Achimota Pararubulavirus 3: A New Bat-Derived Paramyxovirus of the Genus Pararubulavirus
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture Conditions
2.2. Urine Samples
2.3. Isolation Methods
2.4. Isolate Propagation for Genome Sequencing and Electron Microscopy
2.5. Genomic Sequencing and Bioinformatic Analysis
2.6. Electron Microscopy
3. Results
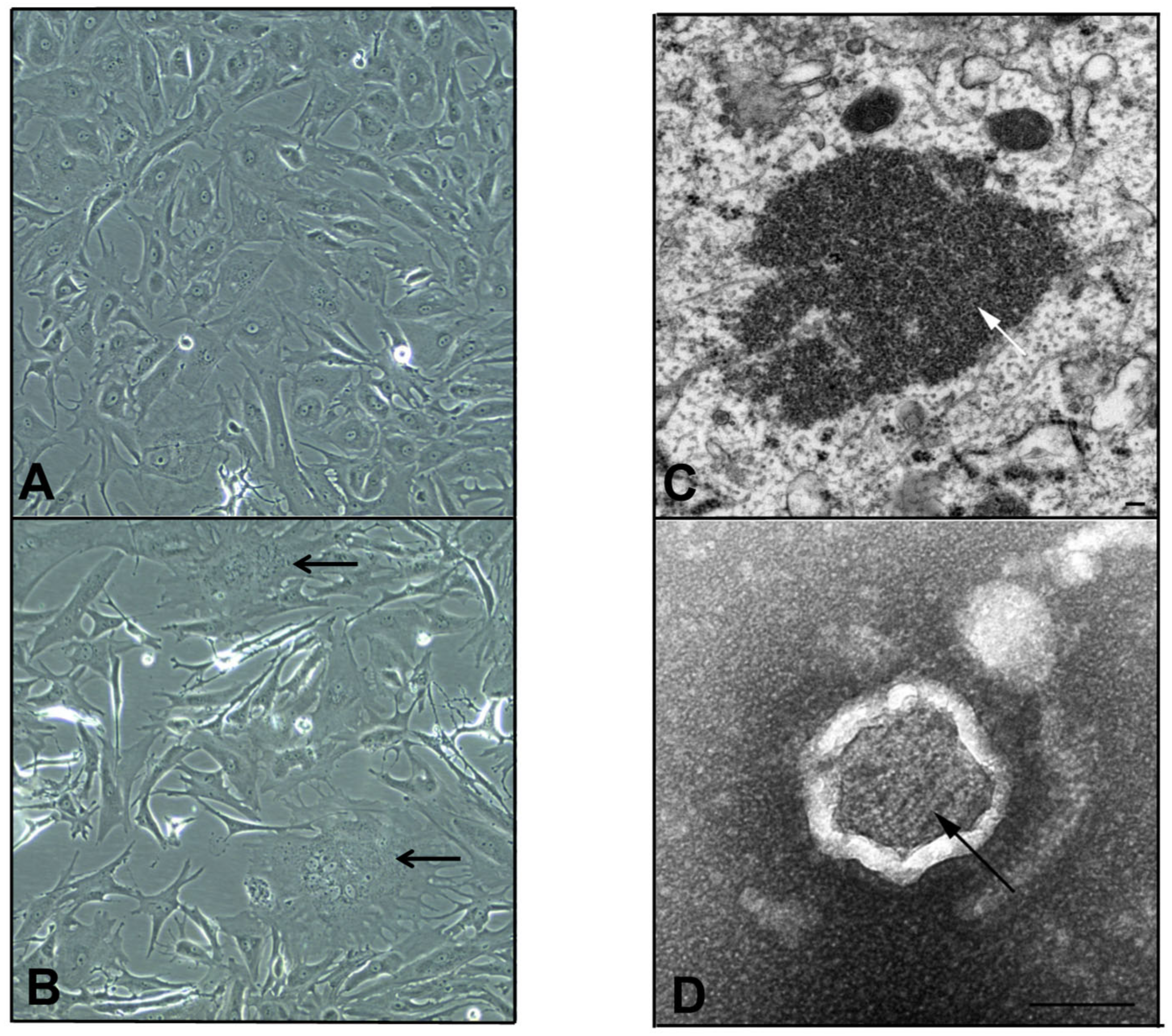
3.1. Isolation of a Novel Paramyxovirus Isolate Using Primary Bat Kidney Cells
3.2. AchPV3 Grows on Both Primary Bat Kidney and Vero Cells
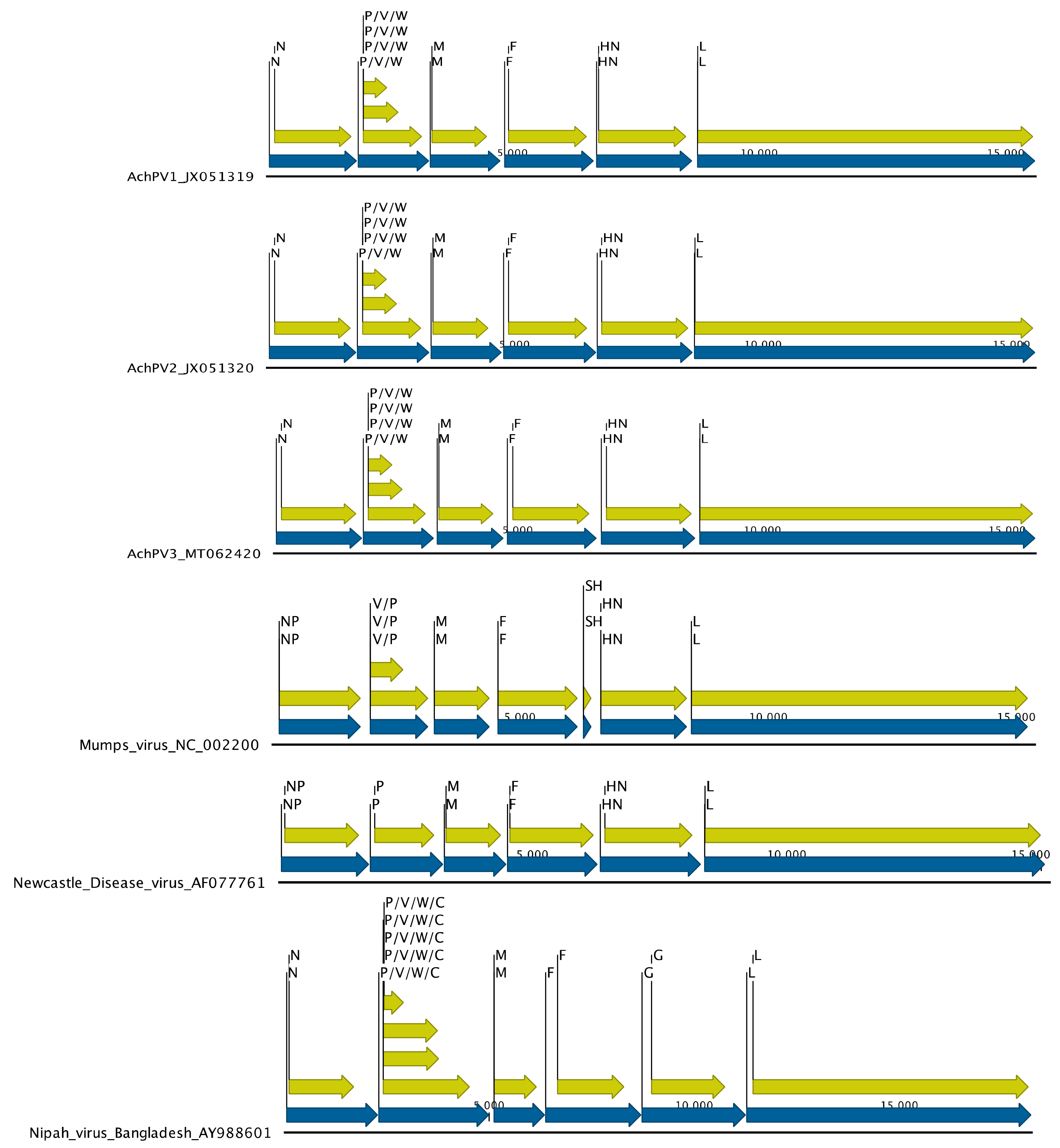
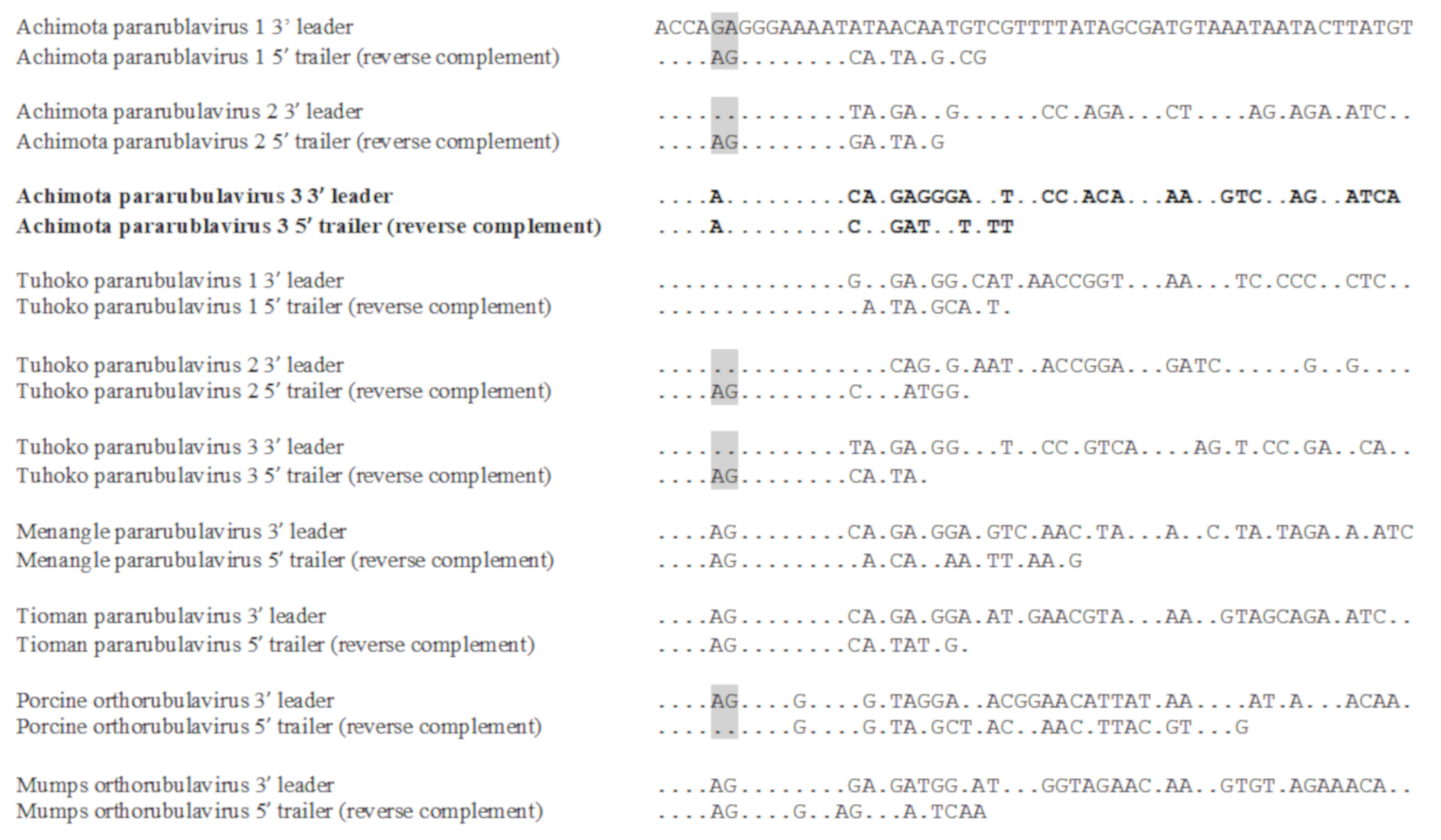
3.3. Genomic Organisation
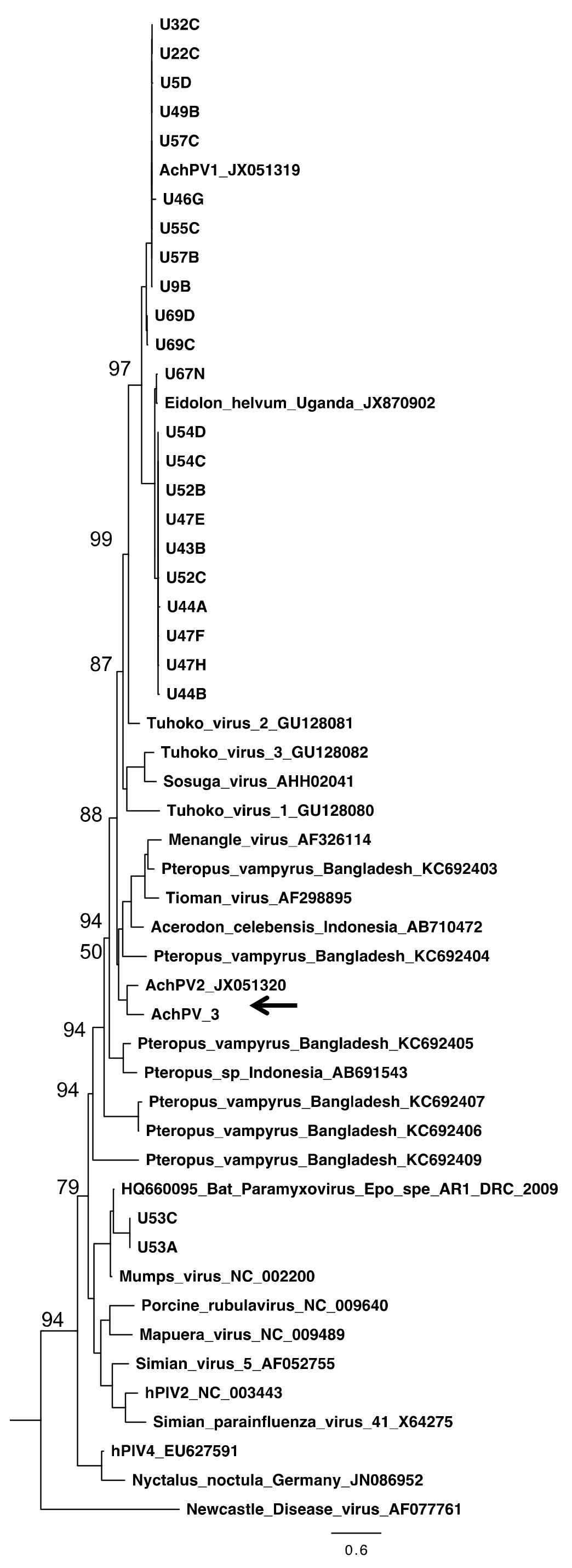
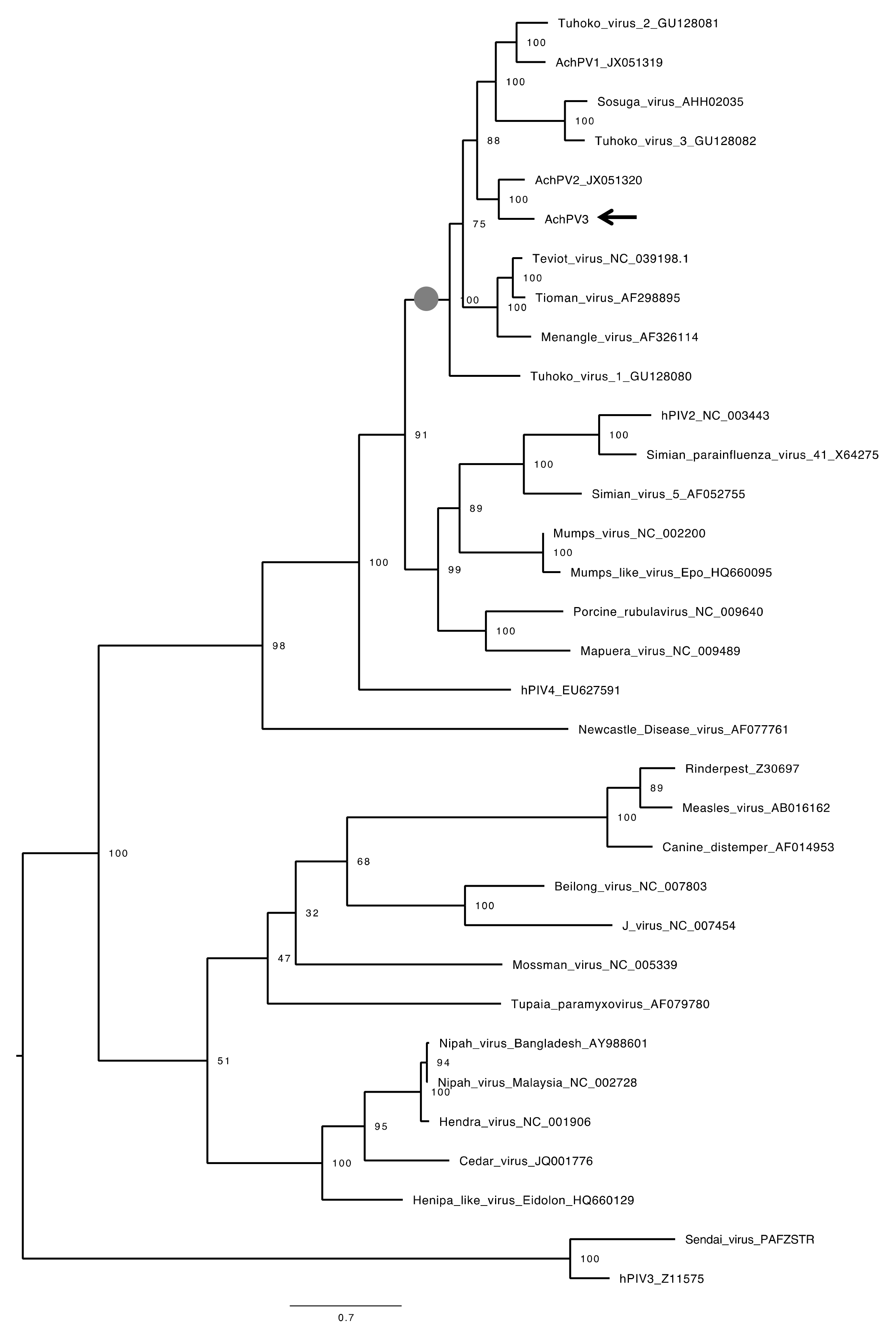
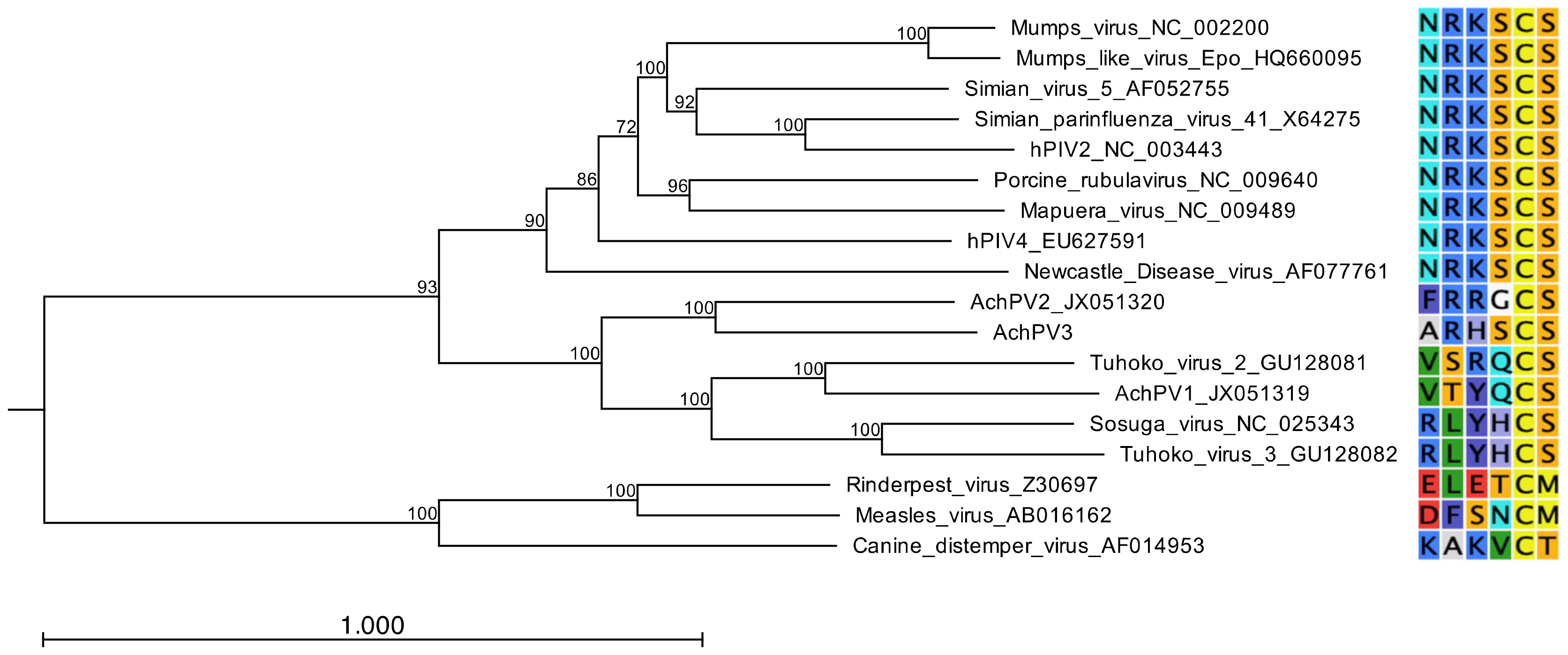
3.4. Relationship with Other Paramyxoviruses
3.5. Electron Microscopy
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Calisher, C.H.; Childs, J.E.; Field, H.E.; Holmes, K.V.; Schountz, T. Bats: Important reservoir hosts of emerging viruses. Clin. Microbiol. Rev. 2006, 19, 531–545. [Google Scholar] [CrossRef] [PubMed]
- Zhou, P.; Yang, X.L.; Wang, X.G.; Hu, B.; Zhang, L.; Zhang, W.; Si, H.R.; Zhu, Y.; Li, B.; Huang, C.L.; et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 2020, 579, 270–273. [Google Scholar] [CrossRef] [PubMed]
- Chan, J.F.; Lau, S.K.; To, K.K.; Cheng, V.C.; Woo, P.C.; Yuen, K.Y. Middle East respiratory syndrome coronavirus: Another zoonotic betacoronavirus causing SARS-like disease. Clin. Microbiol. Rev. 2015, 28, 465–522. [Google Scholar] [CrossRef] [PubMed]
