A Review of Avian Influenza A Virus Associations in Synanthropic Birds
Abstract
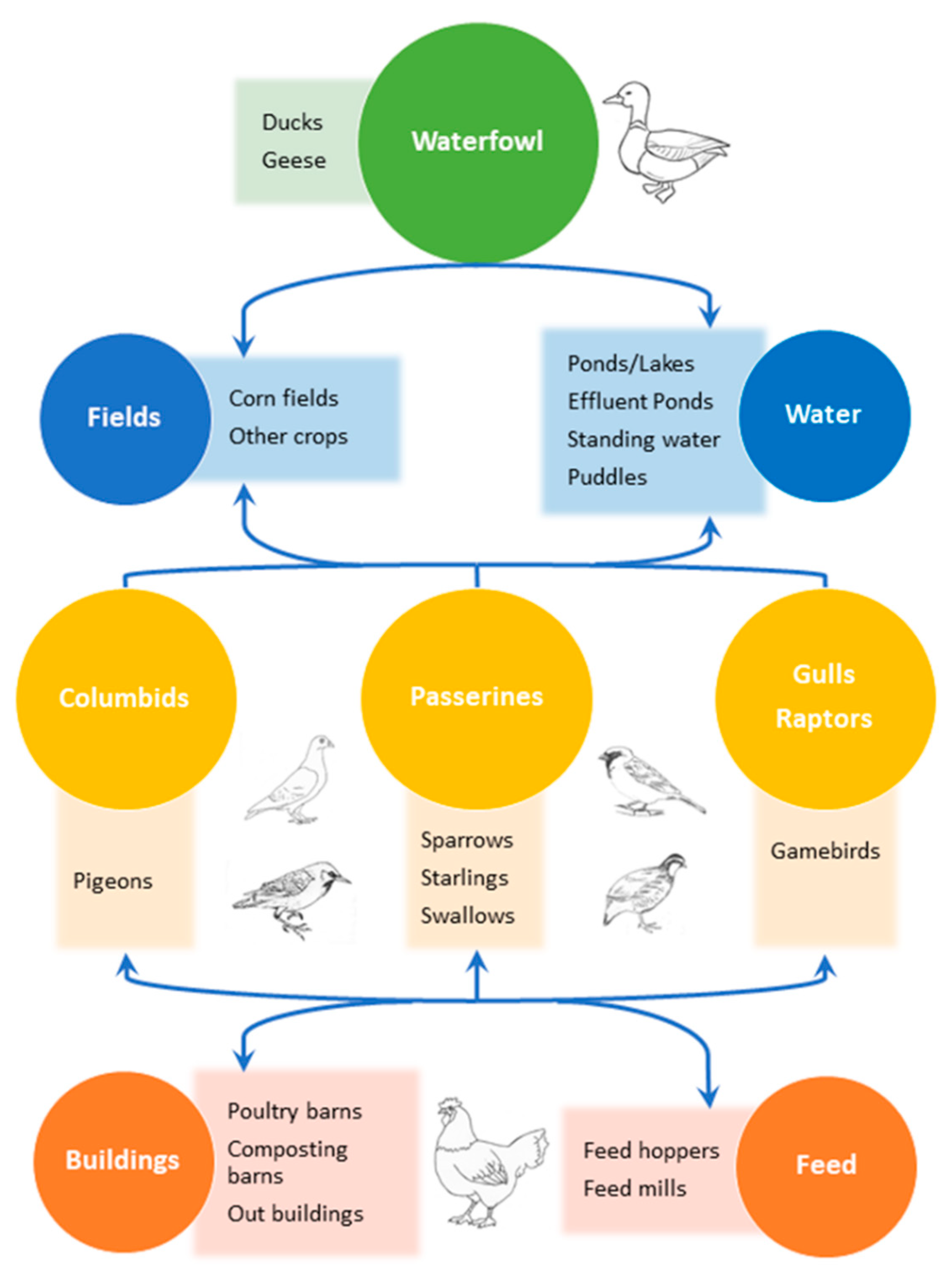
1. Introduction
2. Scavengers and Raptors: Orders Accipitriformes, Cathartiformes, Strigiformes, and Falconiformes
3. Cattle Egrets, Herons, Bitterns: Order Pelecaniformes, Family Aredeidae
4. Gulls: Order Charadriiformes, Family Laridae
5. Pheasants, Turkeys, Peafowl, Old World Quail, New World Quail: Order Galliformes
6. Pigeons, Doves: Order Columbiformes, Family Columbidae
7. Passeriformes: Thrushes, Finches, Swallows, Starlings, Sparrows
7.1. Corvidae: Crows, Ravens, Jays and Magpies
7.2. Hirundae: Swallows
7.3. Sturnidae: Starlings
7.4. Turdidae: Thrushes
7.5. Passeridae: Old World Sparrows
7.6. Fringillidae: Finches
7.7. Icteridae: Blackbirds and Grackles
8. Conclusions and Future Directions
Author Contributions
Funding
Conflicts of Interest
References
- Caron, A.; Grosbois, V.; Etter, E.; Gaidet, N.; de Garine-Wichatitsky, M. Bridge hosts for avian influenza viruses at the wildlife/domestic interface: An eco-epidemiological framework implemented in southern Africa. Prev. Vet. Med. 2014, 117, 590–600. [Google Scholar] [CrossRef] [PubMed]
- Webster, R.G.; Bean, W.J.; Gorman, O.T.; Chambers, T.M.; Kawaoka, Y. Evolution and ecology of influenza A viruses. Microbiol. Rev. 1992, 56, 152–179. [Google Scholar] [CrossRef] [PubMed]
- Röhm, C.; Zhou, N.; Süss, J.; Mackenzie, J.; Webster, R.G. Characterization of a novel influenza hemagglutinin, H15: Criteria for determination of influenza a subtypes. Virology 1996, 217, 508–516. [Google Scholar] [CrossRef] [PubMed]
- Fouchier, R.A.M.; Munster, V.; Wallensten, A.; Bestebroer, T.M.; Herfst, S.; Smith, D.; Rimmelzwaan, G.F.; Olsen, B.; Osterhaus, A.D.M.E. Characterization of a novel influenza A virus hemagglutinin subtype (H16) obtained from black-headed gulls. J. Virol. 2005, 79, 2814–2822. [Google Scholar] [CrossRef]
- Hinshaw, V.S.; Webster, R.G.; Turner, B. The perpetuation of orthomyxoviruses and paramyxoviruses in Canadian waterfowl. Can. J. Microbiol. 1980, 26, 622–629. [Google Scholar] [CrossRef]
- Alexander, D.J. A review of avian influenza in different bird species. Vet. Microbiol. 2000, 74, 3–13. [Google Scholar] [CrossRef]
- Bahl, J.; Pham, T.T.; Hill, N.J.; Hussein, I.T.M.; Ma, E.J.; Easterday, B.C.; Halpin, R.A.; Stockwell, T.B.; Wentworth, D.E.; Kayali, G.; et al. Ecosystem interactions underlie the spread of avian influenza A viruses with pandemic potential. PLoS Pathog. 2016, 12, e1005620. [Google Scholar] [CrossRef]
- Peiris, J.S.M.; De Jong, M.D.; Guan, Y. Avian influenza virus (H5N1): A threat to human health. Clin. Microbiol. Rev. 2007, 20, 243–267. [Google Scholar] [CrossRef]
- Caron, A.; Cappelle, J.; Cumming, G.S.; De Garine-Wichatitsky, M.; Gaidet, N. Bridge hosts, a missing link for disease ecology in multi-host systems. Vet. Res. 2015, 46, 83. [Google Scholar] [CrossRef]
