First Record of Mosquito-Borne Kyzylagach Virus in Central Europe
Abstract
:1. Introduction
2. Materials and Methods
Mosquito Trapping, Their Identification, and Processing
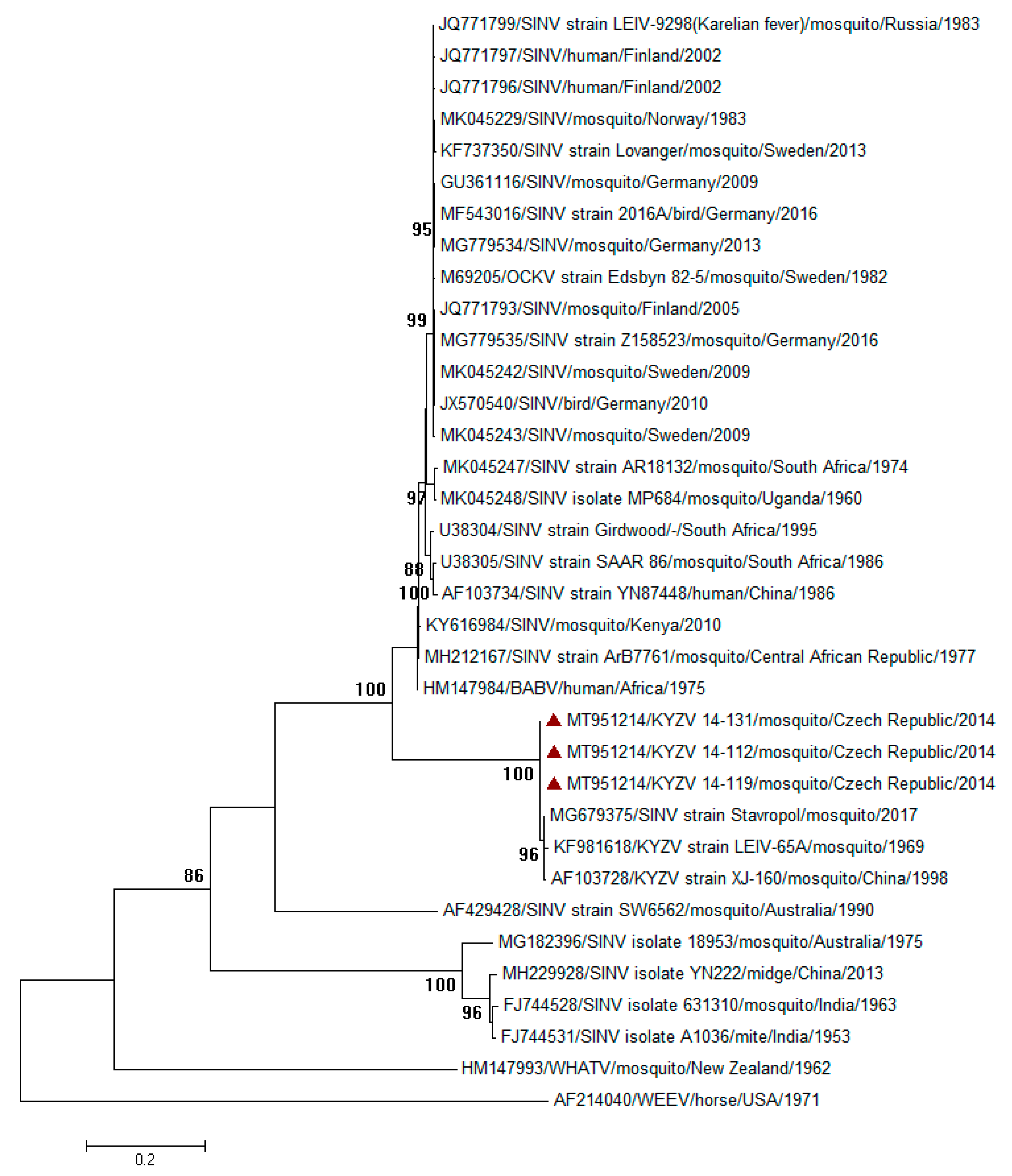
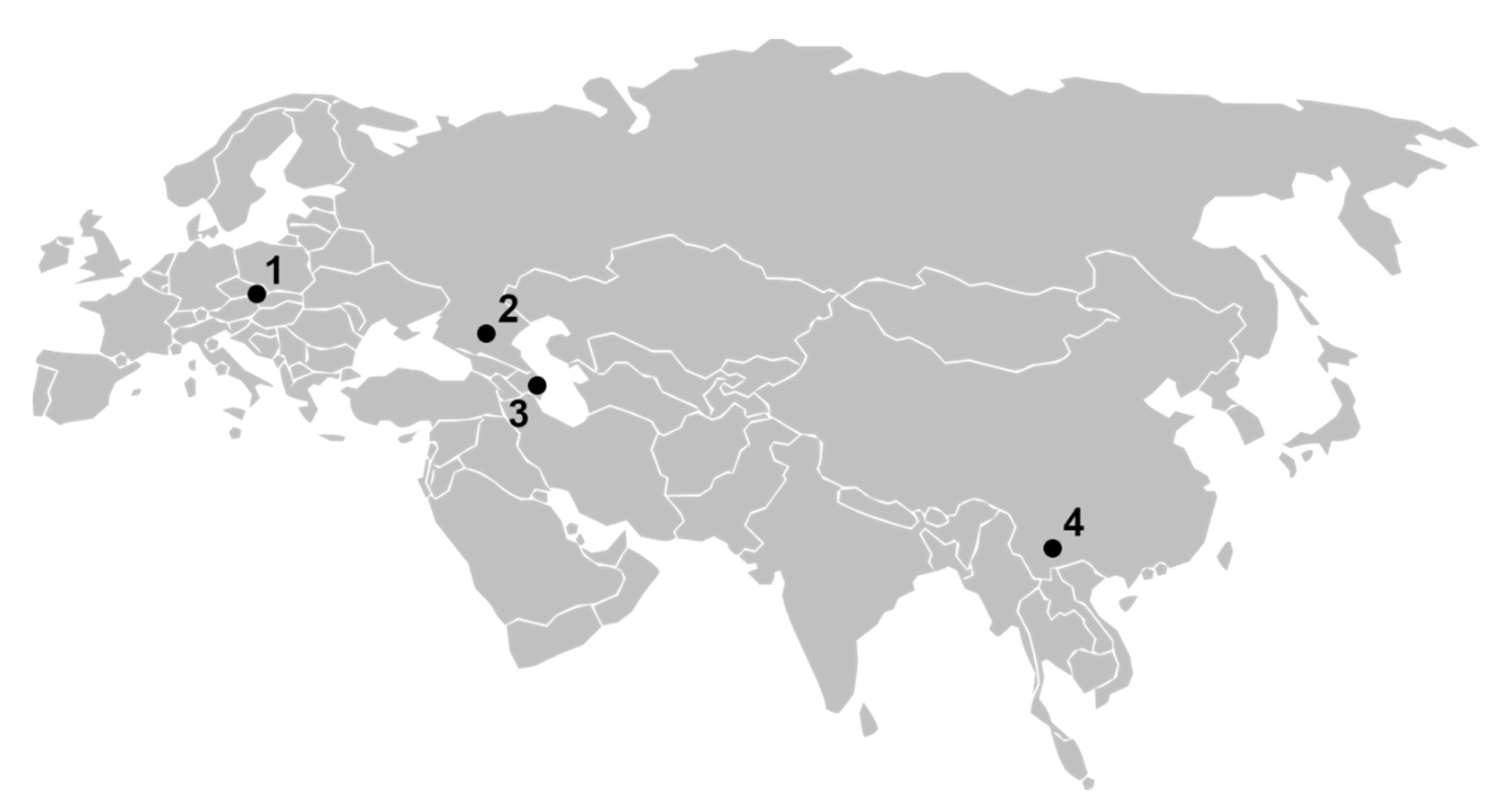
3. Results and Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lwande, O.W.; Obanda, V.; Bucht, G.; Mosomtai, G.; Otieno, V.; Ahlm, C.; Evander, M. Global emergence of Alphaviruses that cause arthritis in humans. Infect. Ecol. Epidemiol. 2015, 5, 29853. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Azar, S.R.; Campos, R.K.; Bergren, N.A.; Camargos, V.N.; Rossi, S.L. Epidemic Alphaviruses: Ecology, Emergence and Outbreaks. Microorganisms 2020, 8, 1167. [Google Scholar] [CrossRef] [PubMed]
- Hubálek, Z.; Rudolf, I. Microbial Zoonoses and Sapronoses; Springer: Dordrecht, The Netherlands, 2011. [Google Scholar]
- Adouchief, S.; Smura, T.; Sane, J.; Vapalahti, O.; Kurkela, S. Sindbis virus as a human pathogen—Epidemiology, clinical picture and pathogenesis. Rev. Med. Virol. 2016, 26, 221–241. [Google Scholar] [CrossRef] [PubMed]
- Becker, N.; Petric, D.; Zgomba, M.; Boase, C.; Madon, M.; Dahl, C.; Kaiser, A. Mosquitoes and Their Control, 2nd ed.; Springer: Berlin/Heidelberg, Germany, 2010. [Google Scholar]
- Votýpka, J.; Šeblová, V.; Rádrová, J. Spread of the West Nile virus vector Culex modestus and the potential malaria vector Anopheles hyrcanus in Central Europe. J. Vector Ecol. 2008, 33, 269–277. [Google Scholar] [CrossRef] [PubMed]
- Šebesta, O.; Gelbič, I.; Peško, J. Seasonal dynamics of mosquito occurrence in the Lower Dyje River Basin at the Czech-Slovak-Austrian border. Ital. J. Zool. 2013, 80, 125–138. [Google Scholar] [CrossRef] [Green Version]
- Rudolf, I.; Bakonyi, T.; Šebesta, O.; Peško, J.; Venclíková, K.; Mendel, J.; Betášová, L.; Blažejová, H.; Straková, P.; Nowotny, N.; et al. West Nile virus lineage 2 isolated from Culex modestus mosquitoes in the Czech Republic, 2013: Expansion of the European WNV endemic area to the North? Eurosurveillance 2014, 19, 20867. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hubálek, Z.; Šebesta, O.; Peško, J.; Betášová, L.; Blažejová, H.; Venclíková, K.; Rudolf, I. Isolation of Ťahyňa virus (California encephalitis group) from Anopheles hyrcanus (Diptera, Culicidae), a mosquito species new to, and expanding in, Central Europe. J. Med. Entomol. 2014, 51, 1264–1267. [Google Scholar] [CrossRef] [PubMed]