- Mari Saez, A.; Weiss, S.; Nowak, K.; Lapeyre, V.; Zimmermann, F.; Dux, A.; Kühl, H.S.; Kaba, M.; Regnaut, S.; Merkel, K.; et al. Investigating the zoonotic origin of the West African Ebola epidemic. EMBO Mol. Med. 2015, 7, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Baker, K.S.; Leggett, R.M.; Bexfield, N.H.; Alston, M.; Daly, G.; Todd, S.; Tachedjian, M.; Holmes, C.E.; Crameri, S.; Wang, L.F.; et al. Metagenomic study of the viruses of African straw-coloured fruit bats: Detection of a chiropteran poxvirus and isolation of a novel adenovirus. Virology 2013, 441, 95–106. [Google Scholar] [CrossRef] [PubMed]
- Drexler, J.F.; Corman, V.M.; Muller, M.A.; Maganga, G.D.; Vallo, P.; Binger, T.; Gloza-Rausch, F.; Cottontail, V.M.; Rasche, A.; Yordanov, S.; et al. Bats host major mammalian paramyxoviruses. Nat. Commun. 2012, 3, 796. [Google Scholar] [CrossRef] [PubMed]
- Eaton, B.T.; Broder, C.C.; Middleton, D.; Wang, L.F. Hendra and Nipah viruses: Different and dangerous. Nat. Rev. Microbiol. 2006, 4, 23–35. [Google Scholar] [CrossRef]
- Philbey, A.W.; Kirkland, P.D.; Ross, A.D.; Davis, R.J.; Gleeson, A.B.; Love, R.J.; Daniels, P.W.; Gould, A.R.; Hyatt, A.D. An apparently new virus (family paramyxoviridae) infectious for pigs, humans and fruit bats. Emerg. Infect. Dis. 1998, 4, 269–271. [Google Scholar] [CrossRef]
