Field Verification of an African Swine Fever Virus Loop-Mediated Isothermal Amplification (LAMP) Assay during an Outbreak in Timor-Leste
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of a Synthetic DNA Positive Control and Modified Internal Amplification Control
2.2. ASFV LAMP
2.3. Sample Matrix Assessment
2.4. Blood Swab Assessment
2.5. Analytical Specificity
2.6. Repeatability
2.7. Limit of Detection
2.8. Colourmetric LAMP
2.9. Sample Collections in Timor-Leste
2.10. ASFV LAMP Sample Preparation in Timor-Leste
2.11. ASF qPCR Assay in Timor-Leste
2.12. Ethics
3. Results
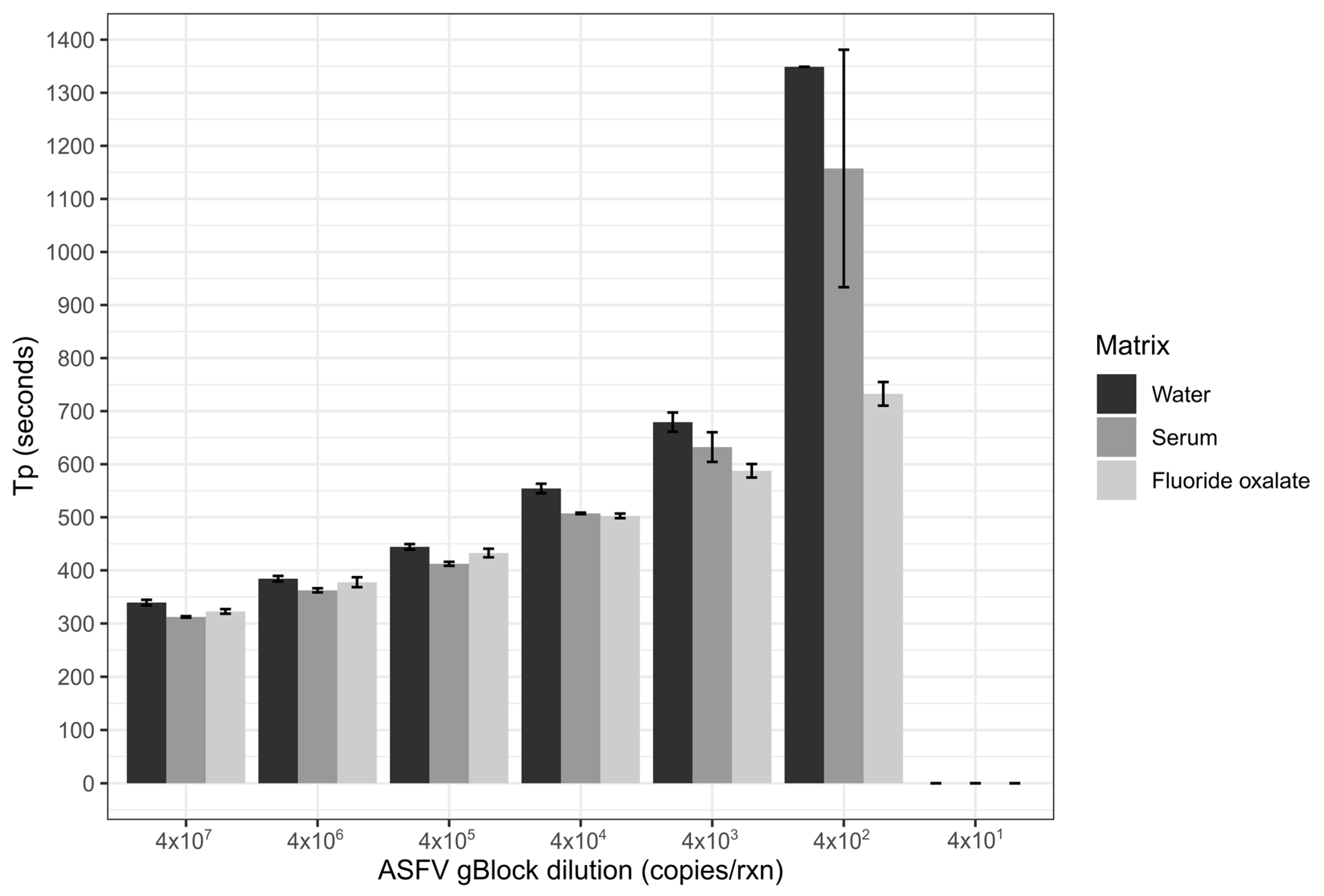
3.1. Assessment of ASFV LAMP
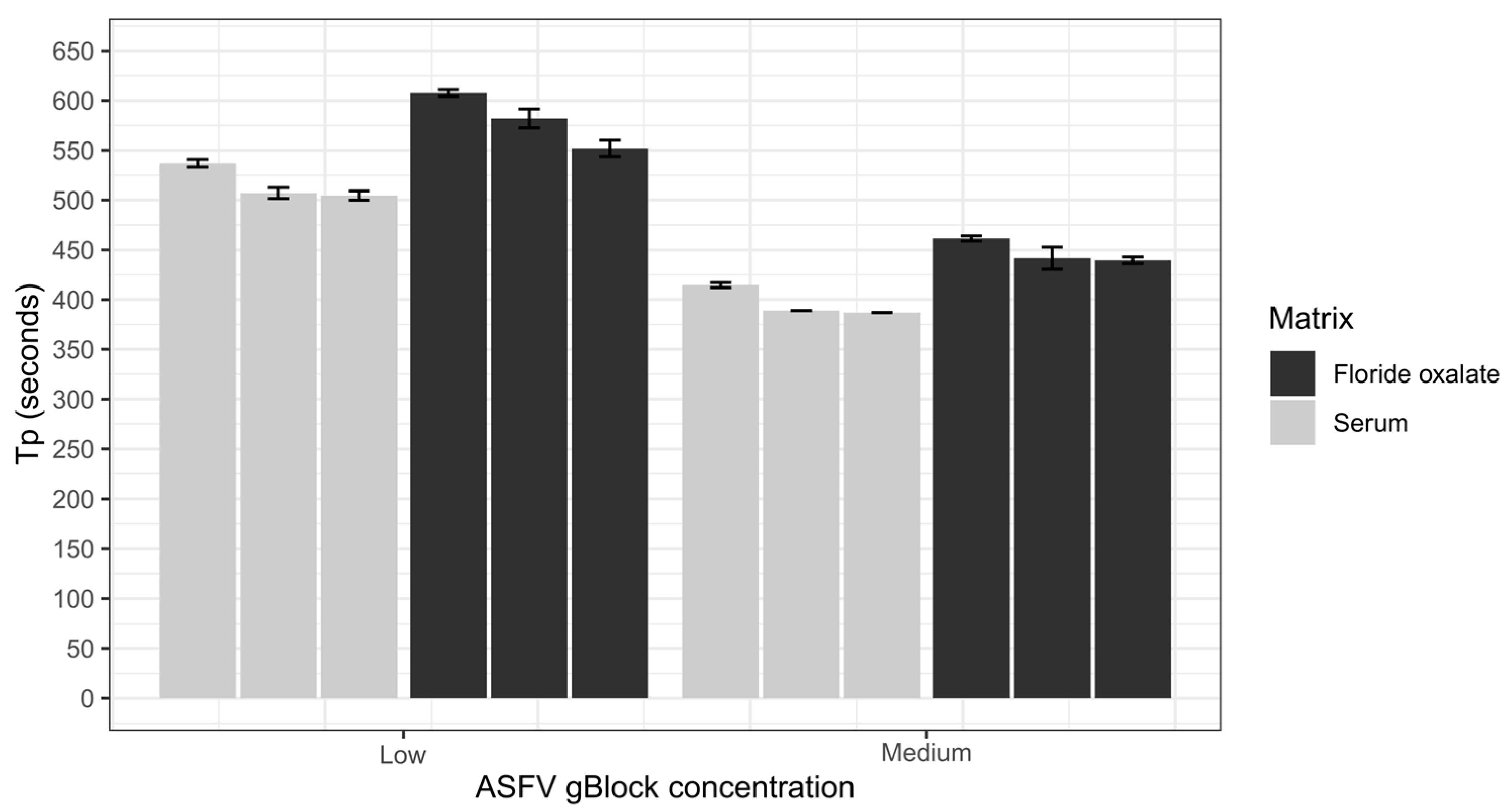
3.2. Comparison of Sample Matrices and Dilution of Anticoagulants
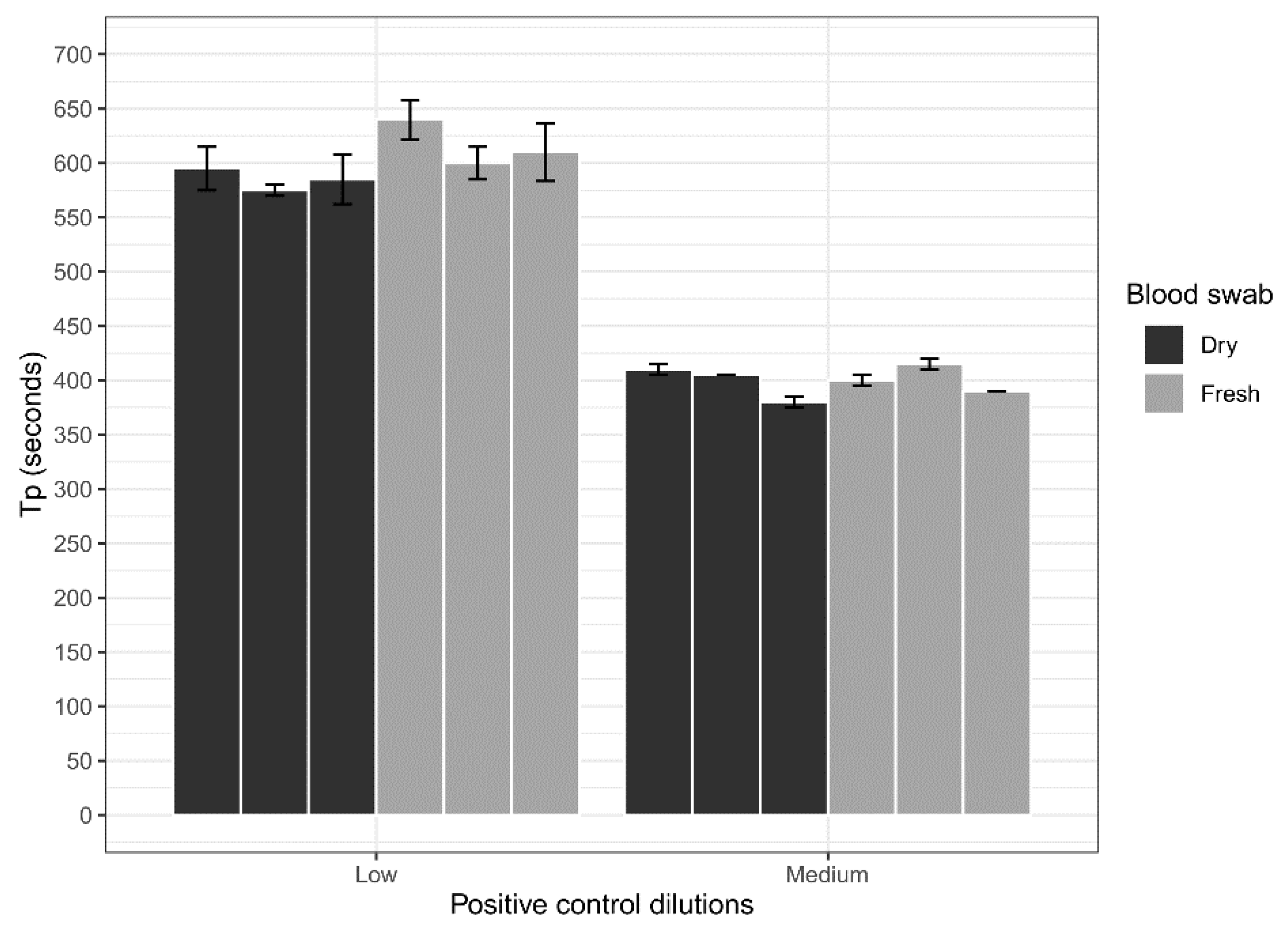
3.3. Blood Swab Assessment
3.4. Analytical Specificity
3.5. ASFV LAMP Results for Serum and Swab Samples Tested in Timor-Leste
3.6. Performance of Internal Amplification Control
3.7. Cross Verification of Positives with qPCR
3.8. Colourmetric LAMP
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dixon, L.; Sun, H.; Roberts, H. African swine fever. Antivir. Res. 2019, 165, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Rowlands, R.J.; Michaud, V.; Heath, L.; Hutchings, G.; Oura, C.; Vosloo, W.; Dwarka, R.; Onashvili, T.; Albina, E.; Dixon, L. African swine fever virus isolate, Georgia. Emerg. Infect. Dis. 2008, 14, 1870–1874. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Li, N.; Luo, Y.; Liu, Y.; Miao, F.; Chen, T.; Zhang, S.; Cao, P.; Li, X.; Tian, K.; et al. Emergence of African swine fever in China. Transbound. Emerg. Dis. 2018, 65, 1482–1484. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Le, V.P.; Jeong, D.G.; Yoon, S.-W.; Kwon, H.-M.; Trinh, T.B.N.; Nguyen, T.L.; Bui, T.T.N.; Oh, J.; Kim, J.B.; Cheong, K.M.; et al. Outbreak of African swine fever, Vietnam. Emerg. Infect. Dis. 2019, 25, 1433–1435. [Google Scholar] [CrossRef] [PubMed]
- FAO. ASF Situation in Asia Update. Available online: http://www.fao.org/ag/againfo/programmes/en/empres/ASF/situation_update.html (accessed on 1 June 2020).
- Kim, H.-J.; Cho, K.-H.; Lee, S.-K.; Kim, D.-Y.; Nah, J.-J.; Kim, H.-J.; Kim, H.-J.; Hwang, J.-Y.; Sohn, H.-J.; Choi, J.G.; et al. Outbreak of African swine fever in South Korea. Transbound. Emerg. Dis. 2020, 67, 473–475. [Google Scholar] [CrossRef]
