus B19 promoters in nonpermissive human cells by adenovirus type 2. J. Virol. 1995, 69, 8096–8101. [Google Scholar] [CrossRef]
- Ozawa, K.; Ayub, J.; Hao, Y.S.; Kurtzman, G.; Shimada, T.; Young, N. Novel transcription map for the B19 (human) pathogenic parvovirus. J. Virol. 1987, 61, 2395–2406. [Google Scholar] [CrossRef] [PubMed]
- Von Kietzell, K.; Pozzuto, T.; Heilbronn, R.; Grössl, T.; Fechner, H.; Weger, S. Antibody-Mediated Enhancement of Parvovirus B19 Uptake into Endothelial Cells Mediated by a Receptor for Complement Factor C1q. J. Virol. 2014, 88, 8102–8115. [Google Scholar] [CrossRef] [PubMed]
- Quattrocchi, S.; Ruprecht, N.; Bnsch, C.; Bieli, S.; Zürcher, C.; Boller, K.; Kempf, C.; Ros, C. Characterization of the early steps of human parvovirus B19 infection. J. Virol. 2012, 86, 9274–9284. [Google Scholar] [CrossRef]
- Dennis, E.A.; Cao, J.; Hsu, Y.H.; Magrioti, V.; Kokotos, G. Phospholipase A2 enzymes: Physical structure, biological function, disease implication, chemical inhibition, and therapeutic intervention. Chem. Rev. 2011, 111, 6130–6185. [Google Scholar] [CrossRef] [PubMed]
- Canaan, S.; Zádori, Z.; Ghomashchi, F.; Bollinger, J.; Sadilek, M.; Moreau, M.E.; Tijssen, P.; Gelb, M.H. Interfacial Enzymology of Parvovirus Phospholipases A2. J. Biol. Chem. 2004, 279, 14502–14508. [Google Scholar] [CrossRef]
- Deng, X.; Dong, Y.; Yi, Q.; Huang, Y.; Zhao, D.; Yang, Y.; Tijssen, P.; Qiu, J.; Liu, K.; Li, Y. The Determinants for the Enzyme Activity of Human Parvovirus B19 Phospholipase A2 (PLA2) and Its Influence on Cultured Cells. PLoS ONE 2013, 8, e61440. [Google Scholar] [CrossRef]
- Lupescu, A.; Bock, C.-T.; Lang, P.A.; Aberle, S.; Kaiser, H.; Kandolf, R.; Lang, F. Phospholipase A2 Activity-Dependent Stimulation of Ca2+ Entry by Human Parvovirus B19 Capsid Protein VP1. J. Virol. 2006, 80, 11370–11380. [Google Scholar] [CrossRef]
- Almilaji, A.; Szteyn, K.; Fein, E.; Pakladok, T.; Munoz, C.; Elvira, B.; Towhid, S.T.; Alesutan, I.; Shumilina, E.; Bock, C.T.; et al. Down-regulation of Na/K + ATPase activity by human parvovirus B19 capsid protein VP1. Cell. Physiol. Biochem. 2013, 31, 638–648. [Google Scholar] [CrossRef]
- Ahmed, M.; Almilaji, A.; Munoz, C.; Elvira, B.; Shumilina, E.; Bock, C.T.; Kandolf, R.; Lang, F. Down-regulation of K+ channels by human parvovirus B19 capsid protein VP1. Biochem. Biophys. Res. Commun. 2014, 450, 1396–1401. [Google Scholar] [CrossRef]
- Ahmed, M.; Honisch, S.; Pelzl, L.; Fezai, M.; Hosseinzadeh, Z.; Bock, C.T.; Kandolf, R.; Lang, F. Up-regulation of epithelial Na+ channel ENaC by human parvovirus B19 capsid protein VP1. Biochem. Biophys. Res. Commun. 2015, 468, 179–184. [Google Scholar] [CrossRef]
- Lu, J.; Zhi, N.; Wong, S.; Brown, K.E. Activation of Synoviocytes by the Secreted Phospholipase A2 Motif in the VP1-Unique Region of Parvovirus B19 Minor Capsid Protein. J. Infect. Dis. 2006, 193, 582–590. [Google Scholar] [CrossRef] [PubMed]
- Lange, A.; Mills, R.E.; Lange, C.J.; Stewart, M.; Devine, S.E.; Corbett, A.H. Classical nuclear localization signals: Definition, function, and interaction with importin α. J. Biol. Chem. 2007, 282, 5101–5105. [Google Scholar] [CrossRef]
