Establishing a Multicolor Flow Cytometry to Characterize Cellular Immune Response in Chickens Following H7N9 Avian Influenza Virus Infection
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Virus and Animal Experiments
2.3. Cell Isolation
2.4. Flow Cytometry
2.5. Statistical Analysis
3. Results
3.1. Establishing a Polychromatic Flow Cytometry to Define Different Immune Cells in Chicken
3.2. The Distribution of Immune Cells in Organs of Chicken at Steady State
3.3. The Dynamic Changes of Chicken Innate Immune Cells after H7N9 Infection
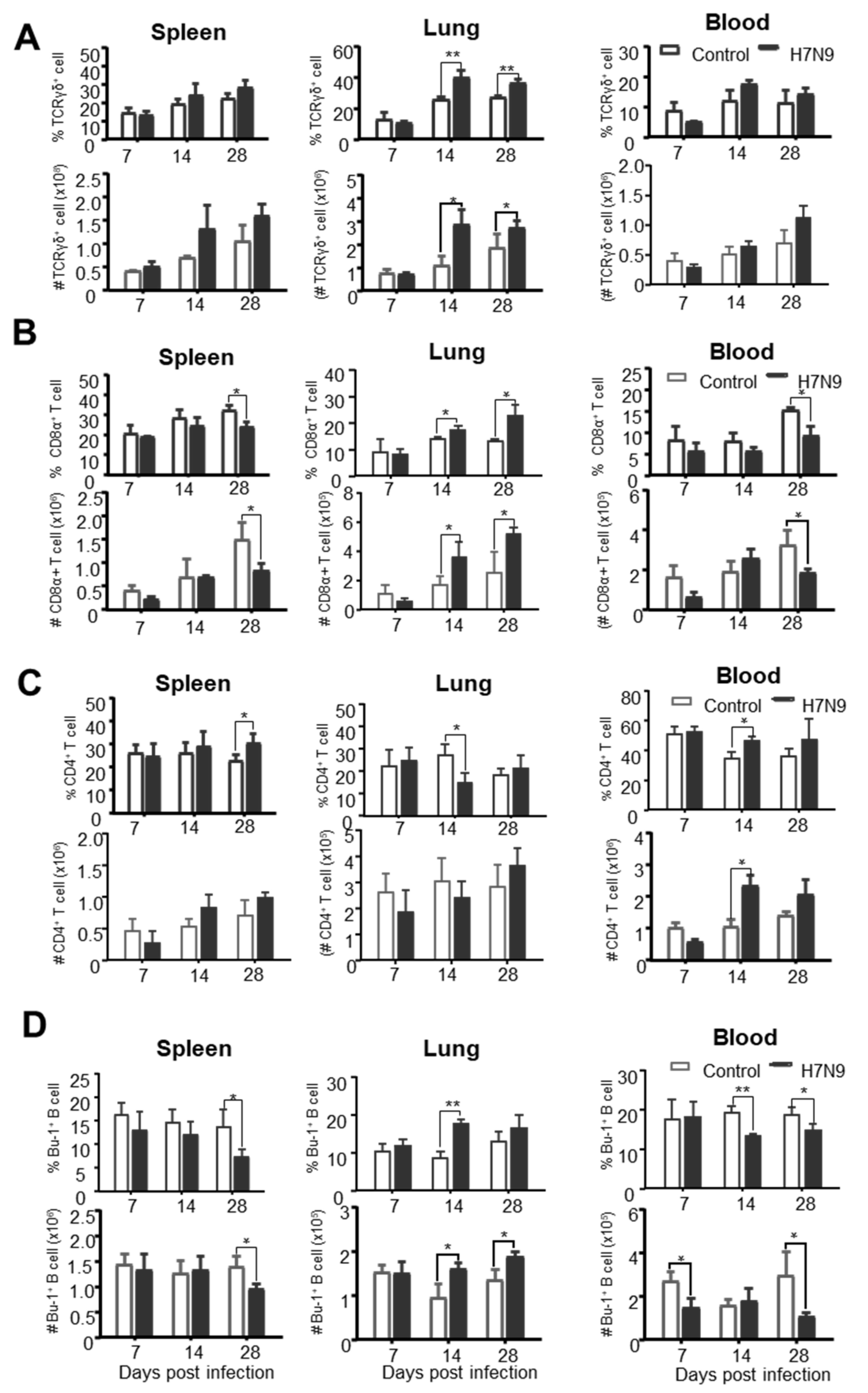
3.4. The Dynamic Changes of Chicken Adaptive Immune Cells after H7N9 Infection
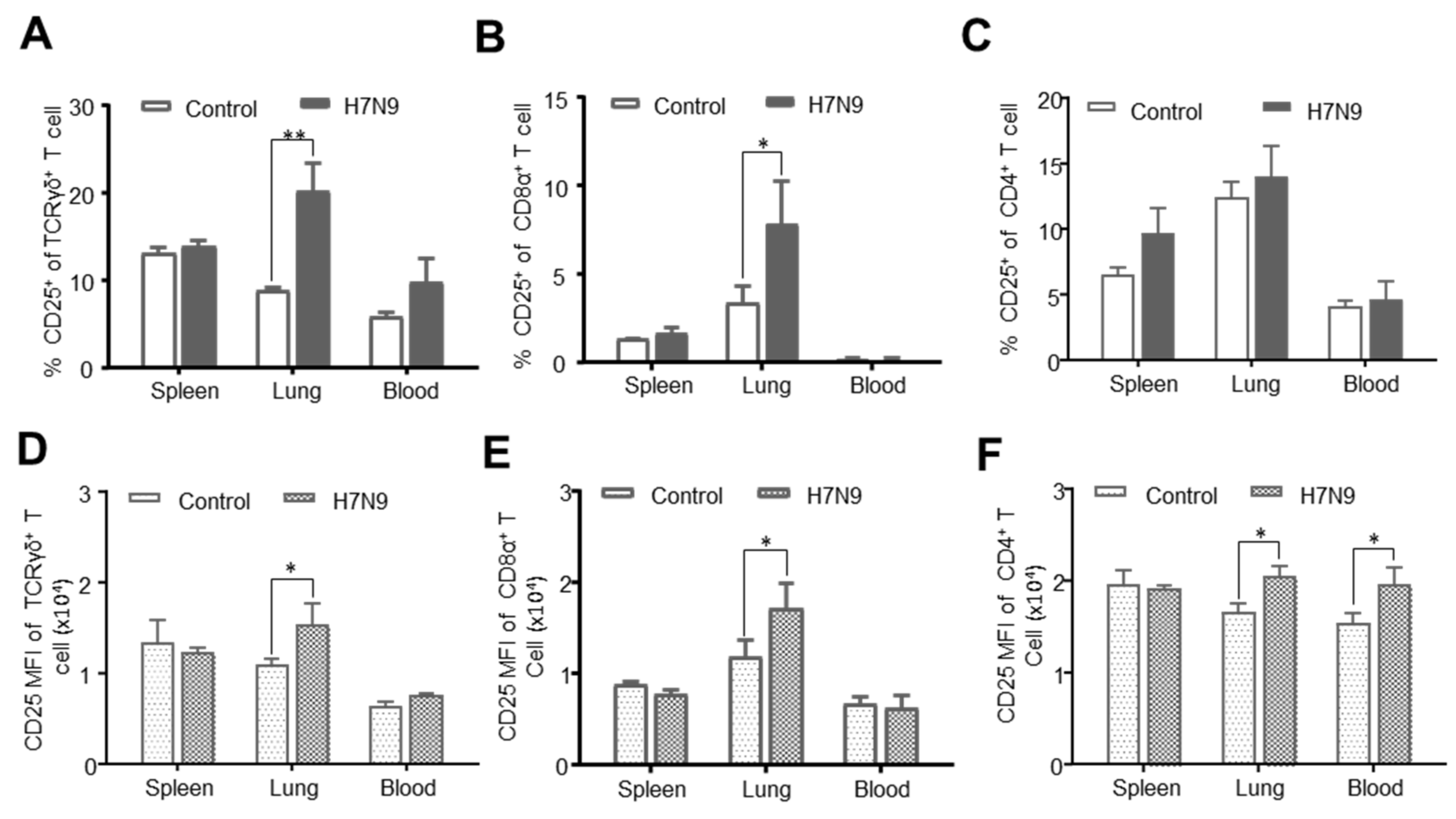
3.5. CD25 Expression on T-Cell Subsets after H7N9 Infection
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wang, X.; Jiang, H.; Wu, P.; Uyeki, M.T.; Feng, L.; Lai, S.; Wang, L.; Huo, X.; Xu, K.; Chen, E.; et al. Epidemiology of avian influenza a H7N9 virus in human beings across five epidemics in mainland China, 2013–2017: An epidemiological study of laboratory-confirmed case series. Lancet Infect. Dis. 2017, 17, 822–832. [Google Scholar] [CrossRef]
- Shi, J.; Deng, G.; Ma, S.; Zeng, X.; Yin, X.; Li, M.; Zhang, B.; Cui, P.; Chen, Y.; Yang, H.; et al. Rapid evolution of H7N9 highly pathogenic viruses that emerged in China in 2017. Cell Host Microbe 2018, 24, 558–568. [Google Scholar] [CrossRef] [Green Version]
- Jiang, W.; Hou, G.; Li, J.; Peng, C.; Wang, S.; Liu, S.; Zhuang, Q.; Yuan, L.; Yu, X.; Li, Y.; et al. Antigenic variant of highly pathogenic avian influenza A(H7N9) virus, China, 2019. Emerg. Infect. Dis. 2020, 26, 379–380. [Google Scholar] [CrossRef] [Green Version]
- Kamal, R.P.; Blanchfield, K.; Belser, J.A.; Music, N.; Tzeng, W.P.; Holiday, C.; Burroughs, A.; Sun, X.; Maines, T.R.; Levine, M.Z.; et al. Inactivated H7 influenza virus vaccines protect mice despite inducing only low levels of neutralizing antibodies. J. Virol. 2017, 91. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stadlbauer, D.; Rajabhathor, A.; Amanat, F.; Kaplan, D.; Masud, A.; Treanor, J.J.; Izikson, R.; Cox, M.M.; Nachbagauer, R.; Krammer, F. Vaccination with a recombinant H7 hemagglutinin-based influenza virus vaccine induces broadly reactive antibodies in humans. mSphere 2017, 2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blanchfield, K.; Kamal, R.P.; Tzeng, W.P.; Music, N.; Wilson, J.R.; Stevens, J.; Lipatov, A.S.; Katz, J.M.; York, I.A. Recombinant influenza H7 hemagglutinins induce lower neutralizing antibody titers in mice than do seasonal hemagglutinins. Influenza Other Respir. Viruses 2014, 8, 628–635. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Jiao, X.; Liu, X. Antibody immunity induced by H7N9 avian influenza vaccines: Evaluation criteria, affecting factors, and implications for rational vaccine design. Front. Microbiol. 2017, 8, 1898. [Google Scholar] [CrossRef] [PubMed]
- Gianchecchi, E.; Torelli, A.; Montomoli, E. The use of cell-mediated immunity for the evaluation of influenza vaccines: An upcoming necessity. Hum. Vaccines Immunother. 2019, 15, 1021–1030. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Hu, Z.; Hu, J.; Liu, D.; He, L.; Liu, J.; Gu, H.; Gan, J.; Wang, X.; Liu, X. Single immunization with newcastle disease virus-vectored H7n9 vaccine confers a complete protection against challenge with highly pathogenic avian influenza H7N9 virus. Avian Dis. 2019, 63, 61–67. [Google Scholar] [CrossRef]
- Hu, Z.; Liu, X.; Jiao, X.; Liu, X. Newcastle disease virus (Ndv) recombinant expressing the hemagglutinin of H7N9 avian influenza virus protects chickens against Ndv and highly pathogenic avian influenza a (H7n9) virus challenges. Vaccine 2017, 35, 6585–6590. [Google Scholar] [CrossRef]
- Yamayoshi, S.; Uraki, R.; Ito, M.; Kiso, M.; Nakatsu, S.; Yasuhara, A.; Oishi, K.; Sasaki, T.; Ikuta, K.; Kawaoka, Y. A broadly reactive human anti-hemagglutinin stem monoclonal antibody that inhibits influenza A virus particle release. EBioMedicine 2017, 17, 182–191. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Dong, M.; Hao, X.; Qin, A.; Shang, S. Revisiting cellular immune response to oncogenic marek’s disease virus: The rising of avian T-cell immunity. Cell. Mol. Life Sci. 2020, 77, 3103–3116. [Google Scholar] [CrossRef] [Green Version]
