Inadequate Immune Humoral Response against JC Virus in Progressive Multifocal Leukoencephalopathy Non-Survivors
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. JCV VP1 Sequencing
2.3. Neutralization Assay
2.4. Statistical Analysis
3. Results
3.1. Patients and Viral Characteristics
3.2. JCV Strain Compartmentalization and Neutralizing Response
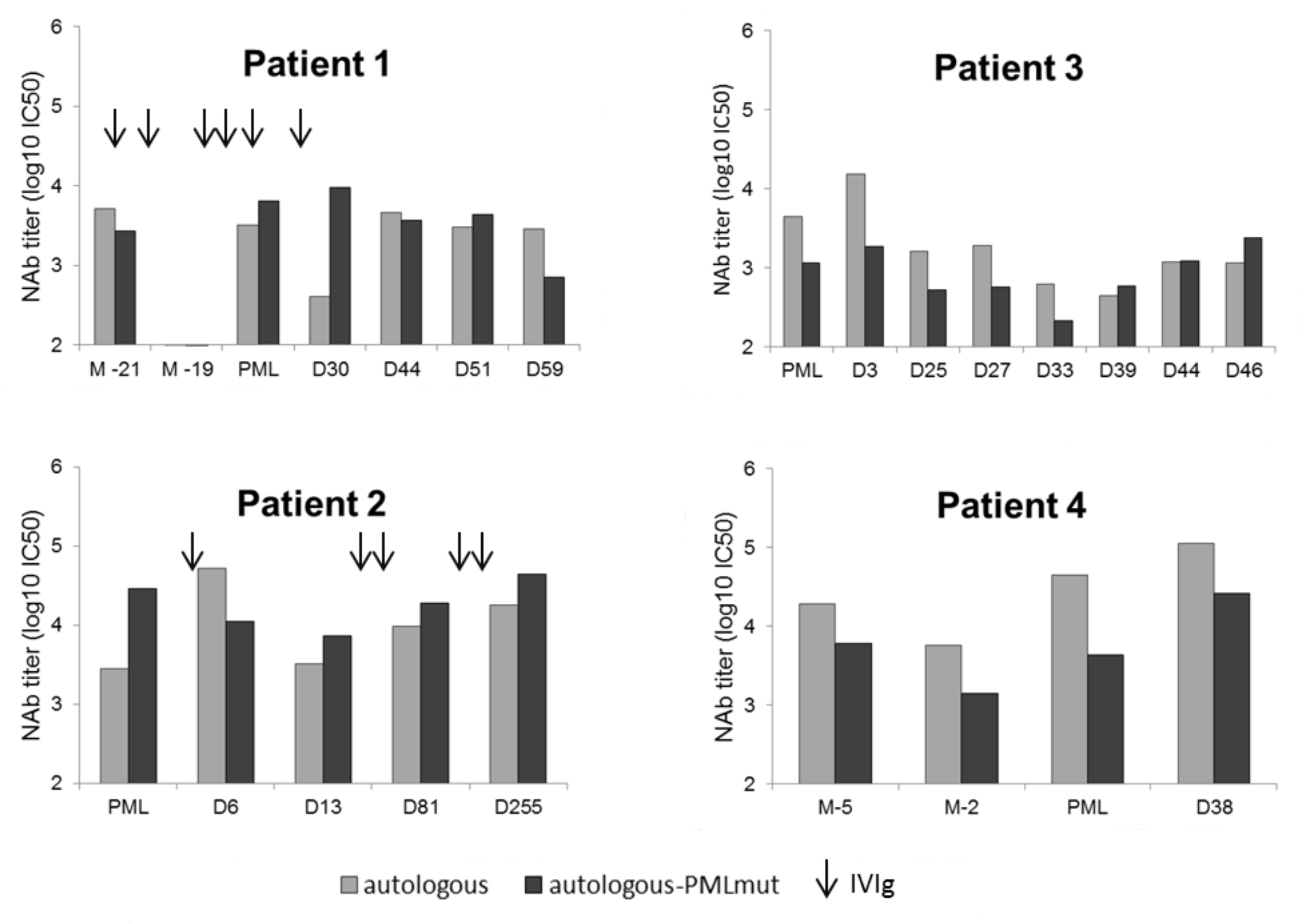
3.3. Lower NAb Titers in Non-Survivor PML Patients
3.4. Absence of “Blind Spots” in JCV Strains Neutralization Profiles at PML Onset
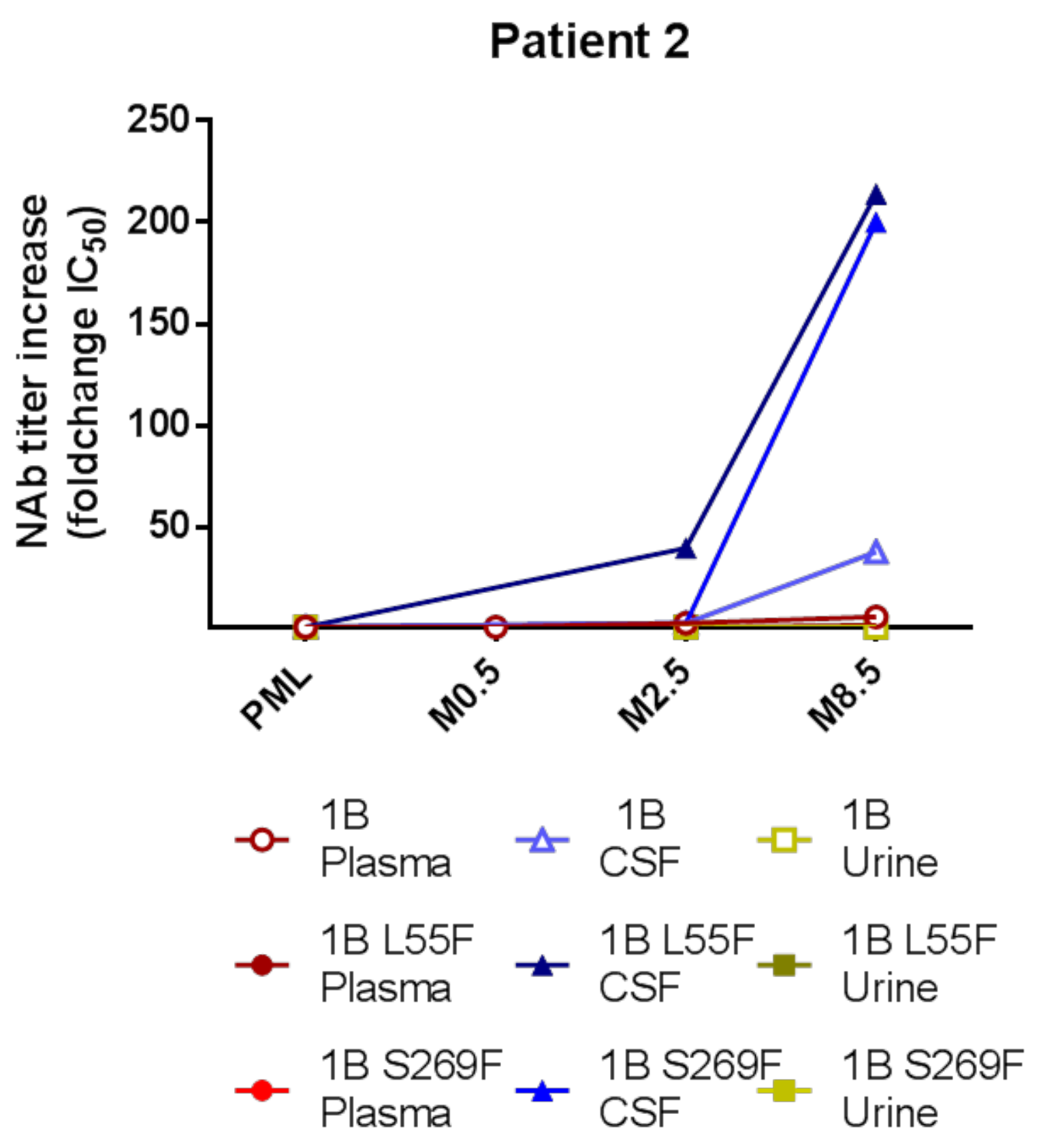
3.5. Intrathecal NAb Synthesis and PML Survival
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Barth, H.; Solis, M.; Lepiller, Q.; Sueur, C.; Soulier, E.; Caillard, S.; Stoll-Keller, F.; Fafi-Kremer, S. 45 years after the discovery of human polyomaviruses BK and JC: Time to speed up the understanding of associated diseases and treatment approaches. Crit. Rev. Microbiol. 2017, 43, 178–195. [Google Scholar] [CrossRef] [PubMed]
- Monaco, M.C.G.; Major, E.O. Immune System Involvement in the Pathogenesis of JC Virus Induced PML: What is Learned from Studies of Patients with Underlying Diseases and Therapies as Risk Factors. Front. Immunol. 2015, 6, 159. [Google Scholar] [CrossRef] [Green Version]
- Chahin, S.; Berger, J.R. A risk classification for immunosuppressive treatment-associated progressive multifocal leukoencephalopathy. J. Neurovirol. 2015, 21, 623–631. [Google Scholar] [CrossRef] [PubMed]
- Guffroy, A.; Solis, M.; Gies, V.; Dieudonne, Y.; Kuhnert, C.; Lenormand, C.; Kremer, L.; Molitor, A.; Carapito, R.; Hansmann, Y.; et al. Progressive multifocal leukoencephalopathy and sarcoidosis under interleukin 7: The price of healing. Neurol. Neuroimmunol. Neuroinflamm. 2020, 7, e862. [Google Scholar] [CrossRef]
- Hadjadj, J.; Guffroy, A.; Delavaud, C.; Taieb, G.; Meyts, I.; Fresard, A.; Streichenberger, N.; L’Honneur, A.-S.; Rozenberg, F.; D’Aveni, M.; et al. Progressive Multifocal Leukoencephalopathy in Primary Immunodeficiencies. J. Clin. Immunol. 2019, 39, 55–64. [Google Scholar] [CrossRef]