- Sánchez-Vizcaíno, J.M.; Mur, L.; Gomez-Villamandos, J.C.; Carrasco, L. An update on the epidemiology and pathology of African swine fever. J. Comp. Pathol. 2015, 152, 9–21. [Google Scholar] [CrossRef]
- Costard, S.; Wieland, B.; De Glanville, W.; Jori, F.; Rowlands, R.; Vosloo, W.; Roger, F.; Pfeiffer, D.U.; Dixon, L. African swine fever: How can global spread be prevented? Philos. Trans. R. Soc. Lond. B Biol. Sci. 2009, 364, 2683–2696. [Google Scholar] [CrossRef] [Green Version]
- King, D.P.; Reid, S.M.; Hutchings, G.H.; Grierson, S.S.; Wilkinson, P.J.; Dixon, L.K.; Bastos, A.D.; Drew, T.W. Development of a TaqMan® PCR assay with internal amplification control for the detection of African swine fever virus. J. Virol. Methods 2003, 107, 53–61. [Google Scholar] [CrossRef]
- Oura, C.; Edwards, L.; Batten, C. Virological diagnosis of African swine fever—Comparative study of available tests. Virus Res. 2013, 173, 150–158. [Google Scholar] [CrossRef]
- Done, S.; Gresham, A.; Potter, R.; Chennells, D. PMWS and PDNS—Two recently recognised diseases of pigs in the UK. Practice 2001, 23, 14–21. [Google Scholar] [CrossRef]
- Smith, D.; Cooper, T.; Pereira, A.; Jong, J.B.D.C. Counting the cost: The potential impact of African swine fever on smallholders in Timor-Leste. One Health 2019, 8, 100109. [Google Scholar] [CrossRef] [PubMed]
- Guinat, C.; Gogin, A.; Blome, S.; Keil, G.; Pollin, R.; Pfeiffer, D.U.; Dixon, L. Transmission routes of African swine fever virus to domestic pigs: Current knowledge and future research directions. Vet. Rec. 2016, 178, 262–267. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guinat, C.; Reis, A.L.; Netherton, C.L.; Goatley, L.; Pfeiffer, D.U.; Dixon, L. Dynamics of African swine fever virus shedding and excretion in domestic pigs infected by intramuscular inoculation and contact transmission. Vet. Res. 2014, 45, 93. [Google Scholar] [CrossRef] [PubMed]
- Sur, J. How far can African swine fever spread? J. Vet. Sci. 2019, 20, e41. [Google Scholar] [CrossRef]
- Mazur-Panasiuk, N.; Żmudzki, J.; Woźniakowski, G. African swine fever virus—Persistence in different environmental conditions and the possibility of its indirect transmission. J. Vet. Res. 2019, 63, 303–310. [Google Scholar] [CrossRef] [Green Version]