- Pizzolla, A.; Wakim, L.M. Memory T cell dynamics in the lung during influenza virus infection. J. Immunol. 2019, 202, 374–381. [Google Scholar] [CrossRef] [Green Version]
- Nussing, S.; Sant, S.; Koutsakos, M.; Subbarao, K.; Nguyen, T.H.O.; Kedzierska, K. Innate and adaptive T cells in influenza disease. Front. Med. 2018, 12, 34–47. [Google Scholar] [CrossRef]
- Koutsakos, M.; Kedzierska, K.; Subbarao, K. Immune responses to avian influenza viruses. J. Immunol. 2019, 202, 382–391. [Google Scholar] [CrossRef] [Green Version]
- Oslund, K.L.; Baumgarth, N. Influenza-induced innate immunity: Regulators of viral replication, respiratory tract pathology & adaptive immunity. Future Virol. 2011, 6, 951–962. [Google Scholar]
- Kim, T.S.; Hufford, M.M.; Sun, J.; Fu, Y.X.; Braciale, T.J. Antigen persistence and the control of local t cell memory by migrant respiratory dendritic cells after acute virus infection. J. Exp. Med. 2010, 207, 1161–1172. [Google Scholar] [CrossRef] [Green Version]
- Kim, T.S.; Braciale, T.J. Respiratory dendritic cell subsets differ in their capacity to support the induction of virus-specific cytotoxic Cd8+ T cell responses. PLoS ONE 2009, 4, e4204. [Google Scholar] [CrossRef] [Green Version]
- Stein-Streilein, J.; Guffee, J. In vivo treatment of mice and hamsters with antibodies to asialo Gm1 increases morbidity and mortality to pulmonary influenza infection. J. Immunol. 1986, 136, 1435–1441. [Google Scholar]
- Gazit, R.; Gruda, R.; Elboim, M.; Arnon, T.I.; Katz, G.; Achdout, H.; Hanna, J.; Qimron, U.; Landau, G.; Greenbaum, E.; et al. Lethal influenza infection in the absence of the natural killer cell receptor gene Ncr1. Nat. Immunol. 2006, 7, 517–523. [Google Scholar] [CrossRef]
- Dong, P.; Ju, X.; Yan, Y.; Zhang, S.; Cai, M.; Wang, H.; Chen, H.; Hu, Y.; Cui, L.; Zhang, J.; et al. Gammadelta T cells provide protective function in highly pathogenic avian H5n1 influenza a virus infection. Front. Immunol. 2018, 9, 2812. [Google Scholar] [CrossRef] [PubMed]
- Qin, G.; Mao, H.; Zheng, J.; Sia, S.F.; Liu, Y.; Chan, P.L.; Lam, K.T.; Peiris, J.S.; Lau, Y.L.; Tu, W. Phosphoantigen-expanded human gammadelta T cells display potent cytotoxicity against monocyte-derived macrophages infected with human and avian influenza viruses. J. Infect. Dis. 2009, 200, 858–865. [Google Scholar] [CrossRef] [PubMed]