- Kocan, A.A.; Snelling, J.; Greiner, E.C. Some infectious and parasitic diseases in Oklahoma raptors. J. Wildl. Dis. 1977, 13, 304–306. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Redig, P.T.; Goyal, S.M. Serologic evidence of exposure of raptors to influenza a virus. Avian Dis. 2012, 56, 411–413. [Google Scholar] [CrossRef] [PubMed]
- Manvell, R.J.; McKinney, P.; Wernery, U.; Frost, K. Isolation of a highly pathogenic influenza A virus of subtype H7N3 from a peregrine falcon (Falco peregrinus). Avian Pathol. 2000, 29, 635–637. [Google Scholar] [CrossRef] [PubMed]
- Couacy-Hymann, E.; Danho, T.; Keita, D.; Bodjo, S.C.; Kouakou, C.; Koffi, Y.M.; Beudje, F.; Tripodi, A.; De Benedictis, P.; Cattoli, G. The first specific detection of a highly pathogenic avian influenza virus (H5N1) in Ivory Coast. Zoonoses Public Health 2009, 56, 10–15. [Google Scholar] [CrossRef] [PubMed]
- Shivakoti, S.; Ito, H.; Otsuki, K.; Ito, T. Characterization of H5N1 highly pathogenic avian influenza virus isolated from a mountain hawk eagle in Japan. J. Vet. Med. Sci. 2010, 72, 459–463. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Goyal, S.M.; Jindal, N.; Chander, Y.; Ramakrishnan, M.A.; Redig, P.T.; Sreevatsan, S. Isolation of mixed subtypes of influenza A virus from a bald eagle (Haliaeetus leucocephalus). Virol. J. 2010, 7, 1–4. [Google Scholar] [CrossRef]
- Choi, J.G.; Kang, H.M.; Jeon, W.J.; Choi, K.S.; Kim, K.I.; Song, B.M.; Lee, H.S.; Kim, J.H.; Lee, Y.J. Characterization of clade 2.3.2.1 H5N1 highly pathogenic avian influenza viruses isolated from wild birds (mandarin duck and Eurasian eagle owl) in 2010 in Korea. Viruses 2013, 5, 1153–1174. [Google Scholar] [CrossRef]
- Van Den Brand, J.M.A.; Krone, O.; Wolf, P.U.; Van De Bildt, M.W.G.; Van Amerongen, G.; Osterhaus, A.D.M.E.; Kuiken, T. Host-specific exposure and fatal neurologic disease in wild raptors from highly pathogenic avian influenza virus H5N1 during the 2006 outbreak in Germany. Vet. Res. 2015, 46, 24. [Google Scholar] [CrossRef]
- Jennelle, C.S.; Carstensen, M.; Hildebrand, E.C.; Cornicelli, L.; Wolf, P.; Grear, D.A.; Ip, H.S.; Vandalen, K.K.; Minicucci, L.A. Surveillance for highly pathogenic avian influenza virus in wild birds during outbreaks in domestic poultry, Minnesota, USA, 2015. Emerg. Infect. Dis. 2016, 22, 1278–1282. [Google Scholar] [CrossRef]
- Globig, A.; Staubach, C.; Sauter-Louis, C.; Dietze, K.; Homeier-Bachmann, T.; Probst, C.; Gethmann, J.; Depner, K.R.; Grund, C.; Harder, T.C.; et al. Highly pathogenic avian influenza H5N8 clade 2.3.4.4b in Germany in 2016/2017. Front. Vet. Sci. 2018, 4, 240. [Google Scholar] [CrossRef]