- Plowright, W.; Parker, J.; Peirce, M.A. African swine fever virus in ticks (Ornithodoros moubata, Murray) collected from animal burrows in Tanzania. Nat. Cell Biol. 1969, 221, 1071–1073. [Google Scholar] [CrossRef] [PubMed]
- Mellor, P.S.; Kitching, R.P.; Wilkinson, P.J. Mechanical transmission of capripox virus and African swine fever virus by Stomoxys calcitrans. Res. Vet. Sci. 1987, 43, 109–112. [Google Scholar] [CrossRef]
- Sanchez, C.B.; Badiola, C. African swine fever virus in Harmatopinus suis. Bull. Off. Int. Epizoot 1966, 66, 699–705. [Google Scholar]
- OIE. 27/09/2019: African Swine Fever, Timor-Leste, (Immediate Notification). 2019. Available online: https://www.oie.int/wahis_2/public/wahid.php/Diseaseinformation/WI/index/newlang/en (accessed on 12 December 2019).
- Gimenez-Lirola, L.G.; Mur, L.; Rivera, B.; Mogler, M.; Sun, Y.; Lizano, S.; Goodell, C.; Harris, D.L.H.; Rowland, R.R.R.; Gallardo, C.; et al. Detection of African swine fever virus antibodies in serum and oral fluid specimens using a recombinant protein 30 (p30) dual matrix indirect ELISA. PLoS ONE 2016, 11, e0161230. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Ji, P.; Fan, H.; Dang, L.; Wan, W.; Liu, S.; Li, Y.; Yu, W.; Li, X.; Ma, X.; et al. CRISPR/Cas12a technology combined with immunochromatographic strips for portable detection of African swine fever virus. Commun. Biol. 2020, 3, 1–8. [Google Scholar] [CrossRef] [Green Version]
- He, Q.; Yu, D.; Bao, M.; Korensky, G.; Chen, J.; Shin, M.; Kim, J.; Park, M.; Qin, P.; Du, K. High-throughput and all-solution phase African swine fever virus (ASFV) detection using CRISPR-Cas12a and fluorescence based point-of-care system. Biosens. Bioelectron. 2020, 154, 112068. [Google Scholar] [CrossRef] [PubMed]
- Notomi, T.; Okayama, H.; Masubuchai, H.; Yonekawa, T.; Watanabe, K.; Amino, N.; Hase, T. Loop-mediated isothermal amplification of DNA. Nucleic Acids Res. 2000, 28, E63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nagamine, K.; Hase, T.; Notomi, T. Accelerated reaction by loop-mediated isothermal amplification using loop primers. Mol. Cell. Probes 2002, 16, 223–229. [Google Scholar] [CrossRef]
