An Update of the Virion Proteome of Kaposi Sarcoma-Associated Herpesvirus
Abstract
:1. Introduction
2. Materials and Methods
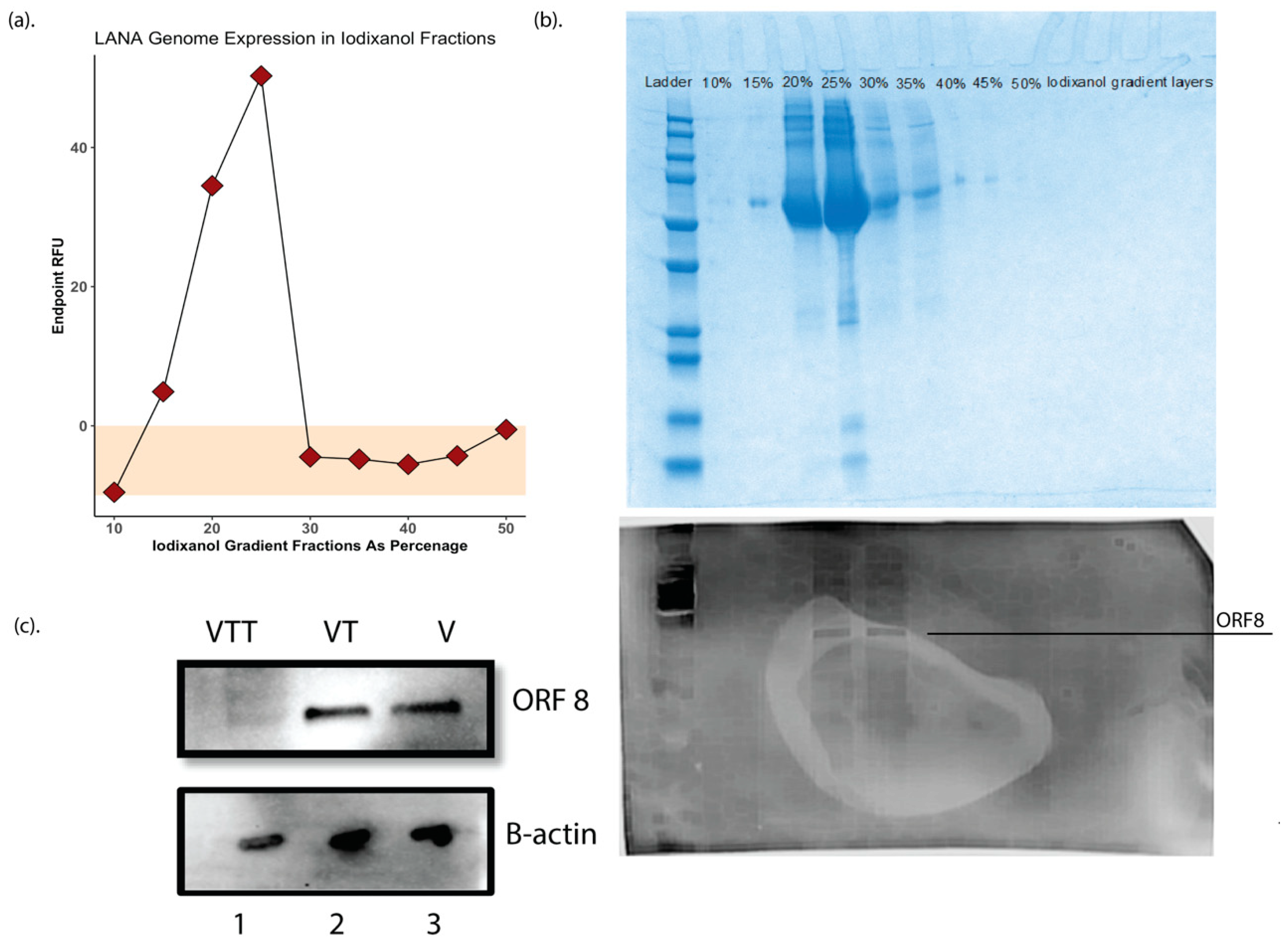
2.1. Purification of Cell-Free KSHV Virions
2.2. Trypsin and Detergent Treatment of Purified Virions
2.3. Mass Spectrometry Analysis
2.4. Antibodies and Western Blotting
3. Results
3.1. Purification of Cell Free KSHV Virions and Virion Protein Fractions
3.2. Mass Spectrometry Quality Control
3.3. KSHV Virion Protein Identification
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chang, Y.; Cesarman, E.; Pessin, M.S.; Lee, F.; Culpepper, J.; Knowles, D.M.A.; Moore, P.S. Identification of herpesvirus-like DNA sequences in AIDS-associated Kaposi’s sarcoma. Science 1994, 266, 1865–1869. [Google Scholar] [CrossRef] [Green Version]
- Moore, P.S.; Gao, S.J.; Dominguez, G.; Cesarman, E.; Lungu, O.; Knowles, D.M.; Garber, R.; Pellett, P.E.; McGeoch, D.J.; Chang, Y. Primary characterization of a herpesvirus agent associated with Kaposi’s sarcomae. J. Virol. 1996, 70, 549–558. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karass, M.; Grossniklaus, E.; Seoud, T.; Jain, S.; Goldstein, D.A. Kaposi Sarcoma Inflammatory Cytokine Syndrome (KICS): A Rare but Potentially Treatable Condition. Oncologist 2017, 22, 623–625. [Google Scholar] [CrossRef] [Green Version]
- Speck, S.H.; Ganem, D. Viral Latency and Its Regulation: Lessons from the γ-Herpesviruses. Cell Host Microbe 2010, 8, 100–115. [Google Scholar] [CrossRef] [Green Version]
- Vieira, J.; O’Hearn, P.; Kimball, L.; Chandran, B.; Corey, L. Activation of Kaposi’s Sarcoma-Associated Herpesvirus (Human Herpesvirus 8) Lytic Replication by Human Cytomegalovirus. J. Virol. 2001, 75, 1378–1386. [Google Scholar] [CrossRef] [Green Version]
- Davis, D.A.; Rinderknecht, A.S.; Zoeteweij, J.P.; Aoki, Y.; Read-Connole, E.L.; Tosato, G.; Blauvelt, A.; Yarchoan, R. Hypoxia induces lytic replication of Kaposi sarcoma–associated herpesvirus. Blood 2001, 97, 3244–3250. [Google Scholar] [CrossRef] [Green Version]
- Ye, F.; Zhou, F.; Bedolla, R.G.; Jones, T.; Lei, X.; Kang, T.; Guadalupe, M.; Gao, S.-J. Reactive Oxygen Species Hydrogen Peroxide Mediates Kaposi’s Sarcoma-Associated Herpesvirus Reactivation from Latency. PLoS Pathog. 2011, 7, e1002054. [Google Scholar] [CrossRef] [Green Version]
- Yu, F.; Harada, J.N.; Brown, H.J.; Deng, H.; Song, M.J.; Wu, T.-T.; Kato-Stankiewicz, J.; Nelson, C.G.; Vieira, J.; Tamanoi, F.; et al. Systematic Identification of Cellular Signals Reactivating Kaposi Sarcoma–Associated Herpesvirus. PLoS Pathog. 2007, 3, e44. [Google Scholar] [CrossRef] [Green Version]