- Seo, S.H.; Peiris, M.; Webster, R.G. Protective cross-reactive cellular immunity to lethal a/Goose/Guangdong/1/96-like H5n1 influenza virus is correlated with the proportion of pulmonary Cd8(+) T cells expressing gamma interferon. J. Virol. 2002, 76, 4886–4890. [Google Scholar] [CrossRef] [Green Version]
- Jansen, C.A.; de Geus, E.D.; van Haarlem, D.A.; van de Haar, P.M.; Löndt, B.Z.; Graham, S.P.; Göbel, T.W.; van Eden, W.; Brookes, S.M.; Vervelde, L. Differential lung Nk cell responses in avian influenza virus infected chickens correlate with pathogenicity. Sci. Rep. 2013, 3, 2478. [Google Scholar] [CrossRef] [PubMed]
- Soloski, M.J.; Chrest, F.J. Multiparameter flow cytometry for discovery of disease mechanisms in rheumatic diseases. Arthritis Rheum. 2013, 65, 1148–1156. [Google Scholar] [CrossRef] [Green Version]
- De Boever, S.; Croubels, S.; Demeyere, K.; Lambrecht, B.; De Backer, P.; Meyer, E. Flow cytometric differentiation of avian leukocytes and analysis of their intracellular cytokine expression. Avian Pathol. 2010, 39, 41–46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taebipour, M.J.; Dadras, H.; Nazifi, S.; Afsar, M.; Ansari-Lari, M. Evaluation of blood monocyte and lymphocyte population in broiler chicken after vaccination and experimental challenge with newcastle disease virus. Vet. Immunol. Immunopathol. 2017, 190, 31–38. [Google Scholar] [CrossRef]
- Dalgaard, T.S.; Norup, L.R.; Pedersen, A.R.; Handberg, K.J.; Jørgensen, P.H.; Juul-Madsen, H.R. Flow cytometric assessment of chicken T cell-mediated immune responses after newcastle disease virus vaccination and challenge. Vaccine 2010, 28, 4506–4514. [Google Scholar] [CrossRef]
- Seliger, C.; Schaerer, B.; Kohn, M.; Pendl, H.; Weigend, S.; Kaspers, B.; Hartle, S. A rapid high-precision flow cytometry based technique for total white blood cell counting in chickens. Vet. Immunol. Immunopathol. 2012, 145, 86–99. [Google Scholar] [CrossRef]