- Blomstrom, A.; Hakhverdyan, M.; Reid, S.M.; Dukes, J.P.; King, D.P.; Belák, S.; Berg, M. A one-step reverse transcriptase loop-mediated isothermal amplification assay for simple and rapid detection of swine vesicular disease virus. J. Virol. Methods 2008, 147, 188–193. [Google Scholar] [CrossRef] [PubMed]
- Poon, L.L.; Wong, B.W.Y.; Ma, E.H.T.; Chan, K.H.; Chow, L.M.C.; Abeyewickreme, W.; Tangpukdee, N.; Yuen, K.Y.; Guan, Y.; Looareesuwan, S.; et al. Sensitive and inexpensive molecular test for falciparum malaria: Detecting Plasmodium falciparum DNA directly from heat-treated blood by loop-mediated isothermal amplification. Clin. Chem. 2006, 52, 303–306. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaneko, H.; Kawana, T.; Fukushima, E.; Suzutani, T. Tolerance of loop-mediated isothermal amplification to a culture medium and biological substances. J. Biochem. Biophys. Methods. 2007, 70, 499–501. [Google Scholar] [CrossRef] [PubMed]
- Fischbach, J.; Xander, N.C.; Frohme, M.; Glökler, J.F. Shining a light on LAMP assays—A comparison of LAMP visualization methods including the novel use of berberine. Biotechniques 2015, 58, 189–194. [Google Scholar] [CrossRef] [Green Version]
- Ohtsuka, K.; Yanagawa, K.; Takatori, K.; Hara-Kudo, Y. Detection of Salmonella enterica in naturally contaminated liquid eggs by loop-mediated isothermal amplification, and characterization of Salmonella isolates. Appl. Environ. Microbiol. 2005, 71, 6730–6735. [Google Scholar] [CrossRef] [Green Version]
- Dukes, J.P.; King, D.P.; Alexandersen, S. Novel reverse transcription loop-mediated isothermal amplification for rapid detection of foot-and-mouth disease virus. Arch. Virol. 2006, 151, 1093–1106. [Google Scholar] [CrossRef]
- Fukuda, S.; Takao, S.; Kuwayama, M.; Shimazu, Y.; Miyazaki, K. Rapid detection of Norovirus from fecal specimens by real-time reverse transcription-loop-mediated isothermal amplification assay. J. Clin. Microbiol. 2006, 44, 1376–1381. [Google Scholar] [CrossRef] [Green Version]
- Okiro, L.A.; Tancos, M.A.; Nyanjom, S.G.; Smart, C.D.; Parker, M.L. Comparative evaluation of LAMP, qPCR, conventional PCR, and ELISA to detect Ralstonia solanacearum in Kenyan potato fields. Plant Dis. 2019, 103, 959–965. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bath, C.; Scott, M.; Sharma, P.M.; Gurung, R.B.; Phuentshok, Y.; Pefanis, S.; Colling, A.; Balasubramanian, N.S.; Firestone, S.M.; Ungvanijban, S.; et al. Further development of a reverse-transcription loop-mediated isothermal amplification (RT-LAMP) assay for the detection of foot-and-mouth disease virus and validation in the field with use of an internal positive control. Transbound. Emerg. Dis. 2020. [Google Scholar] [CrossRef] [PubMed]