- Shin, H.J.; Decotiis, J.; Giron, M.; Palmeri, D.; Lukac, D.M. Histone Deacetylase Classes I and II Regulate Kaposi’s Sarcoma-Associated Herpesvirus Reactivation. J. Virol. 2013, 88, 1281–1292. [Google Scholar] [CrossRef] [Green Version]
- Dyson, O.F.; Walker, L.R.; Whitehouse, A.; Cook, P.P.; Akula, S.M. Resveratrol Inhibits KSHV Reactivation by Lowering the Levels of Cellular EGR-1. PLoS ONE 2012, 7, e33364. [Google Scholar] [CrossRef] [Green Version]
- Grundhoff, A.; Ganem, D. Inefficient establishment of KSHV latency suggests an additional role for continued lytic replication in Kaposi sarcoma pathogenesis. J. Clin. Investig. 2004, 113, 124–136. [Google Scholar] [CrossRef]
- Naranatt, P.P.; Krishnan, H.H.; Svojanovsky, S.R.; Bloomer, C.; Mathur, S.; Chandran, B. Host Gene Induction and Transcriptional Reprogramming in Kaposi’s Sarcoma-Associated Herpesvirus (KSHV/HHV-8)-Infected Endothelial, Fibroblast, and B Cells. Cancer Res. 2004, 64, 72–84. [Google Scholar] [CrossRef] [Green Version]
- Roizman, B.; Carmichael, L.; Deinhardt, F.; De-The, G.; Nahmias, A.; Plowright, W.; Rapp, F.; Sheldrick, P.; Takahashi, M.; Wolf, K. Herpesviridae. Definition, provisional nomenclature, and taxonomy. The Herpesvirus Study Group, the International Committee on Taxonomy of Viruses. Intervirology 1981, 16, 201–217. [Google Scholar] [CrossRef]
- De Oliveira, D.U.; Ballon, G.; Cesarman, E. NF-κB signaling modulation by EBV and KSHV. Trends Microbiol. 2010, 18, 248–257. [Google Scholar] [CrossRef]
- Sathish, N.; Wang, X.; Yuan, Y. Tegument Proteins of Kaposi’s Sarcoma-Associated Herpesvirus and Related Gamma-Herpesviruses. Front. Microbiol. 2012, 3, 98. [Google Scholar] [CrossRef] [Green Version]
- Zhu, F.X.; Sathish, N.; Yuan, Y. Antagonism of Host Antiviral Responses by Kaposi’s Sarcoma-Associated Herpesvirus Tegument Protein ORF45. PLoS ONE 2010, 5, e10573. [Google Scholar] [CrossRef]
- Bergson, S.; Kalt, I.; Itzhak, I.; Brulois, K.F.; Jung, J.U.; Sarid, R. Fluorescent Tagging and Cellular Distribution of the Kaposi’s Sarcoma-Associated Herpesvirus ORF45 Tegument Protein. J. Virol. 2014, 88, 12839–12852. [Google Scholar] [CrossRef] [Green Version]
- Bechtel, J.T.; Winant, R.C.; Ganem, D. Host and Viral Proteins in the Virion of Kaposi’s Sarcoma-Associated Herpesvirus. J. Virol. 2005, 79, 4952–4964. [Google Scholar] [CrossRef] [Green Version]
- Zhu, F.X.; Chong, J.M.; Wu, L.; Yuan, Y. Virion Proteins of Kaposi’s Sarcoma-Associated Herpesvirus. J. Virol. 2005, 79, 800–811. [Google Scholar] [CrossRef] [Green Version]
- Dai, X.; Gong, D.; Wu, T.-T.; Sun, R.; Zhou, Z.H. Organization of Capsid-Associated Tegument Components in Kaposi’s Sarcoma-Associated Herpesvirus. J. Virol. 2014, 88, 12694–12702. [Google Scholar] [CrossRef] [Green Version]