- He, L.; Liu, D.; Hu, J.; Sun, W.; Gao, R.; Shi, L.; He, D.; Li, B.; Wang, X.; Gu, M.; et al. A comprehensive comparison of the fifth-wave highly pathogenic and low-pathogenic H7n9 avian influenza viruses reveals potential threat posed by both types of viruses in mammals. Transbound. Emerg. Dis. 2018, 65, 1459–1473. [Google Scholar] [CrossRef]
- Shang, S.; Siddiqui, S.; Bian, Y.; Zhao, J.; Wang, C.R. Nonclassical Mhc Ib-restricted Cd8+ T cells recognize mycobacterium tuberculosis-derived protein antigens and contribute to protection against infection. PLoS Pathog. 2016, 12, e1005688. [Google Scholar] [CrossRef] [PubMed]
- Dalgaard, T.S.; Norup, L.R.; Rubbenstroth, D.; Wattrang, E.; Juul-Madsen, H.R. Flow cytometric assessment of antigen-specific proliferation in peripheral chicken T cells by Cfse dilution. Vet. Immunol. Immunopathol. 2010, 138, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Rothwell, L.; Young, J.R.; Kaufman, J.; Butter, C.; Kaiser, P. Generation and characterization of chicken bone marrow-derived dendritic cells. Immunology 2010, 129, 133–145. [Google Scholar] [CrossRef]
- Göbel, T.W.; Kaspers, B.; Stangassinger, M. Nk and T cells constitute two major, functionally distinct intestinal epithelial lymphocyte subsets in the chicken. Int. Immunol. 2001, 13, 757–762. [Google Scholar] [CrossRef] [Green Version]
- Qu, C.; Brinck-Jensen, N.S.; Zang, M.; Chen, K. Monocyte-derived dendritic cells: Targets as potent antigen-presenting cells for the design of vaccines against infectious diseases. Int. J. Infect. Dis. 2014, 19, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Shanmugasundaram, R.; Selvaraj, R.K. Regulatory T cell properties of chicken Cd4+Cd25+ cells. J. Immunol. 2011, 186, 1997–2002. [Google Scholar] [CrossRef] [Green Version]
- Gijzen, K.; Liu, W.M.; Visontai, I.; Oftung, F.; van der Werf, S.; Korsvold, G.E.; Pronk, I.; Aaberge, I.S.; Tütto, A.; Jankovics, I.; et al. Standardization and validation of assays determining cellular immune responses against influenza. Vaccine 2010, 28, 3416–3422. [Google Scholar] [CrossRef]
- Misumi, I.; Cook, K.D.; Mitchell, J.E.; Lund, M.M.; Vick, S.C.; Lee, R.H.; Uchimura, T.; Bergmeier, W.; Mieczkowski, P.; Pardo-Manuel de Villena, F.; et al. Identification of a locus in mice that regulates the collateral damage and lethality of virus infection. Cell Rep. 2019, 27, 1387–1396. [Google Scholar] [CrossRef] [Green Version]