- Panté, N.; Kann, M. Nuclear pore complex is able to transport macromolecules with diameters of ∼39 nm. Mol. Biol. Cell 2002, 13, 425–434. [Google Scholar] [CrossRef] [PubMed]
- Mäntylä, E.; Aho, V.; Kann, M.; Vihinen-Ranta, M. Cytoplasmic Parvovirus Capsids Recruit Importin Beta for Nuclear Delivery. J. Virol. 2020, 94, e01532-19. [Google Scholar] [CrossRef]
- Pillet, S.; Annan, Z.; Fichelson, S.; Morinet, F. Identification of a nonconventional motif necessary for the nuclear import of the human parvovirus B19 major capsid protein (VP2). Virology 2003, 306, 25–32. [Google Scholar] [CrossRef]
- Peer, D.; Karp, J.M.; Hong, S.; Farokhzad, O.C.; Margalit, R.; Langer, R. 84 Nat nanotech 2007 R Langer Nanocarriers as an emerging platform for cancer therapy.pdf. Nat. Nanotechnol. 2007, 2, 751–760. [Google Scholar] [CrossRef]
- Wilhelm, S.; Tavares, A.; Dai, Q.; Ohta, S.; Audet, J.; Dvorak, H.F.; Chan, W.C.W. Analysis of nanoparticle delivery to tumours. Nat. Rev. Mater. 2016, 1, 16014. [Google Scholar] [CrossRef]
- Ma, Y.; Nolte, R.J.M.; Cornelissen, J.J.L.M. Virus-based nanocarriers for drug delivery. Adv. Drug Deliv. Rev. 2012, 64, 811–825. [Google Scholar] [CrossRef]
- Somiya, M.; Liu, Q.; Kuroda, S. Current progress of virus-mimicking nanocarriers for drug delivery. Nanotheranostics 2017, 1, 415–429. [Google Scholar] [CrossRef]
- Stachon, A.; Holland-Letz, T.; Krieg, M. High in-hospital mortality of intensive care patients with nucleated red blood cells in blood. Clin. Chem. Lab. Med. 2004, 42, 933–938. [Google Scholar] [CrossRef]
- Stachon, A.; Kempf, R.; Holland-Letz, T.; Friese, J.; Becker, A.; Krieg, M. Daily monitoring of nucleated red blood cells in the blood of surgical intensive care patients. Clin. Chim. Acta 2006, 366, 329–335. [Google Scholar] [CrossRef] [PubMed]
- Buttarello, M.; Plebani, M. Automated blood cell counts: State of the art. Am. J. Clin. Pathol. 2008, 130, 104–116. [Google Scholar] [CrossRef] [PubMed]
- Koury, M.J. Abnormal erythropoiesis and the pathophysiology of chronic anemia. Blood Rev. 2014, 28, 49–66. [Google Scholar] [CrossRef] [PubMed]
- Danise, P.; Maconi, M.; Barrella, F.; Di Palma, A.; Avino, D.; Rovetti, A.; Gioia, M.; Amendola, G. Evaluation of nucleated red blood cells in the peripheral blood of hematological diseases. Clin. Chem. Lab. Med. 2011, 50, 357–360. [Google Scholar] [CrossRef]
- Buoro, S.; Vavassori, M.; Pipitone, S.; Benegiamo, A.; Lochis, E.; Fumagalli, S.; Falanga, A.; Marchetti, M.; Crippa, A.; Ottomano, C.; et al. Evaluation of nucleated red blood cell count by Sysmex XE-2100 in patients with thalassaemia or sickle cell anaemia and in neonates. Blood Transfus. 2015, 13, 588–594. [Google Scholar] [CrossRef]
- Constantino, B.T.; Cogionis, B. Nucleated RBCs—Significance in the peripheral blood film. Lab. Med. 2000, 31, 223–229. [Google Scholar] [CrossRef]