- Wu, J.; Avey, D.; Li, W.; Gillen, J.; Fu, B.; Miley, W.; Whitby, D.; Zhu, F. ORF33 and ORF38 of Kaposi’s Sarcoma-Associated Herpesvirus Interact and Are Required for Optimal Production of Infectious Progeny Viruses. J. Virol. 2015, 90, 1741–1756. [Google Scholar] [CrossRef] [Green Version]
- Gong, D.; Wu, N.C.; Xie, Y.; Feng, J.; Tong, L.; Brulois, K.F.; Luan, H.; Du, Y.; Jung, J.U.; Wang, C.-Y.; et al. Kaposi’s Sarcoma-Associated Herpesvirus ORF18 and ORF30 Are Essential for Late Gene Expression during Lytic Replication. J. Virol. 2014, 88, 11369–11382. [Google Scholar] [CrossRef] [Green Version]
- Butnaru, M.; Gaglia, M.M. The Kaposi’s Sarcoma-Associated Herpesvirus Protein ORF42 Is Required for Efficient Virion Production and Expression of Viral Proteins. Viruses 2019, 11, 711. [Google Scholar] [CrossRef] [Green Version]
- Dünn-Kittenplon, D.D.; Kalt, I.; Lellouche, J.P.; Sarid, R. The KSHV portal protein ORF43 is essential for the production of infectious viral particles. Virology 2019, 529, 205–215. [Google Scholar] [CrossRef]
- Sander, G.; Konrad, A.; Thurau, M.; Wies, E.; Leubert, R.; Kremmer, E.; Dinkel, H.; Schulz, T.; Neipel, F.; Stürzl, M. Intracellular Localization Map of Human Herpesvirus 8 Proteins. J. Virol. 2007, 82, 1908–1922. [Google Scholar] [CrossRef] [Green Version]
- Majerciak, V.; Yamanegi, K.; Zheng, Z.M. Gene structure and expression of Kaposi’s sarcoma-associated herpesvirus ORF56, ORF57, ORF58, and ORF59. J. Virol. 2006, 80, 11968–11981. [Google Scholar] [CrossRef] [Green Version]
- Chow, D.-C.; He, X.; Snow, A.L.; Rose-John, S.; Garcia, K.C. Structure of an Extracellular gp130 Cytokine Receptor Signaling Complex. Science 2001, 291, 2150–2155. [Google Scholar] [CrossRef]
- den Ridder, M.; Daran-Lapujade, P.; Pabst, M. Shot-gun proteomics: Why thousands of unidentified signals matter. FEMS Yeast Res. 2020, 20, foz088. [Google Scholar] [CrossRef]
- Yates, J.R., 3rd. Mass spectrometry and the age of the proteome. J. Mass Spectrom. 1998, 33, 1–19. [Google Scholar] [CrossRef]
- Ford, T.; Graham, J.; Rickwood, D. Iodixanol: A Nonionic Iso-osmotic Centrifugation Medium for the Formation of Self-Generated Gradients. Anal. Biochem. 1994, 220, 360–366. [Google Scholar] [CrossRef]
- Garrigues, H.J.; Rubinchikova, Y.E.; DiPersio, C.M.; Rose, T.M. Integrin αVβ3 Binds to the RGD Motif of Glycoprotein B of Kaposi’s Sarcoma-Associated Herpesvirus and Functions as an RGD-Dependent Entry Receptor. J. Virol. 2008, 82, 1570–1580. [Google Scholar] [CrossRef] [Green Version]