- Frączyk, M.; Woźniakowski, G.; Kowalczyk, A.; Niemczuk, K.; Pejsak, Z. Development of cross-priming amplification for direct detection of the African swine fever virus, in pig and wild boar blood and sera samples. Lett. Appl. Microbiol. 2016, 62, 386–391. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- James, H.E.; Ebert, K.; Mcgonigle, R.; Reid, S.M.; Boonham, N.; Tomlinson, J.A.; Hutchings, G.H.; Denyer, M.; Oura, C.A.; Dukes, J.P.; et al. Detection of African swine fever virus by loop-mediated isothermal amplification. J. Virol. Methods 2010, 164, 68–74. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Yu, J.; Wang, Y.; Zhang, M.; Li, P.; Liu, M.; Liu, Y. Development of a real-time loop-mediated isothermal amplification (LAMP) assay and visual LAMP assay for detection of African swine fever virus (ASFV). J. Virol. Methods 2020, 276, 113775. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Xiao, L.; Wang, Y.; Yang, Z.; Yao, X.; Peng, B. Development of a rapid and sensitive method for detection of African swine fever virus using loop-mediated isothermal amplification. Braz. Arch. Biol. Technol. 2016, 59, 59. [Google Scholar] [CrossRef] [Green Version]
- Kirstein, L.M.; Mellors, J.W.; Rinaldo, C.R.; Margolick, J.B.; Giorgi, J.V.; Phair, J.P.; Dietz, E.; Gupta, P.; Sherlock, C.H.; Hogg, R.; et al. Effects of anticoagulant, processing delay, and assay method (branched DNA versus reverse transcriptase PCR) on measurement of human immunodeficiency virus type 1 RNA levels in plasma. J. Clin. Microbiol. 1999, 37, 2428–2433. [Google Scholar] [CrossRef] [Green Version]
- García, M.E.; Blanco, J.L.; Caballero, J.; Gargallo-Viola, D. Anticoagulants interfere with PCR used to diagnose invasive Aspergillosis. J. Clin. Microbiol. 2002, 40, 1567–1568. [Google Scholar] [CrossRef] [Green Version]
- Office International des Epizooties. Chapter 3.8. African Swine Fever (Infection with African Swine Fever Virus). Available online: https://www.oie.int/fileadmin/Home/eng/Health_standards/tahm/3.08.01_ASF.pdf (accessed on 1 June 2020).