- Rudolf, I.; Bakonyi, T.; Šebesta, O.; Mendel, J.; Peško, J.; Betášová, L.; Blažejová, H.; Venclíková, K.; Straková, P.; Nowotny, N.; et al. Co-circulation of Usutu virus and West Nile virus in a reed bed ecosystem. Parasit. Vectors 2015, 8, 520. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eshoo, M.W.; Whitehouse, C.A.; Zoll, S.T.; Massire, C.; Pennella, T.D.; Blyn, L.B.; Sampath, R.; Hall, T.A.; Ecker, J.A.; Desai, A.; et al. Direct broad-range detection of alphaviruses in mosquito extracts. Virology 2007, 368, 286–295. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jöst, H.; Bialonski, A.; Storch, V.; Günther, S.; Becker, N.; Schmidt-Chanasit, J. Isolation and phylogenetic analysis of Sindbis viruses from mosquitoes in Germany. J. Clin. Microbiol. 2010, 48, 1900–1903. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular Evolutionary Genetics Analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Posada, D. jModeltest: Phylogenetic model averaging. Mol. Biol. Evol. 2008, 25, 1253–1256. [Google Scholar] [CrossRef] [PubMed]
- Lvov, D.K.; Gromashevsky, V.L.; Skvortsova, T.M.; Berezina, L.K.; Zakaryan, V.A. Kyzylagach virus (family Togaviridae, genus Alphavirus), a new arbovirus isolated from Culex modestus mosquitoes trapped in the Azerbaijan SSR. Vopr. Virusol. 1979, 5, 519–523. [Google Scholar]
- Calisher, C.H.; Karabatsos, N.; Lazuick, J.S.; Monath, T.P.; Wolff, K.L. Reevaluation of the western equine encephalitis antigenic complex of alphaviruses (family Togaviridae) as determined by neutralization tests. Am. J. Trop. Med. Hyg. 1988, 38, 447–452. [Google Scholar] [CrossRef] [PubMed]
- King, A.M.Q.; Adams, M.J.; Carstens, E.B.; Lefkowitz, E.J. Genus Alphavirus. In Virus Taxonomy-Classification and Nomenclature of Viruses (Ninth Report of the International Committee on Taxonomy of Viruses); Elsevier: Amsterdam, The Netherlands, 2012; pp. 1105–1108. [Google Scholar]