- Andersen, S.H.; Vervelde, L.; Sutton, K.; Norup, L.R.; Wattrang, E.; Juul-Madsen, H.R.; Dalgaard, T.S. Quantification and phenotypic characterisation of peripheral Ifn-Γ producing leucocytes in chickens vaccinated against newcastle disease. Vet. Immunol. Immunopathol. 2017, 193–194, 18–28. [Google Scholar] [CrossRef]
- Ariaans, M.P.; van de Haar, P.M.; Lowenthal, J.W.; van Eden, W.; Hensen, E.J.; Vervelde, L. Elispot and intracellular cytokine staining: Novel assays for quantifying T Cell responses in the chicken. Dev. Comp. Immunol. 2008, 32, 1398–1404. [Google Scholar] [CrossRef] [Green Version]
- Wattrang, E.; Dalgaard, T.S.; Norup, L.R.; Kjaerup, R.B.; Lunden, A.; Juul-Madsen, H.R. Cd107a as a marker of activation in chicken cytotoxic T cells. J. Immunol. Methods 2015, 419, 35–47. [Google Scholar] [CrossRef] [PubMed]
- Kaspers, B.; Thomas, W.F.G. The avian immune system. In Encyclopedia of Immunobiology; Michael, J.H.R., Ed.; Academic Press: Oxford, UK, 2016; pp. 498–503. [Google Scholar]
- Adrian, L.S.; Thomas, W.G. Chapter 5—Avian T cells: Antigen recognition and lineages. In Avian Immunology, 2nd ed.; Karel, A., Schat, B.K., Kaiser, P., Eds.; Academic Press: Boston, MA, USA, 2014; pp. 91–102. [Google Scholar]
- Mast, J.; Goddeeris, B.M.; Peeters, K.; Vandesande, F.; Berghman, L.R. Characterisation of chicken monocytes, macrophages and interdigitating cells by the monoclonal antibody Kul01. Vet. Immunol. Immunopathol. 1998, 61, 343–357. [Google Scholar] [CrossRef]
- Van den Biggelaar, R.H.G.A.; Arkesteijn, G.J.A.; Rutten, V.P.M.G.; van Eden, W.; Jansen, C.A. In vitro chicken bone marrow-derived dendritic cells comprise subsets at different states of maturation. Front. Immunol. 2020, 11, 141. [Google Scholar] [CrossRef]
- Vu Manh, T.P.; Marty, H.; Sibille, P.; Le Vern, Y.; Kaspers, B.; Dalod, M.; Schwartz-Cornil, I.; Quere, P. Existence of conventional dendritic cells in gallus gallus revealed by comparative gene expression profiling. J. Immunol. 2014, 192, 4510–4517. [Google Scholar] [CrossRef]