- Zerbe, C.S.; Marciano, B.E.; Katial, R.K.; Santos, C.B.; Adamo, N.; Hsu, A.P.; Hanks, M.E.; Darnell, D.N.; Quezado, M.M.; Frein, C.; et al. Progressive Multifocal Leukoencephalopathy in Primary Immune Deficiencies: Stat1 Gain of Function and Review of the Literature. Clin. Infect. Dis. 2016, 62, 986–994. [Google Scholar] [CrossRef]
- White, M.K.; Sariyer, I.K.; Gordon, J.; Delbue, S.; Pietropaolo, V.; Berger, J.R.; Khalili, K. Diagnostic Assays for Polyomavirus JC and Progressive Multifocal Leukoencephalopathy. Rev. Med. Virol. 2016, 26, 102–114. [Google Scholar] [CrossRef] [Green Version]
- Agostini, H.T.; Ryschkewitsch, C.F.; Baumhefner, R.W.; Tourtellotte, W.W.; Singer, E.J.; Komoly, S.; Stoner, G.L. Influence of JC virus coding region genotype on risk of multiple sclerosis and progressive multifocal leukoencephalopathy. J. Neurovirol. 2000, 6 (Suppl. 2), S101–S108. [Google Scholar]
- Zanotta, N.; Delbue, S.; Rossi, T.; Pelos, G.; D’Agaro, P.; Monasta, L.; Ferrante, P.; Comar, M. Molecular epidemiology of JCV genotypes in patients and healthy subjects from Northern Italy. J. Med. Virol. 2013, 85, 1286–1292. [Google Scholar] [CrossRef]
- Dubois, V.; Moret, H.; Lafon, M.E.; Brodard, V.; Icart, J.; Ruffault, A.; Guist’hau, O.; Buffet-Janvresse, C.; Abbed, K.; Dussaix, E.; et al. JC virus genotypes in France: Molecular epidemiology and potential significance for progressive multifocal leukoencephalopathy. J. Infect. Dis. 2001, 183, 213–217. [Google Scholar] [CrossRef] [Green Version]
- Gorelik, L.; Reid, C.; Testa, M.; Brickelmaier, M.; Bossolasco, S.; Pazzi, A.; Bestetti, A.; Carmillo, P.; Wilson, E.; McAuliffe, M.; et al. Progressive multifocal leukoencephalopathy (PML) development is associated with mutations in JC virus capsid protein VP1 that change its receptor specificity. J. Infect. Dis. 2011, 204, 103–114. [Google Scholar] [CrossRef] [PubMed]
- Sunyaev, S.R.; Lugovskoy, A.; Simon, K.; Gorelik, L. Adaptive mutations in the JC virus protein capsid are associated with progressive multifocal leukoencephalopathy (PML). PLoS Genet. 2009, 5, e1000368. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Plavina, T.; Subramanyam, M.; Bloomgren, G.; Richman, S.; Pace, A.; Lee, S.; Schlain, B.; Campagnolo, D.; Belachew, S.; Ticho, B. Anti-JC virus antibody levels in serum or plasma further define risk of natalizumab-associated progressive multifocal leukoencephalopathy. Ann. Neurol. 