- Perez-Riverol, Y.; Csordas, A.; Bai, J.; Bernal-Llinares, M.; Hewapathirana, S.; Kundu, D.J.; Inuganti, A.; Griss, J.; Mayer, G.; Eisenacher, M.; et al. The PRIDE database and related tools and resources in 2019: Improving support for quantification data. Nucleic Acids Res. 2019, 47, 442–450. [Google Scholar] [CrossRef]
- Deutsch, E.W.; Bandeira, N.; Sharma, V.; Perez-Riverol, Y.; Carver, J.J.; Kundu, D.J.; García-Seisdedos, D.; Jarnuczak, A.F.; Hewapathirana, S.; Pullman, B.S.; et al. The ProteomeXchange consortium in 2020: Enabling ‘big data’ approaches in proteomics. Nucleic Acids Res. 2020, 48, 1145–1152. [Google Scholar] [CrossRef] [Green Version]
- Perez-Riverol, Y.; Xu, Q.W.; Wang, R.; Uszkoreit, J.; Griss, J.; Sanchez, A.; Reisinger, F.; Csordas, A.; Ternent, T.; del Toro, N.; et al. PRIDE Inspector Toolsuite: Moving towards a universal visualization tool for proteomics data standard formats and quality assessment of ProteomeXchange datasets. Mol. Cell. Proteom. 2016, 15, 305–317. [Google Scholar] [CrossRef] [Green Version]
- Myoung, J.; Ganem, D. Generation of a doxycycline-inducible KSHV producer cell line of endothelial origin: Maintenance of tight latency with efficient reactivation upon induction. J. Virol. Methods 2011, 174, 12–21. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Xin, L.; Shan, B.; Chen, W.; Xie, M.; Yuen, D.; Zhang, W.; Zhang, Z.; Lajoie, G.A.; Ma, B. PEAKS DB:De NovoSequencing Assisted Database Search for Sensitive and Accurate Peptide Identification. Mol. Cell. Proteom. 2012, 11. [Google Scholar] [CrossRef] [Green Version]
- Katano, H.; Sato, Y.; Kurata, T.; Mori, S.; Sata, T. Expression and localization of human herpesvirus 8-encoded proteins in primary effusion lymphoma, Kaposi’s sarcoma, and multicentric Castleman’s disease. Virology 2000, 269, 335–344. [Google Scholar] [CrossRef] [Green Version]
- Russo, J.J.; Bohenzky, R.A.; Chien, M.-C.; Chen, J.; Yan, M.; Maddalena, D.; Parry, J.P.; Peruzzi, D.; Edelman, I.S.; Chang, Y.; et al. Nucleotide sequence of the Kaposi sarcoma-associated herpesvirus (HHV8). Proc. Natl. Acad. Sci. USA 1996, 93, 14862–14867. [Google Scholar] [CrossRef] [Green Version]
- Chandran, B.; Bloomer, C.; Chan, S.R.; Zhu, L.; Goldstein, E.; Horvat, R. Human herpesvirus-8 ORF K8.1 gene encodes immunogenic glycoproteins generated by spliced transcripts. Virology 1998, 249, 140–149. [Google Scholar] [CrossRef] [Green Version]
- Quiceno, J.L. Characterization of Kaposi’s Sarcoma-Associated Herpesvirus Open Reading Frames 58 and 27. Thesis, Seton Hall University, South Orange, NJ, USA, 2010. Available online: https://scholarship.shu.edu/theses/224 (accessed on 23 August 2020).