- Knight, A.I.; Haines, J.; Zuber, S. Thermal inactivation of animal virus pathogens. Curr. Top. Virol. 2013, 11, 103–119. [Google Scholar]
- Ma, J.; Chen, H.; Gao, X.; Xiao, J.; Wang, H. African swine fever emerging in China: Distribution characteristics and high-risk areas. Prev. Vet. Med. 2020, 175, 104861. [Google Scholar] [CrossRef]
- Jurado, C.; Martínez-Avilés, M.; De La Torre, A.; Štukelj, M.; Ferreira, H.C.D.C.; Cerioli, M.; Sánchez-Vizcaíno, J.M.; Bellini, S. Relevant measures to prevent the spread of African swine fever in the European Union domestic pig sector. Front. Vet. Sci. 2018, 5, 77. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Álvarez, J.; Bicout, D.; Boklund, A.; Bøtner, A.; Depner, K.; More, S.J.; Roberts, H.; Stahl, K.; Thulke, H.; Viltrop, A.; et al. Research gap analysis on African swine fever. EFSA J. 2019, 17, e05811. [Google Scholar] [CrossRef] [Green Version]
- Bastos, A.; Penrith, M.-L.; Crucière, C.; Edrich, J.L.; Hutchings, G.; Roger, F.; Couacy-Hymann, E.; Thomson, G.R. Genotyping field strains of African swine fever virus by partial p72 gene characterisation. Arch. Virol. 2003, 148, 693–706. [Google Scholar] [CrossRef] [PubMed]
- Huggett, J.F.; Novak, T.; Garson, J.; Green, C.; Morris-Jones, S.D.; Miller, R.F.; Zumla, A. Differential susceptibility of PCR reactions to inhibitors: An important and unrecognised phenomenon. BMC Res. Notes 2008, 1, 70. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vaughan-Shaw, P.G.; Walker, M.; Ooi, L.; Gilbert, N.; Farrington, S.M.; Dunlop, M.G. A simple method to overcome the inhibitory effect of heparin on DNA amplification. Cell. Oncol. 2015, 38, 493–495. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Francois, P.; Tangomo, M.; Hibbs, J.; Bonetti, E.-J.; Boehme, C.C.; Notomi, T.; Perkins, M.D.; Schrenzel, J. Robustness of a loop-mediated isothermal amplification reaction for diagnostic applications. FEMS Immunol. Med. Microbiol. 2011, 62, 41–48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mayboroda, O.; Katakis, I.; O’Sullivan, C.K. Multiplexed isothermal nucleic acid amplification. Anal. Biochem. 2018, 545, 20–30. [Google Scholar] [CrossRef] [PubMed]
- Tanner, N.A.; Zhang, Y.; Evans, T.C., Jr. Simultaneous multiple target detection in real-time loop-mediated isothermal amplification. Biotechniques 2012, 53, 81–89. [Google Scholar] [CrossRef] [Green Version]
- Hoorfar, J.; Malorny, B.; Abdulmawjood, A.; Cook, N.; Wagner, M.; Fach, P. Practical considerations in design of internal amplification controls for diagnostic PCR assays. J. Clin. Microbiol. 2004, 42, 1863–1868. [Google Scholar] [CrossRef] [Green Version]
- Hardinge, P.; Murray, J.A. Reduced false positives and improved reporting of loop-mediated isothermal amplification using quenched fluorescent primers. Sci. Rep. 2019, 9, 740. [Google Scholar] [CrossRef]
- Gonçalves, D.D.S.; Hooker, D.J.; Dong, Y.; Baran, N.; Kyrylos, P.; Iturbe-Ormaetxe, I.; Simmons, C.P.; O’Neill, S.L. Detecting wMel Wolbachia in field-collected Aedes aegypti mosquitoes using loop-mediated isothermal amplification (LAMP). Parasites Vectors. 2019, 12, 1–5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Daskou, M.; Tsakogiannis, D.; Dimitriou, T.G.; Amoutzias, G.D.; Mossialos, D.; Kottaridi, C.; Gartzonika, C.; Markoulatos, P. WarmStart colorimetric LAMP for the specific and rapid detection of HPV16 and HPV18 DNA. J. Virol. Methods. 2019, 270, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Thurmond, M.C. Conceptual foundations for infectious disease surveillance. J. Vet. Diagn. Investig. 2003, 15, 501–514. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grau, F.R.; Schroeder, M.E.; Mulhern, E.L.; McIntosh, M.T.; Bounpheng, M.A. Detection of African swine fever, classical swine fever, and foot-and-mouth disease viruses in swine oral fluids by multiplex reverse transcription real-time polymerase chain reaction. J. Vet. Diagn. Investig. 2015, 27, 140–149. [Google Scholar] [CrossRef] [PubMed]