2014, 76, 802–812. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, P.; Plavina, T.; Castro, A.; Berman, M.; Jaiswal, D.; Rivas, S.; Schlain, B.; Subramanyam, M. A second-generation ELISA (STRATIFY JCVTM DxSelectTM) for detection of JC virus antibodies in human serum and plasma to support progressive multifocal leukoencephalopathy risk stratification. J. Clin. Virol. 2013, 57, 141–146. [Google Scholar] [CrossRef] [PubMed]
- Diotti, R.A.; Capra, R.; Moiola, L.; Caputo, V.; De Rossi, N.; Sangalli, F.; Martinelli, V.; Burioni, R.; Clementi, M.; Mancini, N. Divergent Trends of Anti-JCPyV Serum Reactivity and Neutralizing Activity in Multiple Sclerosis (MS) Patients during Treatment with Natalizumab. Viruses 2016, 8, 128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pavlovic, D.; Patera, A.C.; Nyberg, F.; Gerber, M.; Liu, M. Progressive Multifocal Leukeoncephalopathy Consortium Progressive multifocal leukoencephalopathy: Current treatment options and future perspectives. Ther. Adv. Neurol. Disord. 2015, 8, 255–273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walter, O.; Treiner, E.; Bonneville, F.; Mengelle, C.; Vergez, F.; Lerebours, F.; Delobel, P.; Liblau, R.; Martin-Blondel, G. Immune Checkpoint Inhibitors in PML Study Group Treatment of Progressive Multifocal Leukoencephalopathy with Nivolumab. N. Engl. J. Med. 2019, 380, 1674–1676. [Google Scholar] [CrossRef] [PubMed]
- Cortese, I.; Muranski, P.; Enose-Akahata, Y.; Ha, S.-K.; Smith, B.; Monaco, M.; Ryschkewitsch, C.; Major, E.O.; Ohayon, J.; Schindler, M.K.; et al. Pembrolizumab Treatment for Progressive Multifocal Leukoencephalopathy. N. Engl. J. Med. 2019, 380, 1597–1605. [Google Scholar] [CrossRef]
- Ray, U.; Cinque, P.; Gerevini, S.; Longo, V.; Lazzarin, A.; Schippling, S.; Martin, R.; Buck, C.B.; Pastrana, D.V. JC polyomavirus mutants escape antibody-mediated neutralization. Sci. Transl. Med. 2015, 7, 306ra151. [Google Scholar] [CrossRef] [Green Version]
- Pastrana, D.V.; Brennan, D.C.; Çuburu, N.; Storch, G.A.; Viscidi, R.P.; Randhawa, P.S.; Buck, C.B. Neutralization Serotyping of BK Polyomavirus Infection in Kidney Transplant Recipients. PLoS Pathog. 2012, 8, e1002650. [Google Scholar] [CrossRef] [Green Version]