- Da Rin, G.; Vidali, M.; Balboni, F.; Benegiamo, A.; Borin, M.; Ciardelli, M.L.; Dima, F.; Di Fabio, A.; Fanelli, A.; Fiorini, F.; et al. Performance evaluation of the automated nucleated red blood cell count of five commercial hematological analyzers. Int. J. Lab. Hematol. 2017, 39, 663–670. [Google Scholar] [CrossRef]
- Anagnostou, A.; Liu, Z.; Steiner, M.; Chin, K.; Lee, E.S.; Kessimian, N.; Noguchi, C.T. Erythropoietin receptor mRNA expression in human endothelial cells. Proc. Natl. Acad. Sci. USA 1994, 91, 3974–3978. [Google Scholar] [CrossRef]
- Spivak, J.L. The anaemia of cancer: Death by a thousand cuts. Nat. Rev. Cancer 2005, 5, 543–555. [Google Scholar] [CrossRef]
- Jelkmann, W.; Bohlius, J.; Hallek, M.; Sytkowski, A.J. The erythropoietin receptor in normal and cancer tissues. Crit. Rev. Oncol. Hematol. 2008, 67, 39–61. [Google Scholar] [CrossRef]
- Chen, K.; Liu, J.; Heck, S.; Chasis, J.A.; An, X.; Mohandas, N. Resolving the distinct stages in erythroid differentiation based on dynamic changes in membrane protein expression during erythropoiesis. Proc. Natl. Acad. Sci. USA 2009, 106, 17413–17418. [Google Scholar] [CrossRef]
- Hu, J.; Liu, J.; Xue, F.; Halverson, G.; Reid, M.; Guo, A.; Chen, L.; Raza, A.; Galili, N.; Jaffray, J.; et al. Isolation and functional characterization of human erythroblasts at distinct stages: Implications for understanding of normal and disordered erythropoiesis in vivo. Blood 2013, 121, 3246–3253. [Google Scholar] [CrossRef] [PubMed]
- MacHherndl-Spandl, S.; Suessner, S.; Danzer, M.; Proell, J.; Gabriel, C.; Lauf, J.; Sylie, R.; Klein, H.U.; Béné, M.C.; Weltermann, A.; et al. Molecular pathways of early CD105-positive erythroid cells as compared with CD34-positive common precursor cells by flow cytometric cell-sorting and gene expression profiling. Blood Cancer J. 2013, 3, 1–13. [Google Scholar] [CrossRef]
- Origa, R. β-Thalassemia. Genet. Med. 2017, 19, 609–619. [Google Scholar] [CrossRef] [PubMed]
- Kato, G.J.; Piel, F.B.; Reid, C.D.; Gaston, M.H.; Ohene-Frempong, K.; Krishnamurti, L.; Smith, W.R.; Panepinto, J.A.; Weatherall, D.J.; Costa, F.F.; et al. Sickle cell disease. Nat. Rev. Dis. Prim. 2018, 4, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Bayanzay, K.; Alzoebie, L. Reducing the iron burden and improving survival in transfusion-dependent thalassemia patients: Current perspectives. J. Blood Med. 2016, 7, 159–169. [Google Scholar] [CrossRef] [PubMed]
- Coates, T.D.; Wood, J.C. How we manage iron overload in sickle cell patients. Br. J. Haematol. 2017, 177, 703–716. [Google Scholar] [CrossRef]
- Peters, C. Allogeneic Hematopoietic Stem Cell Transplantation to Cure Transfusion-Dependent Thalassemia: Timing Matters! Biol. Blood Marrow Transpl. 2018, 24, 1107–1108. [Google Scholar] [CrossRef]
- Gluckman, G.; Cappelli, B.; Bernaudin, F.; Labopin, M.; Volt, F.; Carreras, J.; Pinto Simoes, B.; Ferster, A.; Dupont, S.; de la Fuente, J.; et al. Sickle cell disease: An international survey of results of HLA-identical sibling hematopoietic stem cell transplantation. Blood 2017, 129, 1548–1556. [Google Scholar] [CrossRef]
- Karponi, G.; Zogas, N. Gene therapy for beta-thalassemia: Updated perspectives. Appl. Clin. Genet. 2019, 12, 167–180. [Google Scholar] [CrossRef]