- Beemer, O.; Remmenga, M.; Gustafson, L.; Johnson, K.; Hsi, D.; Antognoli, M.C. Assessing the value of PCR assays in oral fluid samples for detecting African swine fever, classical swine fever, and foot-and-mouth disease in U.S. swine. PLoS ONE 2019, 14, e0219532. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Assay | Target | Primer | Sequence (5′ > 3′) | Reference |
|---|---|---|---|---|
| qPCR | 3′-end of the VP72 gene | ASF forward | CTGCTCATGGTATCAATCTTATCGA | [9] |
| ASF reverse | GATACCACAAGATCRGCCGT | |||
| ASF probe | FAM-CCACGGGAG/Zen/GAATACCAACCCAGTG-IABkFQ | |||
| LAMP | Topoisomerase II | F3 forward outer | GGCGCAAAATTTTAGCCGG | [36] |
| B3 reverse outer | GCCGAAGCTTCCTATGCC | |||
| FIP forward inner | GCAACGTAGCCCCCGAACTGGAAATGCTTCGCYTCCAACA | |||
| BIP reverse inner | ATCACCATGGCGACATGTCGTGGATAGAGGTGGGAGGAGC | |||
| FLoop forward loop | AAAAACCTTTCGTTCACGGT | |||
| Bloop reverse loop | AAAAGCCGCCCAGTATTACC |
| Synthetic DNA Control | Sequence 5′ > 3′ |
|---|---|
| ASFV gBlock TPII | GGCGCAAAATTTTAGCCGGGGGGTTGAAATGCTTCGCCTCCAACAACCGTGAACGAAAGG TTTTTCAGTTTGGGGGCTACGTTGCGGATCACATGTTTTATCACCATGGCGATATGTCGTTA AACACAAGTATTATAAAAGCCGCCCAGTATTACCCAGGCTCCTCTCACCTCTATCCAGTA TTCATAGGCATAGGAAGCTTCGGC |
| ASFV gBlock IAC | GGCGCAAAATTTTAGCCGGGGGGTTGAAATGCTTCGCCTCCAACAACCGTGAACGAAAGG TTTTTCAGTTTGGGGGCTACGTTGCGCGACGTCACCGACGTGCCGTGATCACCATGGCGATA TGTCGTTAAACACAAGTATTATAAAAGCCGCCCAGTATTACCCAGGCTCCTCTCACCTCTAT CCAGTATTCATAGGCATAGGAAGCTTCGGC |
| Treatment | Replicate | Mean Tp (sec) (n = 6) | ±SD (sec) | CV (%) |
|---|---|---|---|---|
| Low ASFV gBlock in fluoride oxalate blood | 1 | 582 | 21 | 3.64 |
| 2 | 608 | 8 | 1.35 | |
| 3 | 552 | 18 | 3.33 | |
| Low ASFV gBlock in serum | 1 | 537 | 9 | 1.77 |
| 2 | 507 | 13 | 2.65 | |
| 3 | 505 | 11 | 2.24 | |
| Medium ASFV gBlock in fluoride oxalate blood | 1 | 462 | 6 | 1.33 |
| 2 | 442 | 27 | 6.20 | |
| 3 | 440 | 8 | 1.87 | |
| Medium ASFV gBlock in serum | 1 | 415 | 6 | 1.48 |
| 2 | 389 | 0 | 0.00 | |
| 3 | 387 | 0 | 0.00 |
| Treatment | Mean Tp (sec) (n = 18) | ±SD (sec) | CV (%) |
|---|---|---|---|
| Low ASFV gBlock in fluoride oxalate blood | 581 | 28 | 4.79% |
| Low ASFV gBlock in serum | 516 | 18 | 3.50% |
| Medium ASFV gBlock in fluoride oxalate blood | 448 | 12 | 2.71% |
| Medium ASFV gBlock in serum | 397 | 15 | 3.86% |
| Sample | Age (Months) | Sex | ASFV LAMP | IAC LAMP | qPCR | Colourmetric | |||
|---|---|---|---|---|---|---|---|---|---|
| Tp (mm:ss) | Ta (°C) | Tp (mm:ss) | Ta (°C) | Sample (Cq) | Positive (Cq) | ||||
| 1 | 6 | F | - | - | 12:10 | 89.6 | - | 10.65 | NEG |
| 2 | 12 | F | - | - | 11:23 | 89.5 | - | 10.67 | NEG |
| 3 | 18 | M | 1:30 | - | 11:25 | 89.6 | - | 11.83 | NEG |
| 4 | 5 | F | - | - | 12:55 | 89.5 | - | 11.86 | NEG |
| 5 | 48 | F | - | - | 10:43 | 89.6 | - | 11.35 | NEG |
| 6 | 12 | M | 1:30 | 9:55 | 89.5 | - | 11.73 | NEG | |
| 7 ‡ | 18 | F | 10:00 | 87.20 | 9:55 | 89.2 | 27.39 | 10.39 | POS |
| 8 | 6 | M | 1:30 | - | 13:23 | 89.4 | - | 10.47 | IND |
| 9 | 7 | M | 1:45 | - | 13:23 | 89.4 | - | 13.64 | NEG |
| 10 | 48 | F | 6:45 | - | 11:08 | 89.4 | - | 11.39 | NEG |
| 11 | 7 | F | - | - | 9:55 | 89.6 | - | 10.56 | NEG |
| 12 | 12 | F | 1:30 | - | 9:13 | 89.5 | - | 10.87 | NEG |
| 13 ‡ | 18 | F | 7:55 | 87.30 | 10:02 | 89.4 | 22.74 | 11.79 | POS |
| 14 ‡ | 7 | F | 12:10 | 87.40 | 13:55 | 89.4 | 25.4 | 11.57 | POS |
| 15 | 7 | F | - | - | 14:10 | 89.7 | - | 11.83 | NEG |
| 16 | 18 | M | 1:40 | - | 14:25 | 89.6 | - | 11.26 | NEG |
| 17 ‡ | 8 | M | 9:57 | 87.40 | 10:57 | 89.2 | 27.5 | 10.75 | POS |
| 18 | 7 | F | - | - | 11:12 | 89.6 | - | 11.24 | NEG |
| 19 | 8 | M | - | - | 12:57 | 89.4 | - | 11.73 | NEG |
| 20 | 8 | F | 16:10 | 87.20 | 10:38 | 89.6 | - | 11.63 | POS |
| 21 | 8 | F | 22:55 | - | 10:23 | 89.7 | 35.2 | 10.37 | POS |
| 22 | 24 | F | 1:30 | - | 10:27 | 89.6 | - | 11.53 | NEG |