- Shearn-Bochsler, V.I.; Knowles, S.; Ip, H. Lethal infection of wild raptors with highly pathogenic avian influenza H5N8 and H5N2 viruses in the USA, 2014–2015. J. Wildl. Dis. 2019, 55, 164–168. [Google Scholar] [CrossRef]
- Römer, A.; Fiedler, W. Contacts between wild birds and domestic poultry—A serious factor in transmission of avian influenza? Vogelwarte 2011, 49, 149–161. [Google Scholar]
- Elbers, A.R.W.; Gonzales, J.L. Quantification of visits of wild fauna to a commercial free-range layer farm in the Netherlands located in an avian influenza hot-spot area assessed by video-camera monitoring. Transbound. Emerg. Dis. 2020, 67, 661–677. [Google Scholar] [CrossRef]
- Hall, J.S.; Ip, H.S.; Franson, J.C.; Meteyer, C.; Nashold, S.; TeSlaa, J.L.; French, J.; Redig, P.; Brand, C. Experimental infection of a North American raptor, American kestrel (Falco sparverius), with highly pathogenic avian influenza virus (H5N1). PLoS ONE 2009, 4, e7555. [Google Scholar] [CrossRef] [PubMed]
- Uno, Y.; Soda, K.; Tomioka, Y.; Ito, T.; Usui, T.; Yamaguchi, T. Pathogenicity of clade 2.3.2.1 H5N1 highly pathogenic avian influenza virus in American kestrel (Falco sparverius). Avian Pathol. 2020, 49, 515–525. [Google Scholar] [CrossRef] [PubMed]
- Lierz, M.; Hafez, H.M.; Klopfleisch, R.; Luschow, D.; Prusas, C.; Teifke, J.P.; Rudolf, M.; Grund, C.; Kalthoff, D.; Mettenleiter, T.; et al. Protection and virus shedding of falcons vaccinated against highly pathogenic avian influenza A virus (H5N1). Emerg. Infect. Dis. 2007, 13, 1667–1674. [Google Scholar] [CrossRef]
- Bertran, K.; Busquets, N.; Abad, F.X.; de la Fuente, G.J.; Solanes, D.; Cordón, I.; Costa, T.; Dolz, R.; Majó, N. Highly (H5N1) and low (H7N2) pathogenic avian influenza virus infection in falcons via nasochoanal route and ingestion of experimentally infected prey. PLoS ONE 2012, 7, e32107. [Google Scholar] [CrossRef] [PubMed]
- Telfair II, R.C. Cattle Egret (Bubulcus ibis). In Birds of the World; Billerman, S.M., Ed.; Cornell Lab of Ornithology: Ithaca, NY, USA, 2020. [Google Scholar]
- Thompson, P.N.; Sinclair, M.; Ganzevoort, B. Risk factors for seropositivity to H5 avian influenza virus in ostrich farms in the Western Cape Province, South Africa. Prev. Vet. Med. 2008, 86, 139–152. [Google Scholar] [CrossRef]
- Siembieda, J.L.; Johnson, C.K.; Cardona, C.; Anchell, N.; Dao, N.; Reisen, W.; Boyce, W. Influenza A viruses in wild birds of the Pacific flyway, 2005–2008. Vector-Borne Zoonotic Dis. 2010, 10, 793–800. [Google Scholar] [CrossRef]
- Ahmed, S.S.U.; Ersbøll, A.K.; Biswas, P.K.; Christensen, J.P.; Hannan, A.S.M.A.; Toft, N. Ecological determinants of highly pathogenic avian influenza (H5N1) outbreaks in Bangladesh. PLoS ONE 2012, 7, e33938. [Google Scholar] [CrossRef]
- Bárbara, A.; Torrontegi, O.; Camacho, M.C.; Barral, M.; Hernández, J.M.; Höfle, U. Avian influenza virus surveillance in south-central Spain using fecal samples of aquatic birds foraging at landfills. Front. Vet. Sci. 2017, 4, 178. [Google Scholar] [CrossRef]