- Lidonnici, M.R.; Ferrari, G. Gene therapy and gene editing strategies for hemoglobinopathies. Blood Cells Mol. Dis. 2018, 70, 87–101. [Google Scholar] [CrossRef] [PubMed]
- Lino, C.A.; Harper, J.C.; Carney, J.P.; Timlin, J.A. Delivering crispr: A review of the challenges and approaches. Drug Deliv. 2018, 25, 1234–1257. [Google Scholar] [CrossRef] [PubMed]
- Chery, J. RNA therapeutics: RNAi and antisense mechanisms and clinical applications. Postdr. J. 2016, 4, 35–50. [Google Scholar] [CrossRef] [PubMed]
- Galaway, F.A.; Stockley, P.G. MS2 viruslike particles: A robust, semisynthetic targeted drug delivery platform. Mol. Pharm. 2013, 10, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Ashley, C.E.; Carnes, E.C.; Phillips, G.K.; Durfee, P.N.; Buley, M.D.; Lino, C.A.; Padilla, D.P.; Phillips, B.; Carter, M.B.; Willman, C.L.; et al. Cell-specific delivery of diverse cargos by bacteriophage MS2 virus-like particles. ACS Nano 2011, 5, 5729–5745. [Google Scholar] [CrossRef] [PubMed]
- Nai, A.; Lidonnici, M.R.; Rausa, M.; Mandelli, G.; Pagani, A.; Silvestri, L.; Ferrari, G.; Camaschella, C. The second transferrin receptor regulates red blood cell production in mice. Blood 2015, 125, 1170–1179. [Google Scholar] [CrossRef]
- Artuso, I.; Lidonnici, M.R.; Altamura, S.; Mandelli, G.; Pettinato, M.; Muckenthaler, M.U.; Silvestri, L.; Ferrari, G.; Camaschella, C.; Nai, A. Transferrin receptor 2 is a potential novel therapeutic target for β-thalassemia: Evidence from a murine model. Blood 2018, 132, 2286–2297. [Google Scholar] [CrossRef]
- Casu, C.; Pettinato, M.; Liu, A.; Aghajan, M.; Lo Presti, V.; Lidonnici, M.R.; Munoz, K.A.; O’Hara, E.; Olivari, V.; Di Modica, S.M.; et al. Correcting β-thalassemia by combined therapies that restrict iron and modulate erythropoietin activity. Blood 2020, 136, 1968–1979. [Google Scholar] [CrossRef]
- Lohani, N.; Bhargava, N.; Munshi, A.; Ramalingam, S. Pharmacological and molecular approaches for the treatment of β-hemoglobin disorders. J. Cell. Physiol. 2018, 233, 4563–4577. [Google Scholar] [CrossRef]
- Hasserjian, R.P.; Zuo, Z.; Garcia, C.; Tang, G.; Kasyan, A.; Luthra, R.; Abruzzo, L.V.; Kantarjian, H.M.; Medeiros, L.J.; Wang, S.A. Acute erythroid leukemia: A reassessment using criteria refined in the 2008 WHO classification. Blood 2010, 115, 1985–1992. [Google Scholar] [CrossRef]
- Wickrema, A.; Crispino, J.D. Erythroid and megakaryocytic transformation. Oncogene 2007, 26, 6803–6815. [Google Scholar] [CrossRef] [PubMed]
- Mazzella, F.M.; Kowal-Vern, A.; Atef Shrit, M.; Rector, J.T.; Cotelingam, J.D.; Schumacher, H.R. Effects of multidrug resistance gene expression in acute erythroleukemia. Mod. Pathol. 2000, 13, 407–413. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, R.S. Paul Ehrlich’s magic bullets. N. Engl. J. Med. 2004, 350, 1079–1080. [Google Scholar] [CrossRef]
- Boddu, P.; Benton, C.B.; Wang, W.; Borthakur, G.; Khoury, J.D.; Pemmaraju, N. Erythroleukemia-historical perspectives and recent advances in diagnosis and management. Blood Rev. 2018, 32, 96–105. [Google Scholar] [CrossRef] [PubMed]