| 23 | 24 | F | - | - | 9:27 | 89.7 | - | 11.73 | NEG |
| 24 | 12 | F | - | - | 9:57 | 89.6 | 39.1 | 11.43 | NEG |
| 25 | 12 | F | - | - | 10:25 | 89.6 | - | 10.87 | NEG |
| 26 ‡ | 18 | F | 9:25 | 87.34 | 8:55 | 89.7 | 25.4 | 11.4 | POS |
| 27 ‡ | 1 | M | 8:20 | 87.20 | 10:10 | 89.2 | 23.2 | 11.63 | POS |
| 28 | 24 | F | 1:40 | - | 9:55 | 89.6 | - | 11.73 | POS |
| 29 | 12 | M | - | - | 9:10 | 89.6 | - | 11.92 | NEG |
| 30 | 12 | M | 2:55 | - | 9:10 | 89.6 | - | 12.67 | NEG |
| 31 ‡ | 18 | F | 7:38 | 87.54 | 8:53 | 89.1 | 25.58 | 11.12 | POS |
| 32 | 12 | M | - | - | 9:38 | 89.7 | - | 12.73 | NEG |
| 33 | 12 | M | - | - | 8:38 | 89.7 | - | 11.34 | IND |
| 34 ‡ | 24 | F | 8:57 | 87.4 | 8:27 | 89.8 | 24.23 | 11.4 | POS |
| 35 ‡ | 12 | M | 8:45 | 87.53 | 8:42 | 89.8 | 21.2 | 11.73 | POS |
| 36 | 6 | M | - | - | 9:27 | 89.7 | - | 11.64 | NEG |
| 37 | 24 | F | 10:08 | 87.30 | 9:00 | 89.1 | - | 11.37 | POS |
| Sample | Age (Months) | Sex | Serum ASFV LAMP | Swab ASFV LAMP | ||
|---|---|---|---|---|---|---|
| Tp (mm:ss) | Ta (°C) | Tp (mm:ss) | Ta (°C) | |||
| 1 | 6 | F | - | - | - | - |
| 2 | 12 | F | - | - | - | - |
| 3 | 18 | M | 1:30 | - | 18:30 | - |
| 4 | 5 | F | - | - | - | - |
| 5 | 48 | F | - | - | - | - |
| 6 | 12 | M | 1:30 | - | - | |
| 7 | 18 | F | 10:00 | 87.2 | 9:53 | 87.58 |
| 8 | 6 | M | 1:30 | - | - | - |
| 9 | 7 | M | 1:45 | - | - | - |
| 10 | 48 | F | 6:45 | - | - | - |
| 11 | 7 | F | - | - | - | - |
| 12 | 12 | F | 1:30 | - | - | - |
| 13 | 18 | F | 7:55 | 87.3 | - | - |
| 14 | 7 | F | 12:10 | 87.4 | 14:00 | 87.6 |
| 15 | 7 | F | - | - | - | - |
| 16 | 18 | M | 1:40 | - | 18:00 | |
| 17 | 8 | M | 9:57 | 87.4 | 17:00 | 87.6 |
| 18 | 7 | F | - | - | - | - |
| 19 | 8 | M | - | - | - | - |
| 20 | 8 | F | 16:10 | 87.2 | - | - |
| 21 | 8 | F | 22:55 | - | - | - |
| 22 | 24 | F | 1:30 | - | - | - |
| 23 | 24 | F | - | - | - | - |
| 24 | 12 | F | - | - | 20:00 | 89.1 |
| 25 | 12 | F | - | - | - | - |
| 26 | 18 | F | 9:25 | 87.34 | 8:15 | 87.62 |
| 27 | 1 | M | 8:20 | 87.2 | 7:30 | 87.54 |
| 28 | 24 | F | 1:40 | - | - | - |
| 29 | 12 | M | - | - | - | - |
| 30 | 12 | M | 2:55 | - | - | - |
| 31 | 18 | F | 7:38 | 87.54 | 8:15 | 87.5 |
| 32 | 12 | M | - | - | - | - |
| 33 | 12 | M | - | - | - | - |
| 34 | 24 | F | 8:57 | 87.4 | 10:45 | 87.49 |
| 35 | 12 | M | 8:45 | 87.53 | 11:00 | 87.54 |
| 36 | 6 | M | - | - | 12:30 | 87.47 |
| 37 | 24 | F | 10:08 | 87.3 | 9:30 | 87.47 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mee, P.T.; Wong, S.; O’Riley, K.J.; da Conceição, F.; Bendita da Costa Jong, J.; Phillips, D.E.; Rodoni, B.C.; Rawlin, G.T.; Lynch, S.E. Field Verification of an African Swine Fever Virus Loop-Mediated Isothermal Amplification (LAMP) Assay during an Outbreak in Timor-Leste. Viruses 2020, 12, 1444. https://doi.org/10.3390/v12121444
Mee PT, Wong S, O’Riley KJ, da Conceição F, Bendita da Costa Jong J, Phillips DE, Rodoni BC, Rawlin GT, Lynch SE. Field Verification of an African Swine Fever Virus Loop-Mediated Isothermal Amplification (LAMP) Assay during an Outbreak in Timor-Leste. Viruses. 2020; 12(12):1444. https://doi.org/10.3390/v12121444
Chicago/Turabian StyleMee, Peter T., Shani Wong, Kim J. O’Riley, Felisiano da Conceição, Joanita Bendita da Costa Jong, Dianne E. Phillips, Brendan C. Rodoni, Grant T. Rawlin, and Stacey E. Lynch. 2020. "Field Verification of an African Swine Fever Virus Loop-Mediated Isothermal Amplification (LAMP) Assay during an Outbreak in Timor-Leste" Viruses 12, no. 12: 1444. https://doi.org/10.3390/v12121444
APA StyleMee, P. T., Wong, S., O’Riley, K. J., da Conceição, F., Bendita da Costa Jong, J., Phillips, D. E., Rodoni, B. C., Rawlin, G. T., & Lynch, S. E. (2020). Field Verification of an African Swine Fever Virus Loop-Mediated Isothermal Amplification (LAMP) Assay during an Outbreak in Timor-Leste. Viruses, 12(12), 1444. https://doi.org/10.3390/v12121444