- Pastrana, D.V.; Ray, U.; Magaldi, T.G.; Schowalter, R.M.; Cuburu, N.; Buck, C.B. BK Polyomavirus Genotypes Represent Distinct Serotypes with Distinct Entry Tropism. J. Virol. 2013, 87, 10105–10113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Solis, M.; Velay, A.; Porcher, R.; Domingo-Calap, P.; Soulier, E.; Joly, M.; Meddeb, M.; Kack-Kack, W.; Moulin, B.; Bahram, S.; et al. Neutralizing Antibody-Mediated Response and Risk of BK Virus-Associated Nephropathy. J. Am. Soc. Nephrol. JASN 2018, 29, 326–334. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferretti, F.; Bestetti, A.; Yiannoutsos, C.T.; Musick, B.S.; Gerevini, S.; Passeri, L.; Bossolasco, S.; Boschini, A.; Franciotta, D.; Lazzarin, A.; et al. Diagnostic and Prognostic Value of JC Virus DNA in Plasma in Progressive Multifocal Leukoencephalopathy. Clin. Infect. Dis. 2018, 67, 65–72. [Google Scholar] [CrossRef]
- Delbue, S.; Guerini, F.R.; Mancuso, R.; Caputo, D.; Mazziotti, R.; Saresella, M.; Ferrante, P. JC virus viremia in interferon-beta -treated and untreated Italian multiple sclerosis patients and healthy controls. J. Neurovirol. 2007, 13, 73–77. [Google Scholar] [CrossRef]
- Major, E.O.; Frohman, E.; Douek, D. JC Viremia in Natalizumab-Treated Patients with Multiple Sclerosis. N. Engl. J. Med. 2013, 368, 2240–2241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reid, C.E.; Li, H.; Sur, G.; Carmillo, P.; Bushnell, S.; Tizard, R.; McAuliffe, M.; Tonkin, C.; Simon, K.; Goelz, S.; et al. Sequencing and Analysis of JC Virus DNA From Natalizumab-Treated PML Patients. J. Infect. Dis. 2011, 204, 237–244. [Google Scholar] [CrossRef]
- Weber, F.; Goldmann, C.; Krämer, M.; Kaup, F.J.; Pickhardt, M.; Young, P.; Petry, H.; Weber, T.; Lüke, W. Cellular and humoral immune response in progressive multifocal leukoencephalopathy. Ann. Neurol. 2001, 49, 636–642. [Google Scholar] [CrossRef]
- Koralnik, I.J. Overview of the cellular immunity against JC virus in progressive multifocal leukoencephalopathy. J. Neurovirol. 2002, 8 (Suppl. 2), 59–65. [Google Scholar] [CrossRef] [Green Version]