- Epstein, J.H.; McKee, J.; Shaw, P.; Hicks, V.; Micalizzi, G.; Daszak, P.; Kilpatrick, A.M.; Kaufman, G. The Australian white ibis (Threskiornis molucca) as a reservoir of zoonotic and livestock pathogens. EcoHealth 2006, 3, 290–298. [Google Scholar] [CrossRef]
- Niqueux, E.; Guionie, O.; Schmitz, A.; Hars, J.; Jestin, V. Presence of serum antibodies to influenza a subtypes H5 and N1 in swans and ibises in French wetlands, irrespective of highly pathogenic H5N1 natural infection. Avian Dis. 2010, 54, 502–508. [Google Scholar] [CrossRef] [PubMed]
- Abolnik, C.; Olivier, A.; Reynolds, C.; Henry, D.; Cumming, G.; Rauff, D.; Romito, M.; Petty, D.; Falch, C. Susceptibility and status of avian influenza in ostriches. Avian Dis. 2016, 60, 286–295. [Google Scholar] [CrossRef]
- Valdez-Gómez, H.E.; Navarro-López, R.; Vázquez-Mendoza, L.F.; Zalapa-Hernández, M.; Guerrero-Hernández, I.; Fonseca-Delgado, V.; Márquez-Ruiz, M.; Afonso, C.L. Risk factors for the transmission of infectious diseases agents at the wild birds-commercial birds interface. A pilot study in the region of the Altos de Jalisco, Mexico. Bull. Acad. Vet. Fr. 2017, 170, 142–150. [Google Scholar] [CrossRef]
- Bahnson, C.S.; Hernandez, S.M.; Poulson, R.L.; Cooper, R.E.; Curry, S.E.; Ellison, T.J.; Adams, H.C.; Welch, C.N.; Stallknecht, D.E. Experimental infections and serology indicate that American white IBIS (Eudociumus albus) are competent reservoirs for type a influenza virus. J. Wildl. Dis. 2020, 56, 530–537. [Google Scholar] [CrossRef]
- Swayne, D.E.; Suarez, D.L. Highly pathogenic avian influenza. OIE Rev. Sci. Tech. 2000, 19, 463–482. [Google Scholar] [CrossRef] [PubMed]
- Verhagen, J.; Majoor, F.; Lexmond, P.; Vuong, O.; Kasemir, G.; Lutterop, D.; Osterhaus, A.D.M.E.; Fouchier, R.A.M.; Kuiken, T. Epidemiology of influenza A Virus among black-headed gulls, the Netherlands, 2006–2010. Emerg. Infect. Dis. 2014, 20, 138. [Google Scholar] [CrossRef]
- Froberg, T.; Cuthbert, F.; Jennelle, C.S.; Cardona, C.; Culhane, M. Avian influenza prevalence and viral shedding routes in Minnesota ring-billed gulls (Larus delawarensis). Avian Dis. 2019, 63, 120–125. [Google Scholar] [CrossRef]
- Velarde, R.; Calvin, S.E.; Ojkic, D.; Barker, I.K.; Nagy, É. Avian influenza virus H13 circulating in ring-billed gulls (Larus delawarensis) in Southern Ontario, Canada. Avian Dis. 2010, 54, 411–419. [Google Scholar] [CrossRef]
- Brown, J.; Poulson, R.; Carter, D.; Lebarbenchon, C.; Pantin-Jackwood, M.; Spackman, E.; Shepherd, E.; Killian, M.; Stallknecht, D. Susceptibility of avian species to North American H13 low pathogenic avian influenza viruses. Avian Dis. 2012, 56, 969–975. [Google Scholar] [CrossRef]