- Jenner, R.G.; Albà, M.M.; Boshoff, C.; Kellam, P. Kaposi’s sarcoma-associated herpesvirus latent and lytic gene expression as revealed by DNA arrays. J. Virol. 2001, 75, 891–902. [Google Scholar] [CrossRef] [Green Version]
- Majerciak, V.; Pripuzova, N.; McCoy, J.P.; Gao, S.J.; Zheng, Z.M. Targeted disruption of Kaposi’s sarcoma-associated herpesvirus ORF57 in the viral genome is detrimental for the expression of ORF59, K8alpha, and K8.1 and the production of infectious virus. J. Virol. 2007, 81, 1062–1071. [Google Scholar] [CrossRef] [Green Version]
- Shevchenko, A.; Tomas, H.; Havlis, J.; Olsen, J.V.; Mann, M. In-gel digestion for mass spectrometric characterization of proteins and proteomes. Nat. Protoc. 2006, 1, 2856–2860. [Google Scholar] [CrossRef]
- Zhang, Y.; Fonslow, B.R.; Shan, B.; Baek, M.-C.; Yates, J.R. Protein Analysis by Shotgun/Bottom-up Proteomics. Chem. Rev. 2013, 113, 2343–2394. [Google Scholar] [CrossRef] [Green Version]
- Thakur, S.S.; Geiger, T.; Chatterjee, B.; Bandilla, P.; Fröhlich, F.; Cox, J.; Mann, M. Deep and highly sensitive proteome coverage by LC-MS/MS without prefractionation. Mol Cell Proteom. 2011, 10. [Google Scholar] [CrossRef] [Green Version]
- Brulois, K.F.; Chang, H.; Lee, A.S.-Y.; Ensser, A.; Wong, L.-Y.; Toth, Z.; Lee, S.H.; Lee, H.-R.; Myoung, J.; Ganem, D.; et al. Construction and manipulation of a new Kaposi’s sarcoma-associated herpesvirus bacterial artificial chromosome clone. J. Virol. 2012, 86, 9708–9720. [Google Scholar] [CrossRef] [Green Version]
- Borza, C.M.; Hutt-Fletcher, L.M. Alternate replication in B cells and epithelial cells switches tropism of Epstein-Barr virus. Nat. Med. 2002, 8, 594–599. [Google Scholar] [CrossRef]
- Nishimura, M.; Watanabe, T.; Yagi, S.; Yamanaka, T.; Fujimuro, M. Kaposi’s sarcoma-associated herpesvirus ORF34 is essential for late gene expression and virus production. Sci. Rep. 2017, 7, 329. [Google Scholar] [CrossRef] [Green Version]
- Ohno, S.; Steer, B.; Sattler, C.; Adler, H. ORF23 of murine gammaherpesvirus 68 is non-essential for in vitro and in vivo infection. J. Gen. Virol. 2012, 93 Pt 5, 1076–1080. [Google Scholar] [CrossRef]
- Bergson, S.; Itzhak, I.; Wasserman, T.; Gelgor, A.; Kalt, I.; Sarid, R. The Kaposi’s-sarcoma-associated herpesvirus orf35 gene product is required for efficient lytic virus reactivation. Virology 2016, 499, 91–98. [Google Scholar] [CrossRef]
- Hikita, S.I.; Yanagi, Y.; Ohno, S. Murine gammaherpesvirus 68 ORF35 is required for efficient lytic replication and latency. J. Gen. Virol. 2015, 96, 3624–3634. [Google Scholar] [CrossRef]
- Qi, J.; Han, C.; Gong, D.; Liu, P.; Zhou, S.; Deng, H. Murine Gammaherpesvirus 68 ORF48 Is an RTA-Responsive Gene Product and Functions in both Viral Lytic Replication and Latency during In Vivo Infection. J. Virol. 2015, 89, 5788–5800. [Google Scholar] [CrossRef] [Green Version]
- Wu, F.Y.; Ahn, J.-H.; Alcendor, D.J.; Jang, W.-J.; Xiao, J.; Hayward, S.D.; Hayward, G.S. Origin-independent assembly of Kaposi’s sarcoma-associated herpesvirus DNA replication compartments in transient cotransfection assays and association with the ORF-K8 protein and cellular PML. J. Virol. 2001, 75, 1487–1506. [Google Scholar] [CrossRef] [Green Version]
- Holzerlandt, R.; Orengo, C.; Kellam, P.; Albà, M.M. Identification of new herpesvirus gene homologs in the human genome. Genome Res. 2002, 12, 1739–1748. [Google Scholar] [CrossRef] [Green Version]