- Barr, J.A.; Smith, C.; Marsh, G.A.; Field, H.; Wang, L.F. Evidence of bat origin for Menangle virus, a zoonotic paramyxovirus first isolated from diseased pigs. J. Gen. Virol. 2012, 93, 2590–2594. [Google Scholar] [CrossRef] [PubMed]
- Chua, K.B.; Wang, L.F.; Lam, S.K.; Crameri, G.S.; Yu, M.; Wise, T.; Boyle, D.; Hyatt, A.D.; Eaton, B.T. Tioman virus, a novel paramyxovirus isolated from fruit bats in Malaysia. Virology 2001, 283, 215–229. [Google Scholar] [CrossRef]
- Baker, K.S.; Todd, S.; Marsh, G.A.; Crameri, G.; Barr, J.; Kamins, A.O.; Peel, A.J.; Yu, M.; Hayman, D.T.; Nadjm, B.; et al. Novel, potentially zoonotic paramyxoviruses from the African straw-colored fruit bat Eidolon helvum. J. Virol. 2013, 87, 1348–1358. [Google Scholar] [CrossRef] [PubMed]
- Barr, J.; Todd, S.; Crameri, G.; Foord, A.; Marsh, G.; Frazer, L.; Payne, J.; Harper, J.; Baker, K.S.; Cunningham, A.A.; et al. Animal infection studies of two recently discovered African bat paramyxoviruses, Achimota 1 and Achimota 2. Sci. Rep. 2018, 8, 12744. [Google Scholar] [CrossRef] [PubMed]
- Johnson, R.I.; Tachedjian, M.; Clayton, B.A.; Layton, R.; Bergfeld, J.; Wang, L.F.; Marsh, G.A. Characterization of Teviot virus, an Australian bat-borne paramyxovirus. J. Gen. Virol. 2019, 100, 403–413. [Google Scholar] [CrossRef] [PubMed]
- Albarino, C.G.; Foltzer, M.; Towner, J.S.; Rowe, L.A.; Campbell, S.; Jaramillo, C.M.; Bird, B.H.; Reeder, D.M.; Vodzak, M.E.; Rota, P.; et al. Novel paramyxovirus associated with severe acute febrile disease, South Sudan and Uganda, 2012. Emerg. Infect. Dis. 2014, 20, 211–216. [Google Scholar] [CrossRef]