- Guinn, K.; Fojtik, A.; Davis-Fields, N.; Poulson, R.L.; Krauss, S.; Webster, R.G.; Stallknecht, D.E. Antibodies to influenza a viruses in gulls at Delaware Bay, USA. Avian Dis. 2016, 60, 341–345. [Google Scholar] [CrossRef]
- Ellis, T.M.; Bousfield, R.B.; Bissett, L.A.; Dyrting, K.C.; Luk, G.S.M.; Tsim, S.T.; Sturm-Ramirez, K.; Webster, R.G.; Guan, Y.; Peiris, J.S.M. Investigation of outbreaks of highly pathogenic H5N1 avian influenza in waterfowl and wild birds in Hong Kong in late 2002. Avian Pathol. 2004, 33, 492–505. [Google Scholar] [CrossRef] [PubMed]
- Wood, J.M.; Webster, R.G.; Nettles, V.F. Host range of A/Chicken/Pennsylvania/83 (H5N2) influenza virus. Avian Dis. 1985, 29, 198–207. [Google Scholar] [CrossRef]
- Mathieu, C.; Moreno, V.; Pedersen, J.; Jeria, J.; Agredo, M.; Gutiérrez, C.; García, A.; Vásquez, M.; Avalos, P.; Retamal, P. Avian influenza in wild birds from Chile, 2007–2009. Virus Res. 2015, 199, 42–45. [Google Scholar] [CrossRef]
- De Marco, M.A.; Campitelli, L.; Delogu, M.; Raffini, E.; Foni, E.; Di Trani, L.; Scaffidi, M.; Donatelli, I. Serological evidences showing the involvement of free-living pheasants in the influenza ecology. Ital. J. Anim. Sci. 2005, 4, 287–291. [Google Scholar] [CrossRef]
- Ferro, P.J.; Khan, O.; Vuong, C.; Reddy, S.M.; Lacoste, L.; Rollins, D.; Lupiani, B. Avian influenza virus investigation in wild bobwhite quail from Texas. Avian Dis. 2012, 56, 858–860. [Google Scholar] [CrossRef]
- Webby, R.J.; Webster, R.G.; Richt, J.A. Influenza viruses in animal wildlife populations. Curr. Top. Microbiol. Immunol. 2007, 315, 67–83. [Google Scholar] [PubMed]
- Rocke, T.E.; Yuill, T.M. Microbial infections in a declining wild turkey population in Texas. J. Wildl. Manag. 1987, 51, 778–782. [Google Scholar] [CrossRef]
- Davidson, W.R.; Yoder, H.W.; Brugh, M.; Nettles, V.F. Serological monitoring of eastern wild turkeys for antibodies to Mycoplasma spp. and avian influenza viruses. J. Wildl. Dis. 1988, 24, 348–351. [Google Scholar] [CrossRef]
- Hopkins, B.A.; Skeeles, J.K.; Houghten, G.E.; Slagle, D.; Gardner, K. A survey of infectious diseases in wild turkeys from Arkansas. J. Wildl. Dis. 1990, 26, 468–472. [Google Scholar] [CrossRef] [PubMed]
- Charlton, K.G. Antibodies to selected disease agents in translocated wild turkeys in California. J. Wildl. Dis. 2000, 36, 161–164. [Google Scholar] [CrossRef] [PubMed]
- Peterson, M.J.; Aguirre, R.; Ferro, P.J.; Jones, D.A.; Lawyer, T.A.; Peterson, M.N.; Silvy, N.J. Infectious disease survey of Rio Grande wild turkeys in the Edwards Plateau of Texas. J. Wildl. Dis. 2002, 38, 826–833. [Google Scholar] [CrossRef] [PubMed]
- Ingram, D.R.; Miller, D.L.; Baldwin, C.A.; Turco, J.; Mitchell Lockhart, J. Serologic survey of wild turkeys (Meleagris gallopavo) and evidence of exposure to avian encephalomyelitis virus in Georgia and Florida, USA. J. Wildl. Dis. 2015, 51, 374–379. [Google Scholar] [CrossRef] [PubMed]