- Yu, K.; Gu, M.J.; Pyung, Y.J.; Song, K.D.; Park, T.S.; Han, S.H.; Yun, C.H. Characterization of splenic Mrc1(Hi)Mhcii(Lo) and Mrc1(Lo)Mhcii(Hi) cells from the monocyte/macrophage lineage of white leghorn chickens. Vet. Res. 2020, 51, 73. [Google Scholar] [CrossRef]
- Jansen, C.A.; van de Haar, P.M.; van Haarlem, D.; van Kooten, P.; de Wit, S.; van Eden, W.; Viertlböck, B.C.; Göbel, T.W.; Vervelde, L. Identification of new populations of chicken natural killer (Nk) cells. Dev. Comp. Immunol. 2010, 34, 759–767. [Google Scholar] [CrossRef] [Green Version]
- Göbel, T.W.; Chen, C.L.; Shrimpf, J.; Grossi, C.E.; Bernot, A.; Bucy, R.P.; Auffray, C.; Cooper, M.D. Characterization of avian natural killer cells and their intracellular Cd3 protein complex. Eur. J. Immunol. 1994, 24, 1685–1691. [Google Scholar] [CrossRef] [Green Version]
- Qiang, F.; Youxiang, D. The effects of H9n2 influenza a on the immune system of broiler chickens in the Shandong province. Transbound. Emerg. Dis. 2011, 58, 145–151. [Google Scholar] [CrossRef]
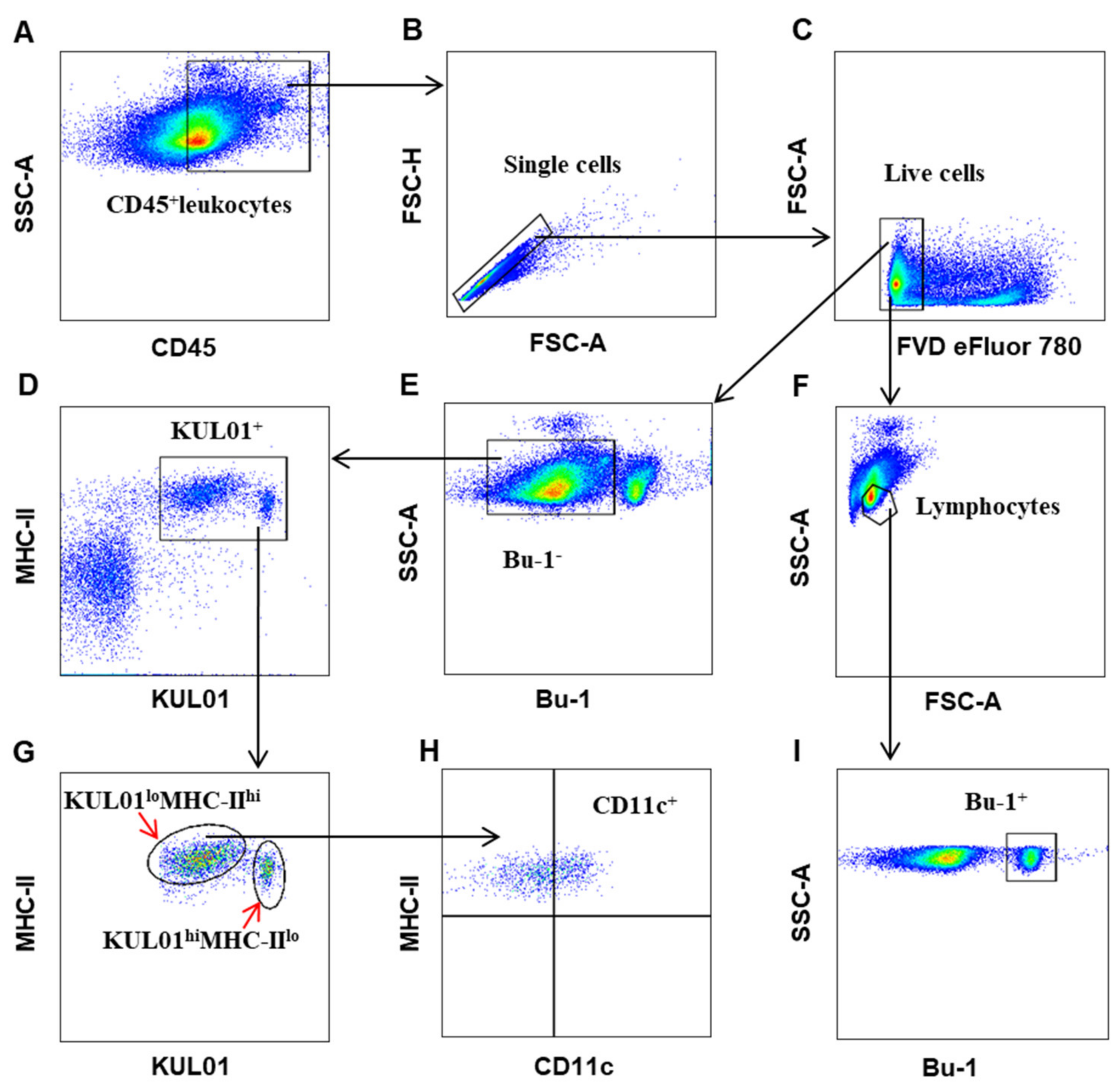
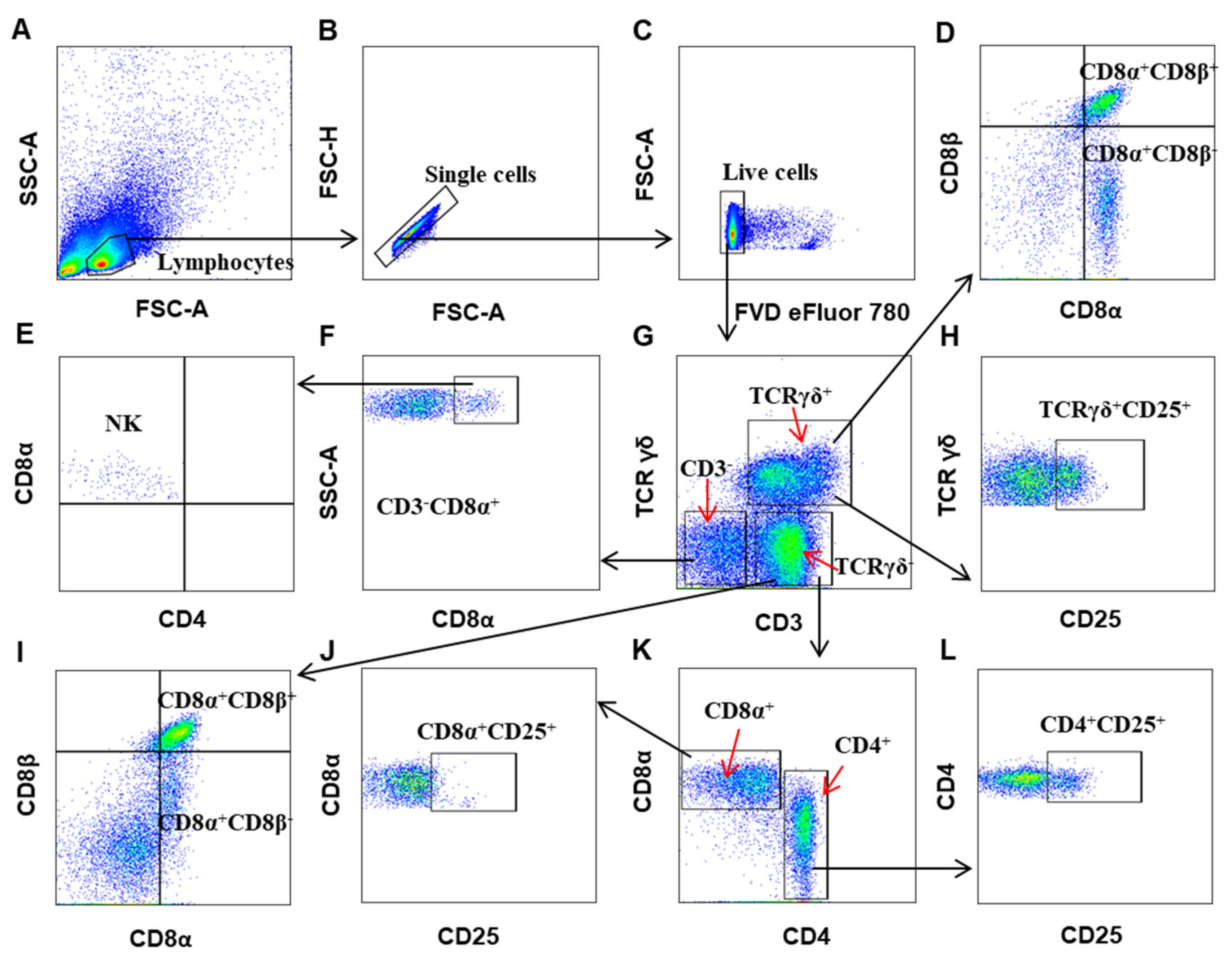
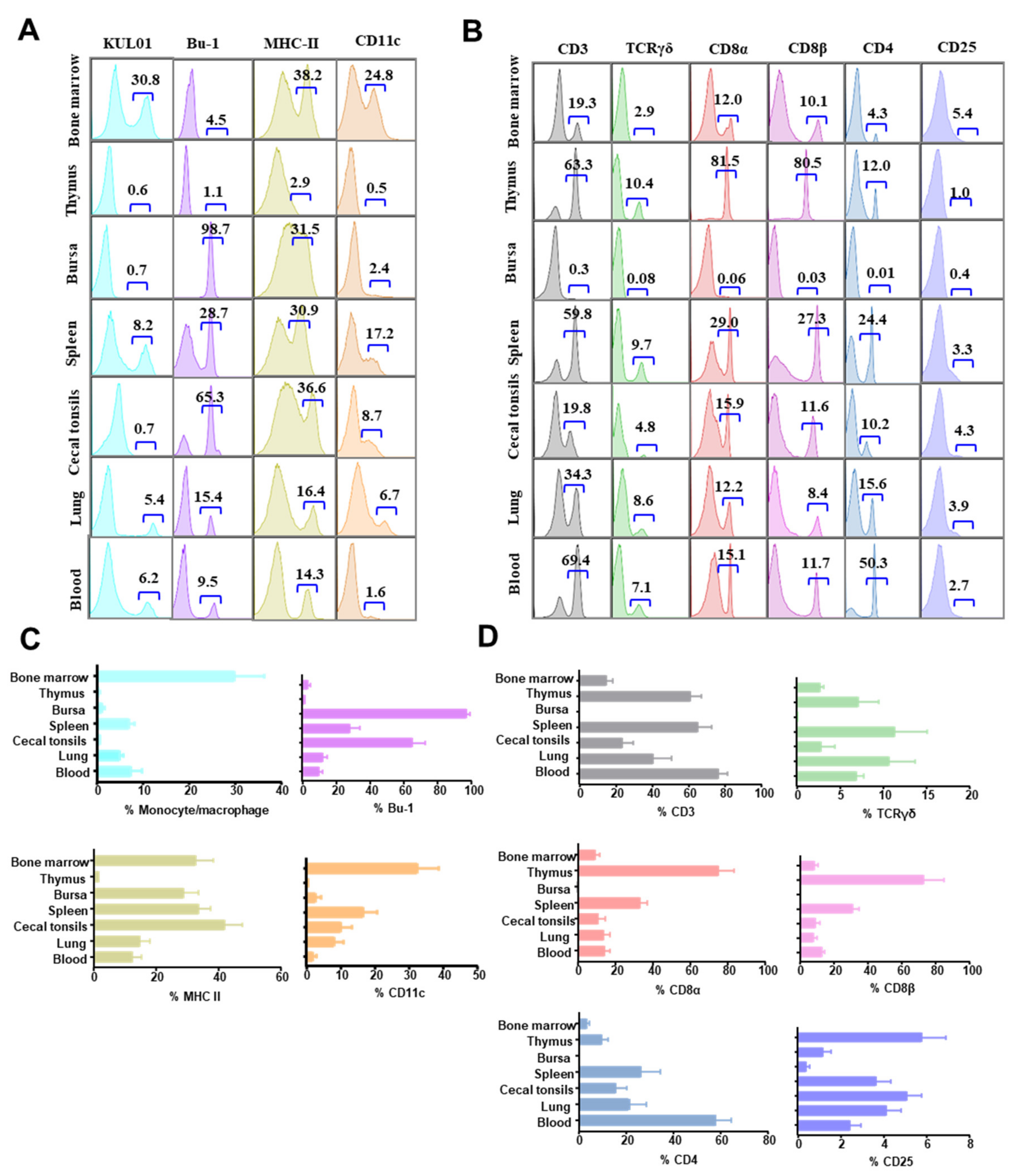
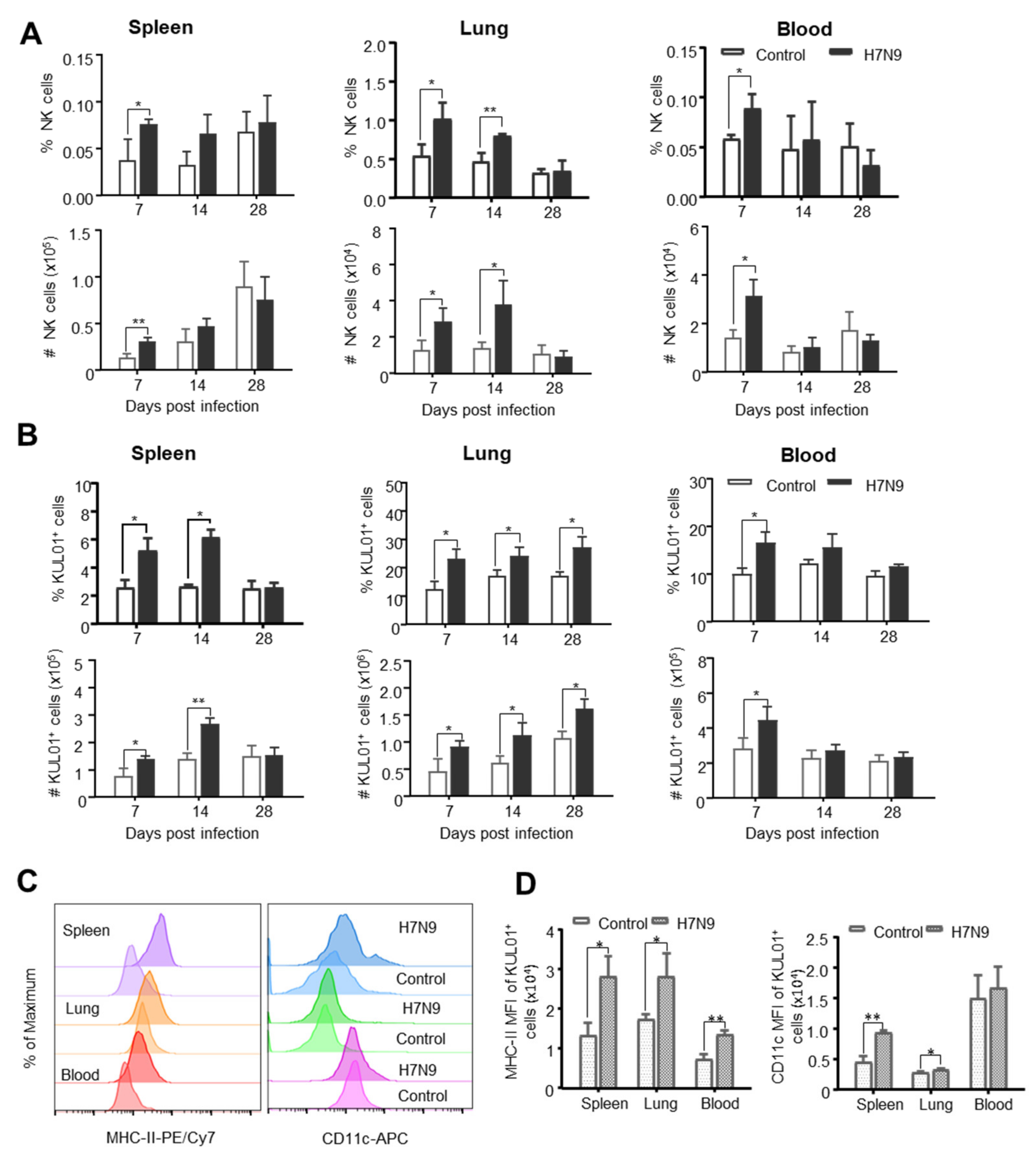
| Marker | Clone | Isotype | Conjugate | Purpose |
|---|---|---|---|---|
| Panel 1 | ||||
| CD45 | LT40 | mouse IgM | PerCP-Cy5.5 | Leukocytes |
| Bu-1 | AV20 | mouse IgG1 | FITC | B cells |
| Monocyte/macrophage | KUL01 | mouse IgG1 | PE | Monocytes and macrophages |
| MHC Class II | 2G11 | mouse IgG1 | PE-Cy7 | DC lineage marker |
| CD11c | polyclonal | rabbit IgG | APC | DC lineage marker |
| Dead Cell Stain | - | - | FVD eFluor 780 | Live cell |
| Panel 2 | ||||
| CD3 | CT-3 | mouse IgG1 | PerCP-Cy5.5 | T cells |
| TCRγδ | TCR-1 | mouse IgG1 | Biotin | γδ T cells |
| CD8α | CT-8 | mouse IgG1 | AF700 | CD8+ T cells |
| CD8β | EP42 | mouse IgG2a | PE | CD8+ T cells |
| CD4 | CT-4 | mouse IgG1 | Pacific blue | CD4+ T cells |
| CD25 | AV142 | mouse IgG1 | FITC | Activation |
| Streptavidin | - | - | BV510 | - |
| Dead Cell Stain | - | - | FVD eFluor 780 | Live cell |
| Leucocyte Type | Percentage (%) | ||||||
|---|---|---|---|---|---|---|---|
| Bone Marrow | Thymus | Bursa | Spleen | Cecal Tonsil | Lung | Blood | |