- Baker, K.S.; Todd, S.; Marsh, G.; Fernandez-Loras, A.; Suu-Ire, R.; Wood, J.L.N.; Wang, L.F.; Murcia, P.R.; Cunningham, A.A. Co-circulation of diverse paramyxoviruses in an urban African fruit bat population. J. Gen. Virol. 2012, 93, 850–856. [Google Scholar] [CrossRef]
- Wilkinson, D.A.; Temmam, S.; Lebarbenchon, C.; Lagadec, E.; Chotte, J.; Guillebaud, J.; Ramasindrazana, B.; Héraud, J.M.; De Lamballerie, X.; Goodman, S.M.; et al. Identification of novel paramyxoviruses in insectivorous bats of the Southwest Indian Ocean. Virus Res. 2012, 170, 159–163. [Google Scholar] [CrossRef]
- Yuan, L.; Li, M.; Li, L.; Monagin, C.; Chmura, A.A.; Schneider, B.S.; Epstein, J.H.; Mei, X.; Shi, Z.; Daszak, P.; et al. Evidence for retrovirus and paramyxovirus infection of multiple bat species in china. Viruses 2014, 6, 2138–2154. [Google Scholar] [CrossRef]
- Anthony, S.J.; Epstein, J.H.; Murray, K.A.; Navarrete-Macias, I.; Zambrana-Torrelio, C.M.; Solovyov, A.; Ojeda-Flores, R.; Arrigo, N.C.; Islam, A.; Khan, S.A.; et al. A strategy to estimate unknown viral diversity in mammals. mBio 2013, 4, e00598–e00613. [Google Scholar] [CrossRef]
- Kurth, A.; Kohl, C.; Brinkmann, A.; Ebinger, A.; Harper, J.A.; Wang, L.F.; Mühldorfer, K.; Wibbelt, G. Novel paramyxoviruses in free-ranging European bats. PLoS ONE 2012, 7, e38688. [Google Scholar] [CrossRef]