- Jennelle, C.S.; Carstensen, M.; Hildebrand, E.C.; Wolf, P.C.; Grear, D.A.; Ip, H.S.; Cornicelli, L. Surveillance for highly pathogenic avian influenza in wild turkeys (Meleagris gallopavo) of Minnesota, USA during 2015 outbreaks in domestic poultry. J. Wildl. Dis. 2017, 53, 616–620. [Google Scholar] [CrossRef]
- Macdonald, A.M.; Jardine, C.M.; Bowman, J.; Susta, L.; Nemeth, N.M. Detection of lymphoproliferative disease virus in Canada in a survey for viruses in Ontario wild Turkeys (Meleagris gallopavo). J. Wildl. Dis. 2019, 55, 113–122. [Google Scholar] [CrossRef]
- Nettles, V.F.; Wood, J.M.; Webster, R.G. Wildlife surveillance associated with an outbreak of lethal H5N2 avian influenza in domestic poultry. Avian Dis. 1985, 29, 733–741. [Google Scholar] [CrossRef]
- Abolnik, C. A current review of avian influenza in pigeons and doves (Columbidae). Vet. Microbiol. 2014, 170, 181–196. [Google Scholar] [CrossRef]
- Shriner, S.A.; Root, J.J.; Mooers, N.L.; Ellis, J.W.; Stopak, S.R.; Sullivan, H.J.; VanDalen, K.K.; Franklin, A.B. Susceptibility of rock doves to low-pathogenic avian influenza A viruses. Arch. Virol. 2016, 161, 715–720. [Google Scholar] [CrossRef]
- Uchida, Y.; Kanehira, K.; Takemae, N.; Hikono, H.; Saito, T. Susceptibility of chickens, quail, and pigeons to an H7N9 human influenza virus and subsequent egg-passaged strains. Arch. Virol. 2017, 162, 103–116. [Google Scholar] [CrossRef]
- Kalthoff, D.; Bogs, J.; Grund, C.; Tauscher, K.; Teifke, J.P.; Starick, E.; Harder, T.; Beer, M. Avian influenza H7N9/13 and H7N7/13: A comparative virulence study in chickens, pigeons, and ferrets. J. Virol. 2014, 88, 9153–9165. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, Z.; Wang, X.; Chen, J.; Yao, J.; Song, Y.; Lin, J.; Han, C.; Duan, H.; Zhao, J.; et al. Pigeons are resistant to experimental infection with H7N9 avian influenza virus. Avian Pathol. 2015, 44, 342–346. [Google Scholar] [CrossRef] [PubMed]
- Bosco-Lauth, A.M.; Bowen, R.A.; Root, J.J. Limited transmission of emergent H7N9 influenza a virus in a simulated live animal market: Do chickens pose the principal transmission threat? Virology 2016, 495, 161–166. [Google Scholar] [CrossRef]
- Elgendy, E.M.; Watanabe, Y.; Daidoji, T.; Arai, Y.; Ikuta, K.; Ibrahim, M.S.; Nakaya, T. Genetic characterization of highly pathogenic avian influenza H5N1 viruses isolated from naturally infected pigeons in Egypt. Virus Genes 2016, 52, 867–871. [Google Scholar] [CrossRef] [PubMed]