- Eis, P.S.; Bruno, C.D.; Richmond, T.A.; Koralnik, I.J.; Hanson, B.A.; Major, E.O.; Chow, C.R.; Hendel-Chavez, H.; Stankoff, B.; Gasnault, J.; et al. Germline Genetic Risk Variants for Progressive Multifocal Leukoencephalopathy. Front. Neurol. 2020, 11, 186. [Google Scholar] [CrossRef] [Green Version]

| Patient | PML Context | JCV Genotype | JCV Mutant | Survivor | IVIg Treatment |
|---|---|---|---|---|---|
| 1 | Granulomatous disease in common variable immunodeficiency treated by rituximab (last dose 14 months before PML onset) | 1B | Double population wildtype/L55F (CSF/plasma) | no | yes |
| 2 | Waldenström disease treated by rituximab (last dose 10 months before PML onset) | 1B | none detected (urine) | yes | yes |
| 3 | HIV | 1B | S269F (CSF/plasma) | no | no |
| 4 | MPA for cardiac transplantation complicated by graft rejection treated by rituximab (last dose 9 months before PML onset) | 3B | S269F (CSF/plasma) | no | no |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Solis, M.; Guffroy, A.; Lersy, F.; Soulier, E.; Gallais, F.; Renaud, M.; Douiri, N.; Argemi, X.; Hansmann, Y.; De Sèze, J.; et al. Inadequate Immune Humoral Response against JC Virus in Progressive Multifocal Leukoencephalopathy Non-Survivors. Viruses 2020, 12, 1380. https://doi.org/10.3390/v12121380
Solis M, Guffroy A, Lersy F, Soulier E, Gallais F, Renaud M, Douiri N, Argemi X, Hansmann Y, De Sèze J, et al. Inadequate Immune Humoral Response against JC Virus in Progressive Multifocal Leukoencephalopathy Non-Survivors. Viruses. 2020; 12(12):1380. https://doi.org/10.3390/v12121380
Chicago/Turabian StyleSolis, Morgane, Aurélien Guffroy, François Lersy, Eric Soulier, Floriane Gallais, Mathilde Renaud, Nawal Douiri, Xavier Argemi, Yves Hansmann, Jérôme De Sèze, and et al. 2020. "Inadequate Immune Humoral Response against JC Virus in Progressive Multifocal Leukoencephalopathy Non-Survivors" Viruses 12, no. 12: 1380. https://doi.org/10.3390/v12121380
APA StyleSolis, M., Guffroy, A., Lersy, F., Soulier, E., Gallais, F., Renaud, M., Douiri, N., Argemi, X., Hansmann, Y., De Sèze, J., Kremer, S., & Fafi-Kremer, S. (2020). Inadequate Immune Humoral Response against JC Virus in Progressive Multifocal Leukoencephalopathy Non-Survivors. Viruses, 12(12), 1380. https://doi.org/10.3390/v12121380