- Peel, A.J.; Sargan, D.R.; Baker, K.S.; Hayman, D.T.; Barr, J.A.; Crameri, G.; Suu-Ire, R.; Broder, C.C.; Lembo, T.; Wang, L.F.; et al. Continent-wide panmixia of an African fruit bat facilitates transmission of potentially zoonotic viruses. Nat. Commun. 2013, 4, 2770. [Google Scholar] [CrossRef]
- Lau, S.K.; Woo, P.C.; Wong, B.H.; Wong, A.Y.; Tsoi, H.W.; Wang, M.; Lee, P.; Xu, H.; Poon, R.W.; Guo, R.; et al. Identification and complete genome analysis of three novel paramyxoviruses, Tuhoko virus 1, 2 and 3, in fruit bats from China. Virology 2010, 404, 106–116. [Google Scholar] [CrossRef] [PubMed]
- Crameri, G.; Todd, S.; Grimley, S.; McEachern, J.A.; Marsh, G.A.; Smith, C.; Tachedjian, M.; De Jong, C.; Virtue, E.R.; Yu, M.; et al. Establishment, immortalisation and characterisation of pteropid bat cell lines. PLoS ONE 2009, 4, e8266. [Google Scholar] [CrossRef] [PubMed]
- Hayman, D.T.S.; McCrea, R.; Restif, O.; Suu-Ire, R.; Fooks, A.R.; Wood, J.L.N.; Cunningham, A.A.; Rowcliffe, J.M. Demography of straw-colored fruit bats in Ghana. J. Mammal. 2012, 93, 1393–1404. [Google Scholar] [CrossRef] [PubMed]
- Tong, S.; Chern, S.W.; Li, Y.; Pallansch, M.A.; Anderson, L.J. Sensitive and broadly reactive reverse transcription-PCR assays to detect novel paramyxoviruses. J. Clin. Microbiol. 2008, 46, 2652–2658. [Google Scholar] [CrossRef]