- Liang, G.D.; Li, L.; Zhou, G.L.; Fu, S.H.; Li, Q.P.; Li, F.S.; He, H.H.; Jin, Q.; He, Y.; Chen, B.Q.; et al. Isolation and complete nucleotide sequence of a Chinese Sindbis-like virus. J. Gen. Virol. 2000, 81, 1347–1351. [Google Scholar] [CrossRef]
- Alkhovsky, S.V.; Lvov, D.K.; Shchelkanov, M.Y.; Shchetinin, A.M.; Deryabin, P.G.; Gitelman, A.K.; Botikov, A.G.; Samokhvalov, E.I. Complete genome characterization of the Kyzylagach virus (KYZV) (Togaviridae, Alphavirus, Sindbis serogroup) isolated from mosquitoes Culex modestus Ficalbi, 1889 (Culicinae) collected in a colony of herons (Ardeidae Leach, 1820) in Azerbaijan. Vopr. Virusol. 2014, 59, 27–31. [Google Scholar]
- Lundstrom, J.O.; Pfeffer, M. Phylogeographic structure and evolutionary history of Sindbis virus. Vector. Borne Zoonot. Dis. 2010, 10, 889–907. [Google Scholar] [CrossRef]
- Lvov, D.K.; Shchelkanov, M.; Alkhovsky, S.V.; Deryabin, P.G. Zoonotic Viruses of Northern Eurasia: Taxonomy and Ecology; Elsevier: Amsterdam, The Netherlands, 2015. [Google Scholar]
- Ling, J.; Smura, T.; Lundström, J.O.; Pettersson, J.H.O.; Sironen, T.; Vapalahti, O.; Lundkvist, Å.; Hesson, J.C. Introduction and Dispersal of Sindbis Virus from Central Africa to Europe. J. Virol. 2019, 93, e00620-19. [Google Scholar] [CrossRef] [Green Version]
- Karabatsos, N. International Catalogue of Arboviruses, Including Certain Other Viruses of Vertebrates, 3rd ed.; American Society of Tropical Medicine and Hygiene: San Antonio, TX, USA, 1985. [Google Scholar]
- Hubálek, Z.; Juřicová, Z.; Halouzka, J.; Pellantová, J.; Hudec, K. Arboviruses associated with birds in southern Moravia, Czechoslovakia. Acta Sci. Nat. Brno 1989, 23, 1–50. [Google Scholar]
- Balenghien, T.; Vazeille, M.; Grandadam, M.; Schaffner, F.; Zeller, H.; Reiter, P.; Sabatier, P.; Fouque, F.; Bicout, D.J. Vector competence of some French Culex and Aedes mosquitoes for West Nile Virus. Vector-Borne Zoonot Dis. 2008, 8, 589–595. [Google Scholar] [CrossRef]
- Cotar, A.I.; Falcuta, E.; Prioteasa, L.F.; Dinu, S.; Ceieanu, C.S.; Paz, S. Transmission dynamics of the West Nile virus in mosquito vector populations under the influence of weather factors in the Danube delta, Romania. EcoHealth 2016, 13, 796–807. [Google Scholar] [CrossRef] [PubMed]