- Chang, Y.; Moore, P.S.; Talbot, S.J.; Boshoff, C.; Zarkowska, T.; Godden-Kent, D.; Paterson, H.; Weiss, R.A.; Mittnacht, S. Cyclin encoded by KS herpesvirus. Nature 1996, 382, 410. [Google Scholar] [CrossRef]
- Li, M.; Lee, H.; Yoon, D.W.; Albrecht, J.C.; Fleckenstein, B.; Neipel, F.; Jung, J.U. Kaposi’s sarcoma-associated herpesvirus encodes a functional cyclin. J. Virol. 1997, 71, 1984–1991. [Google Scholar] [CrossRef] [Green Version]
- Moore, P.S.; Boshoff, C.; Weiss, R.A.; Chang, Y. Molecular mimicry of human cytokine and cytokine response pathway genes by KSHV. Science 1996, 274, 1739–1744. [Google Scholar] [CrossRef]
- Jones, T.; Ramos da Silva, S.; Bedolla, R.; Ye, F.; Zhou, F.; Gao, S.J. Viral cyclin promotes KSHV-induced cellular transformation and tumorigenesis by overriding contact inhibition. Cell Cycle 2014, 13, 845–858. [Google Scholar] [CrossRef] [Green Version]
- Godden-Kent, D.; Talbot, S.J.; Boshoff, C.; Chang, Y.; Moore, P.; Weiss, R.A.; Mittnacht, S. The cyclin encoded by Kaposi’s sarcoma-associated herpesvirus stimulates cdk6 to phosphorylate the retinoblastoma protein and histone H1. J. Virol. 1997, 71, 4193–4198. [Google Scholar] [CrossRef] [Green Version]
- Swanton, C.; Mann, D.J.; Fleckenstein, B.; Neipel, F.; Peters, G.; Jones, N. Herpes viral cyclin/Cdk6 complexes evade inhibition by CDK inhibitor proteins. Nature 1997, 390, 184–187. [Google Scholar] [CrossRef]
- Chang, P.C.; Li, M. Kaposi’s sarcoma-associated herpesvirus K-cyclin interacts with Cdk9 and stimulates Cdk9-mediated phosphorylation of p53 tumor suppressor. J. Virol. 2008, 82, 278–290. [Google Scholar] [CrossRef] [Green Version]
- Neipel, F.; Albrecht, J.C.; Ensser, A.; Huang, Y.Q.; Li, J.J.; Friedman-Kien, A.E.; Fleckenstein, B. Human herpesvirus 8 encodes a homolog of interleukin-6. J. Virol. 1997, 71, 839–842. [Google Scholar] [CrossRef] [Green Version]
- Sakakibara, S.; Tosato, G. Viral interleukin-6: Role in Kaposi’s sarcoma-associated herpesvirus: Associated malignancies. J. Interferon Cytokine Res. 2011, 31, 791–801. [Google Scholar] [CrossRef] [Green Version]
- Meads, M.B.; Medveczky, P.G. Kaposi’s sarcoma-associated herpesvirus-encoded viral interleukin-6 is secreted and modified differently than human interleukin-6: Evidence for a unique autocrine signaling mechanism. J. Biol. Chem. 2004, 279, 51793–51803. [Google Scholar] [CrossRef] [Green Version]
- Giffin, L.; West, J.A.; Damania, B. Kaposi’s Sarcoma-Associated Herpesvirus Interleukin-6 Modulates Endothelial Cell Movement by Upregulating Cellular Genes Involved in Migration. mBio 2015, 6, e01499-15. [Google Scholar] [CrossRef] [Green Version]
- Lorenzo, M.E.; Jung, J.U.; Ploegh, H.L. Kaposi’s sarcoma-associated herpesvirus K3 utilizes the ubiquitin-proteasome system in routing class major histocompatibility complexes to late endocytic compartments. J. Virol. 2002, 76, 5522–5531. [Google Scholar] [CrossRef] [Green Version]
- Lang, S.M.; Bynoe, M.O.; Karki, R.; Tartell, M.A.; Means, R.E. Kaposi’s sarcoma-associated herpesvirus K3 and K5 proteins down regulate both DC-SIGN and DC-SIGNR. PLoS ONE 2013, 8, e58056. [Google Scholar] [CrossRef]
- Coscoy, L.; Ganem, D. Kaposi’s sarcoma-associated herpesvirus encodes two proteins that block cell surface display of MHC class I chains by enhancing their endocytosis. Proc. Natl. Acad. Sci. USA 2000, 97, 8051–8056. [Google Scholar] [CrossRef] [Green Version]
- Brulois, K.; Toth, Z.; Wong, L.-Y.; Feng, P.; Gao, S.-J.; Ensser, A.; Jung, J.U. Kaposi’s sarcoma-associated herpesvirus K3 and K5 ubiquitin E3 ligases have stage-specific immune evasion roles during lytic replication. J. Virol. 2014, 88, 9335–9349. [Google Scholar] [CrossRef] [Green Version]