- Johnson, R.I.; Tachedjian, M.; Rowe, B.; Clayton, B.A.; Layton, R.; Bergfeld, J.; Wang, L.F.; Marsh, G.A. Alston Virus, a Novel Paramyxovirus Isolated from Bats Causes Upper Respiratory Tract Infection in Experimentally Challenged Ferrets. Viruses 2018, 10, 675. [Google Scholar] [CrossRef]
- Hayward, J.A.; Tachedjian, M.; Kohl, C.; Johnson, A.; Dearnley, M.; Jesaveluk, B.; Langer, C.; Solymosi, P.D.; Hille, G.; Nitsche, A.; et al. Infectious KoRV-related retroviruses circulating in Australian bats. Proc. Natl. Acad. Sci. USA 2020, 117, 9529–9536. [Google Scholar] [CrossRef]
- Mirza, A.M.; Deng, R.; Iorio, R.M. Site-directed mutagenesis of a conserved hexapeptide in the paramyxovirus hemagglutinin-neuraminidase glycoprotein: Effects on antigenic structure and function. J. Virol. 1994, 68, 5093–5099. [Google Scholar] [CrossRef]
- Kolakofsky, D.; Roux, L.; Garcin, D.; Ruigrok, R.W. Paramyxovirus mRNA editing, the “rule of six” and error catastrophe: A hypothesis. J. Gen. Virol. 2005, 86, 1869–1877. [Google Scholar] [CrossRef]
- Stelfox, A.J.; Bowden, T.A. A structure-based rationale for sialic acid independent host-cell entry of Sosuga virus. Proc. Natl. Acad. Sci. USA 2019, 116, 21514–21520. [Google Scholar] [CrossRef]
| Target | Primer | Sequence |
|---|---|---|
| Paramyxovirinae | Forward 1 | GAAGGITATTGTCAIAARNTNTGGAC |
| Forward 2 | GTTGCTTCAATGGTTCARGGNGAY AA | |
| Reverse | GCTGAAGTTACIGGITCICCDATRTTNC | |
| Respirovirus Morbilliovirus Henipavirus | Forward 1 | TCITTCTTTAGAACITTYGGNCAYCC |
| Forward 2 | GCCATATTTTGTGGAATAATHATHAAYGG | |