- Leisi, R.; Von Nordheim, M.; Kempf, C.; Ros, C. Specific targeting of proerythroblasts and erythroleukemic cells by the VP1u region of parvovirus B19. Bioconjug. Chem. 2015, 26, 1923–1930. [Google Scholar] [CrossRef]
- Rapti, K.; Louis-Jeune, V.; Kohlbrenner, E.; Ishikawa, K.; Ladage, D.; Zolotukhin, S.; Hajjar, R.J.; Weber, T. Neutralizing antibodies against AAV serotypes 1, 2, 6, and 9 in sera of commonly used animal models. Mol. Ther. 2012, 20, 73–83. [Google Scholar] [CrossRef]
- Rutledge, E.A.; Halbert, C.L.; Russell, D.W. Infectious Clones and Vectors Derived from Adeno-Associated Virus (AAV) Serotypes Other Than AAV Type 2. J. Virol. 1998, 72, 309–319. [Google Scholar] [CrossRef]
- Gao, G.; Vandenberghe, L.H.; Alvira, M.R.; Lu, Y.; Calcedo, R.; Zhou, X.; Wilson, J.M. Clades of Adeno-Associated Viruses Are Widely Disseminated in Human Tissues. J. Virol. 2004, 78, 6381–6388. [Google Scholar] [CrossRef]
- Zinn, E.; Pacouret, S.; Khaychuk, V.; Turunen, H.T.; Carvalho, L.S.; Andres-Mateos, E.; Shah, S.; Shelke, R.; Maurer, A.C.; Maurer, E.; et al. In silico reconstruction of the viral evolutionary lineage yields a potent gene therapy vector. Cell Rep. 2015, 12, 1056–1068. [Google Scholar] [CrossRef]
- Yeh, P.; Landais, D.; Lemaître, M.; Maury, I.; Crenne, J.Y.; Becquart, J.; Murry-Brelier, A.; Boucher, F.; Montay, G.; Fleer, R.; et al. Design of yeast-secreted albumin derivatives for human therapy: Biological and antiviral properties of a serum albumin-CD4 genetic conjugate. Proc. Natl. Acad. Sci. USA 1992, 89, 1904–1908. [Google Scholar] [CrossRef]
- Czajkowsky, D.M.; Hu, J.; Shao, Z.; Pleass, R.J. Fc-fusion proteins: New developments and future perspectives. EMBO Mol. Med. 2012, 4, 1015–1028. [Google Scholar] [CrossRef] [PubMed]
- Shen, B.Q.; Xu, K.; Liu, L.; Raab, H.; Bhakta, S.; Kenrick, M.; Parsons-Reponte, K.L.; Tien, J.; Yu, S.F.; Mai, E.; et al. Conjugation site modulates the in vivo stability and therapeutic activity of antibody-drug conjugates. Nat. Biotechnol. 2012, 30, 184–189. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Brown, W.L.; Stockley, P.G. Cell-Specific Delivery of Bacteriophage-Encapsidated Ricin a Chain. Bioconjug. Chem. 1995, 6, 587–595. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ros, C.; Bieri, J.; Leisi, R. The VP1u of Human Parvovirus B19: A Multifunctional Capsid Protein with Biotechnological Applications. Viruses 2020, 12, 1463. https://doi.org/10.3390/v12121463
Ros C, Bieri J, Leisi R. The VP1u of Human Parvovirus B19: A Multifunctional Capsid Protein with Biotechnological Applications. Viruses. 2020; 12(12):1463. https://doi.org/10.3390/v12121463
Chicago/Turabian StyleRos, Carlos, Jan Bieri, and Remo Leisi. 2020. "The VP1u of Human Parvovirus B19: A Multifunctional Capsid Protein with Biotechnological Applications" Viruses 12, no. 12: 1463. https://doi.org/10.3390/v12121463
APA StyleRos, C., Bieri, J., & Leisi, R. (2020). The VP1u of Human Parvovirus B19: A Multifunctional Capsid Protein with Biotechnological Applications. Viruses, 12(12), 1463. https://doi.org/10.3390/v12121463