- Pozharskaya, V.P.; Weakland, L.L.; Zimring, J.C.; Krug, L.T.; Unger, E.R.; Neisch, A.; Joshi, H.; Inoue, N.; Offermann, M.K. Short duration of elevated vIRF-1 expression during lytic replication of human herpesvirus 8 limits its ability to block antiviral responses induced by alpha interferon in BCBL-1 cells. J. Virol. 2004, 78, 6621–6635. [Google Scholar] [CrossRef] [Green Version]
- Gao, S.J.; Boshoff, C.; Jayachandra, S.; Weiss, R.A.; Chang, Y.; Moore, P.S. KSHV ORF K9 (vIRF) is an oncogene which inhibits the interferon signaling pathway. Oncogene 1997, 15, 1979–1985. [Google Scholar] [CrossRef] [Green Version]
- Wies, E.; Mori, Y.; Hahn, A.; Kremmer, E.; Stürzl, M.; Fleckenstein, B.; Neipel, F. The viral interferon-regulatory factor-3 is required for the survival of KSHV-infected primary effusion lymphoma cells. Blood J. Am. Soc. Hematol. 2008, 111, 320–327. [Google Scholar] [CrossRef]
- Schmidt, K.; Wies, E.; Neipel, F. Kaposi’s sarcoma-associated herpesvirus viral interferon regulatory factor 3 inhibits gamma interferon and major histocompatibility complex class II expression. J. Virol. 2011, 85, 4530–4537. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.-R.; Doğanay, S.; Chung, B.; Toth, Z.; Brulois, K.; Lee, S.; Kanketayeva, Z.; Feng, P.; Ha, T.; Jung, J.U. Kaposi’s sarcoma-associated herpesvirus viral interferon regulatory factor 4 (vIRF4) targets expression of cellular IRF4 and the Myc gene to facilitate lytic replication. J. Virol. 2014, 88, 2183–2194. [Google Scholar] [CrossRef] [Green Version]
- Hwang, S.W.; Kim, D.; Jung, J.U.; Lee, H.R. KSHV-encoded viral interferon regulatory factor 4 (vIRF4) interacts with IRF7 and inhibits interferon alpha production. Biochem. Biophys. Res. Commun. 2017, 486, 700–705. [Google Scholar] [CrossRef] [Green Version]
- Zhu, F.X.; King, S.M.; Smith, E.J.; Levy, D.E.; Yuan, Y. A Kaposi’s sarcoma-associated herpesviral protein inhibits virus-mediated induction of type I interferon by blocking IRF-7 phosphorylation and nuclear accumulation. Proc. Natl. Acad. Sci. USA 2002, 99, 5573–5578. [Google Scholar] [CrossRef] [Green Version]
- Paulose-Murphy, M.; Ha, N.-K.; Xiang, C.; Chen, Y.; Gillim, L.; Yarchoan, R.; Meltzer, P.; Bittner, M.; Trent, J.; Zeichner, S.L. Transcription Program of Human Herpesvirus 8 (Kaposi’s Sarcoma-Associated Herpesvirus). J. Virol. 2001, 75, 4843–4853. [Google Scholar] [CrossRef] [Green Version]



| Gene | Protein | aa Size | Previous Report by MS | Number of Unique Peptide Hits via Trypsin Digest | Number of Unique Peptide Hits via Chymotrypsin Digest |
|---|---|---|---|---|---|
| ORF6 | Major DNA-binding protein (MBP) | 1133 | Zhu et al. [19] | 7 | 6 |
| ORF7 | Tripartite terminase subunit 1 (TRM1) | 695 | Zhu et al. [19] | 6 | 3 |
| ORF8 | Envelope glycoprotein B (gB) | 845 | Zhu et al. [19], Bechtel et al. [18] | 10 | 20 |
| ORF11 | ORF11 | 407 | Zhu et al. [19] | 9 | 5 |
| ORF17 | Capsid Scaffolding protein | 534 | Zhu et al. [19] | 3 | 3 |
| ORF21 | Thymidine kinase | 580 | Zhu et al. [19], Bechtel et al. [18] | 8 | 3 |
| ORF22 | Envelope glycoprotein H (gH) | 730 | Zhu et al. [19], Bechtel et al. [18] | 7 | 5 |
| ORF24 | ORF24 | 752 | Bechtel et al. [18] | 4 | 7 |
| ORF25 | Major capsid protein (MCP) | 1376 | Zhu et al. [19], Bechtel et al. [18] | 5 | 6 |
| ORF26 | Triplex capsid protein 2 (TRX-2) | 305 | Zhu et al. [19], Bechtel et al. [18] | 5 | 3 |
| ORF27 | ORF27 | 290 | Zhu et al. [19] | 6 | 4 |