| Reverse | CTCATTTTGTAIGTCATYTTNGCRAA |
| Gene | Start | Stop | IGR Sequence Boundaries | IGR Length (nt) |
|---|---|---|---|---|
| Consensus a | gGGCCcGA | tTTTAAgAAAAAA | ||
| N | AGGCCCGA | TTTTAAGAAAAAA | GGGAAAATAAAGGT | 14 |
| P | GGGCCCGA | TTTAAGAAAAAA | TGTGAAC...TGGAAGACT | 54 |
| M | GGGCCCGA | TTTTAAGAAAAAA | TATGCCC...CACCATAGT | 63 |
| F | GGGCCCGA | TTTTAATAAAAAA | CTGAGTTCT...AATAAAGGT | 82 |
| HN | GGGCCCGA | TTTAAGAAAAAA | GTGACATTA...TGTCATACT | 75 |
| L | GGGCCAGA | TTTAAGAAAAAA | TATCTAG...5′ trailer | 21 |
| Achimota Pararubulavirus 3 | |||
|---|---|---|---|
| Genus | N | P | |
| Pararubulavirus | Achimota virus 1 | 66 | 43 |
| Achimota virus 2 | 75 | 47 | |
| Tuhoko virus 1 | 60 | 38 | |
| Tuhoko virus 2 | 66 | 39 | |
| Tuhoko virus 3 | 60 | 41 | |
| Menangle virus | 64 | 41 | |
| Tioman virus | 65 | 39 | |
| Sosuga virus | 58 | 42 | |
| Teviot virus | 77 | 29 | |
| Orthorubulavirus | Mumps virus | 51 | 24 |
| Mapuera virus | 47 | 26 | |
| Simian virus 41 | 45 | 25 | |
| Human parainfluenzavirus 2 | 44 | 25 | |
| Parainfluenzavirus 5 | 47 | 25 | |
| Porcine rubulavirus | 49 | 25 | |
| Human parainfluenzavirus 4 | 42 | 24 | |
| Morbillivirus | Rinderpest virus | 22 | 9 |
| Measles virus | 23 | 8 | |
| Canine distemper virus | 23 | 10 | |
| Henipavirus | Hendra virus | 27 | 7 |
| Nipah virus (Malaysia) | 27 | 7 | |
| Nipah virus (Bangladesh) | 27 | 8 | |
| Avulavirus | Newcastle disease virus | 31 | 20 |
| Respirovirus | Sendai virus | 19 | 5 |