| CD3−CD8α+NK cells a | 0.5 ± 0.2 c | ND d | <0.1 | 0.3 ± 0.2 | 1.0 ± 1.1 | 0.8 ± 0.4 | 0.2 ± 0.1 |
| TCRγδ+ T cells a | 5.4 ± 2.0 | 6.9 ± 2.3 | 0.12 ± 0.03 | 12.2 ± 3.9 | 4.8 ± 1.9 | 13.6 ± 3.2 | 6.3 ± 2.3 |
| TCRγδ+CD8αβ+T cells a | 0.8 ± 0.1 | 0.7 ± 0.3 | <0.1 | 3.3 ± 2.2 | 0.4 ± 0.3 | 2.4 ± 1.3 | 0.5 ± 0.4 |
| TCRγδ+CD8αα+T cells a | 1.1 ± 0.3 | <0.1 | <0.1 | 3.8 ± 1.6 | 1.1 ± 0.8 | 2.9 ± 1.1 | 0.6 ± 0.3 |
| TCRγδ−CD3+CD8αβ+T cells a | 7.4 ± 1.6 | 43.6 ± 7.7 | <0.1 | 23.4 ± 8.6 | 8.4 ± 3.8 | 5.5 ± 1.9 | 11.7 ± 4.7 |
| TCRγδ−CD3+CD8αα+T cells a | 0.9 ± 0.5 | 13.1± 9.9 | <0.1 | 5.7 ± 2.7 | 2.4 ± 0.9 | 2.2 ± 0.9 | 0.8 ± 0.3 |
| TCRγδ−CD3+CD4+T cells a | 2.7 ± 1.1 | 5.9 ± 2.8 | <0.1 | 26.3 ± 7.8 | 10.4 ± 8.2 | 20.5 ± 7.2 | 43.8 ± 6.8 |
| TCRγδ−CD4+CD25+T cells b | 9.8 ± 4.6 | 4.9 ± 2.7 | ND d | 6.5 ± 3.5 | 8.5 ± 5.9 | 9.4 ± 2.9 | 1.8 ± 1.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hao, X.; Li, S.; Chen, L.; Dong, M.; Wang, J.; Hu, J.; Gu, M.; Wang, X.; Hu, S.; Peng, D.; et al. Establishing a Multicolor Flow Cytometry to Characterize Cellular Immune Response in Chickens Following H7N9 Avian Influenza Virus Infection. Viruses 2020, 12, 1396. https://doi.org/10.3390/v12121396
Hao X, Li S, Chen L, Dong M, Wang J, Hu J, Gu M, Wang X, Hu S, Peng D, et al. Establishing a Multicolor Flow Cytometry to Characterize Cellular Immune Response in Chickens Following H7N9 Avian Influenza Virus Infection. Viruses. 2020; 12(12):1396. https://doi.org/10.3390/v12121396
Chicago/Turabian StyleHao, Xiaoli, Shuai Li, Lina Chen, Maoli Dong, Jiongjiong Wang, Jiao Hu, Min Gu, Xiaoquan Wang, Shunlin Hu, Daxin Peng, and et al. 2020. "Establishing a Multicolor Flow Cytometry to Characterize Cellular Immune Response in Chickens Following H7N9 Avian Influenza Virus Infection" Viruses 12, no. 12: 1396. https://doi.org/10.3390/v12121396
APA StyleHao, X., Li, S., Chen, L., Dong, M., Wang, J., Hu, J., Gu, M., Wang, X., Hu, S., Peng, D., Liu, X., & Shang, S. (2020). Establishing a Multicolor Flow Cytometry to Characterize Cellular Immune Response in Chickens Following H7N9 Avian Influenza Virus Infection. Viruses, 12(12), 1396. https://doi.org/10.3390/v12121396