| ORF28 | ORF28 | 102 | Zhu et al. [19] | 2 | 3 |
| ORF33 | Cytoplasmic envelopment protein 2 (CEP-2) | 334 | Zhu et al. [19], Bechtel et al. [18] | 7 | 7 |
| ORF39 | Envelope glycoprotein M (gM) | 400 | Zhu et al. [19] | 6 | 3 |
| ORF45 | ORF45 | 407 | Zhu et al. [19] | 5 | 4 |
| ORF47 | Envelope glycoprotein L (gL) | 528 | Zhu et al. [19] | 5 | 3 |
| ORF52 | ORF52 | 131 | Zhu et al. [19] | 9 | 5 |
| ORF53 | Envelope glycoprotein N (gN) | 110 | Zhu et al. [19] | 1 | 2 |
| ORF62 | Triplex capsid protein 1 (TRX-1) | 331 | Zhu et al. [19], Bechtel et al. [18] | 4 | 3 |
| ORF63 | Inner tegument protein | 927 | Zhu et al. [19], Bechtel et al. [18] | 7 | 5 |
| ORF64 | Large tegument protein deneddylase | 2635 | Zhu et al. [19] | 6 | 12 |
| ORF65 | Small capsomere-interacting protein (SCP) | 170 | Zhu et al. [19] | 4 | 3 |
| ORF68 | Packaging protein UL32 homolog | 545 | Zhu et al. [19] | 3 | 4 |
| ORF75 | ORF75 | 1296 | Zhu et al. [19], Bechtel et al. [18] | 8 | 6 |
| ORF K8.1 | gp35/37 | 228 | Zhu et al. [19] | 4 | 4 |
| Gene | Protein | aa Size | Previous Report by MS | Unique Peptide Hits via Trypsin Digest | Unique Peptide Hits via Chymotrypsin Digest |
|---|---|---|---|---|---|
| ORF18 | Protein UL79 homolog | 257 | Gong et al. [22] | 4 | 4 |
| ORF32 | Capsid vertex component 1 (CVC-1) | 454 | Dai et al. [20] | 7 | 4 |
| ORF38 | Cytoplasmic envelopment protein 3 (CEP-3) | 61 | Wu et al. [21] | 4 | 3 |
| ORF42 | Cytoplasmic envelopment protein 1 (CEP-1) | 278 | Butnaru et al. [23] | 3 | 3 |
| ORF43 | Portal Protein | 605 | Dünn-Kittenplon et al. [24] | 6 | 5 |
| ORF48 | ORF48 | 402 | Sander et al. [25] | 6 | 3 |
| ORF56 | DNA primase | 843 | Majerciak. [26] | 6 | 4 |
| ORF K2 | Viral Interleukin 6 Homolog | 204 | Katano, H. et al. [37] | 12 | 7 |
| Gene | Protein | aa Size | Unique Peptide Hits via Trypsin Digest | −10lgP (Trypsin) | Unique Peptide Hits via Chymotrypsin Digest | −10lgP (Chymotrypsin) |
|---|---|---|---|---|---|---|
| ORF9 | DPOL | 1012 | 6 | 45.15 | 4 | 34.08 |
| ORF23 | ORF23 | 404 | 6 | 26.45 | 4 | 32.21 |
| ORF35 | ORF35 | 150 | 4 | 26.23 | 3 | 37.48 |
| ORF58 | ORF58 | 357 | 5 | 42.45 | 4 | 41.66 |
| ORF72 | viral cyclin homolog | 257 | 4 | 42.46 | 4 | 45.19 |
| ORF K3 | E3 ubiquitin-protein ligase MIR1 | 333 | 5 | 35.01 | 3 | 32.7 |
| vIRF-1 | Viral IRF-like protein 1 | 449 | 5 | 39.12 | 4 | 26.26 |
| vIRF-3 | Viral IRF-like protein 3 | 566 | 7 | 27.51 | 3 | 26.50 |
| vIRF-4 | Viral IRF-like protein 4 | 911 | 11 | 37.95 | 5 | 30.02 |
| ORF44 | DNA Replication helicase | 788 | 0 | N/A | 5 | 35.06 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nabiee, R.; Syed, B.; Ramirez Castano, J.; Lalani, R.; Totonchy, J.E. An Update of the Virion Proteome of Kaposi Sarcoma-Associated Herpesvirus. Viruses 2020, 12, 1382. https://doi.org/10.3390/v12121382
Nabiee R, Syed B, Ramirez Castano J, Lalani R, Totonchy JE. An Update of the Virion Proteome of Kaposi Sarcoma-Associated Herpesvirus. Viruses. 2020; 12(12):1382. https://doi.org/10.3390/v12121382
Chicago/Turabian StyleNabiee, Ramina, Basir Syed, Jesus Ramirez Castano, Rukhsana Lalani, and Jennifer E. Totonchy. 2020. "An Update of the Virion Proteome of Kaposi Sarcoma-Associated Herpesvirus" Viruses 12, no. 12: 1382. https://doi.org/10.3390/v12121382
APA StyleNabiee, R., Syed, B., Ramirez Castano, J., Lalani, R., & Totonchy, J. E. (2020). An Update of the Virion Proteome of Kaposi Sarcoma-Associated Herpesvirus. Viruses, 12(12), 1382. https://doi.org/10.3390/v12121382