| Jeilongvirus | Beilong virus | 25 | 10 |
| J-virus | 22 | 9 | |
| Narmovirus | Mossman virus | 25 | 8 |
| Tupaia paramyxovirus | 23 | 8 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baker, K.S.; Tachedjian, M.; Barr, J.; Marsh, G.A.; Todd, S.; Crameri, G.; Crameri, S.; Smith, I.; Holmes, C.E.G.; Suu-Ire, R.; et al. Achimota Pararubulavirus 3: A New Bat-Derived Paramyxovirus of the Genus Pararubulavirus. Viruses 2020, 12, 1236. https://doi.org/10.3390/v12111236
Baker KS, Tachedjian M, Barr J, Marsh GA, Todd S, Crameri G, Crameri S, Smith I, Holmes CEG, Suu-Ire R, et al. Achimota Pararubulavirus 3: A New Bat-Derived Paramyxovirus of the Genus Pararubulavirus. Viruses. 2020; 12(11):1236. https://doi.org/10.3390/v12111236
Chicago/Turabian StyleBaker, Kate S., Mary Tachedjian, Jennifer Barr, Glenn A. Marsh, Shawn Todd, Gary Crameri, Sandra Crameri, Ina Smith, Clare E.G. Holmes, Richard Suu-Ire, and et al. 2020. "Achimota Pararubulavirus 3: A New Bat-Derived Paramyxovirus of the Genus Pararubulavirus" Viruses 12, no. 11: 1236. https://doi.org/10.3390/v12111236
APA StyleBaker, K. S., Tachedjian, M., Barr, J., Marsh, G. A., Todd, S., Crameri, G., Crameri, S., Smith, I., Holmes, C. E. G., Suu-Ire, R., Fernandez-Loras, A., Cunningham, A. A., Wood, J. L. N., & Wang, L.-F. (2020). Achimota Pararubulavirus 3: A New Bat-Derived Paramyxovirus of the Genus Pararubulavirus. Viruses, 12(11), 1236. https://doi.org/10.3390/v12111236