- Danielová, V. To the problem of the vector of Lednice virus. Folia Parasitol. 1984, 31, 379–382. [Google Scholar]
- Hubálek, Z.; Halouzka, J. Arthropod-borne viruses of vertebrates in Europe. Acta Sci. Nat. Brno 1996, 30, 1–95. [Google Scholar]
- Brugman, V.A.; Hernandez-Triana, L.M.; England, M.E.; Medlock, J.M.; Mertens, P.P.C.; Logan, J.G.; Wilson, A.J.; Fooks, A.R.; Johnson, N.; Carpenter, S. Blood-feeding patterns of native mosquitoes and insights into their potential role as pathogen vectors in the Thames estuary region of the United Kingdom. Parasit. Vectors 2017, 10, 163. [Google Scholar] [CrossRef] [Green Version]
- Rádrová, J.; Šeblová, V.; Votýpka, J. Feeding behaviour and spatial distribution of Culex mosquitoes (Diptera: Culicidae) in wetland areas of the Czech Republic. J. Med. Entomol. 2013, 50, 1097–1104. [Google Scholar] [CrossRef]
- Balenghien, T.; Fouque, F.; Sabatier, P.; Bicout, D.J. Horse-, bird-, and human-seeking behaviour and seasonal abundance of mosquitoes in a West Nile virus focus of southern France. J. Med. Entomol. 2006, 43, 936–946. [Google Scholar] [CrossRef]
- Brugman, V.A.; England, M.E.; Stoner, J.; Tugwell, L.; Harrup, L.E.; Wilson, A.J.; Medlock, J.M.; Logan, J.G.; Fooks, A.R.; Mertens, P.P.C.; et al. How often do mosquitoes bite humans in southern England? A standardised summer trial at four sites reveals spatial, temporal and site-related variation in biting rates. Parasit. Vectors 2017, 10, 420. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Šikutová, S.; Dočkal, P.; Straková, P.; Mendel, J.; Šebesta, O.; Betášová, L.; Blažejová, H.; Hubálek, Z.; Rudolf, I. First Record of Mosquito-Borne Kyzylagach Virus in Central Europe. Viruses 2020, 12, 1445. https://doi.org/10.3390/v12121445
Šikutová S, Dočkal P, Straková P, Mendel J, Šebesta O, Betášová L, Blažejová H, Hubálek Z, Rudolf I. First Record of Mosquito-Borne Kyzylagach Virus in Central Europe. Viruses. 2020; 12(12):1445. https://doi.org/10.3390/v12121445
Chicago/Turabian StyleŠikutová, Silvie, Patrik Dočkal, Petra Straková, Jan Mendel, Oldřich Šebesta, Lenka Betášová, Hana Blažejová, Zdeněk Hubálek, and Ivo Rudolf. 2020. "First Record of Mosquito-Borne Kyzylagach Virus in Central Europe" Viruses 12, no. 12: 1445. https://doi.org/10.3390/v12121445