- Kwon, J.H.; Noh, Y.K.; Lee, D.H.; Yuk, S.S.; Erdene-Ochir, T.O.; Noh, J.Y.; Hong, W.T.; Jeong, J.H.; Jeong, S.; Gwon, G.B.; et al. Experimental infection with highly pathogenic H5N8 avian influenza viruses in the Mandarin duck (Aix galericulata) and domestic pigeon (Columba livia domestica). Vet. Microbiol. 2017, 203, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Bosco-Lauth, A.M.; Marlenee, N.L.; Hartwig, A.E.; Bowen, R.A.; Root, J.J. Shedding of clade 2.3.4.4 H5N8 and H5N2 highly pathogenic avian influenza viruses in peridomestic wild birds in the U.S. Transbound. Emerg. Dis. 2019, 66, 1301–1305. [Google Scholar] [CrossRef]
- Liu, K.; Gao, R.; Wang, X.; Han, W.; Ji, Z.; Zheng, H.; Gu, M.; Hu, J.; Liu, X.; Hu, S.; et al. Pathogenicity and transmissibility of clade 2.3.4.4 highly pathogenic avian influenza virus subtype H5N6 in pigeons. Vet. Microbiol. 2020, 247. [Google Scholar] [CrossRef]
- Winkler, D.W.; Billerman, S.M.; Lovette, I.J. Corvidae; Cornell Lab of Ornithology: Ithaca, NY, USA, 2020. [Google Scholar]
- Burns, T.E.; Ribble, C.; Stephen, C.; Kelton, D.; Toews, L.; Osterhold, J.; Wheeler, H. Use of observed wild bird activity on poultry farms and a literature review to target species as high priority for avian influenza testing in 2 regions of Canada. Can. Vet. J. 2012, 53, 158–166. [Google Scholar]
- Boender, G.J.; Hagenaars, T.J.; Bouma, A.; Nodelijk, G.; Elbers, A.R.W.; De Jong, M.C.M.; Van Boven, M. Risk maps for the spread of highly pathogenic avian influenza in poultry. PLoS Comput. Biol. 2007, 3, 704–712. [Google Scholar] [CrossRef]
- Biswas, P.K.; Rahman, M.H.; Das, A.; Ahmed, S.S.U.; Giasuddin, M.; Christensen, J.P. Risk for highly pathogenic avian influenza H5N1 virus infection in chickens in small-scale commercial farms, in a high-risk area, Bangladesh, 2008. Transbound. Emerg. Dis. 2011, 58, 519–525. [Google Scholar] [CrossRef]
- Mase, M.; Tsukamoto, K.; Imada, T.; Imai, K.; Tanimura, N.; Nakamura, K.; Yamamoto, Y.; Hitomi, T.; Kira, T.; Nakai, T.; et al. Characterization of H5N1 influenza a viruses isolated during the 2003–2004 influenza outbreaks in Japan. Virology 2005, 332, 167–176. [Google Scholar] [CrossRef]
- Tanimura, N.; Tsukamoto, K.; Okamatsu, M.; Mase, M.; Imada, T.; Nakamura, K.; Kubo, M.; Yamaguchi, S.; Irishio, W.; Hayashi, M.; et al. Pathology of fatal highly pathogenic H5N1 avian influenza virus infection in large-billed crows (Corvus macrorhynchos) during the 2004 outbreak in Japan. Vet. Pathol. 2006, 43, 500–509. [Google Scholar] [CrossRef] [PubMed]
- Nagarajan, S.; Tosh, C.; Murugkar, H.V.; Venkatesh, G.; Katare, M.; Jain, R.; Behera, P.; Khandia, R.; Tripathi, S.; Kulkarni, D.D.; et al. Isolation and molecular characterization of a H5N1 virus isolated from a Jungle crow (Corvus macrohynchos) in India. Virus Genes 2010, 41, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.U.; Berman, L.S.; Haider, N.; Gerloff, N.; Rahman, M.Z.; Shu, B.; Rahman, M.; Dey, T.K.; Davis, T.C.; Das, B.C.; et al. Investigating a crow die-off in January-February 2011 during the introduction of a new clade of highly pathogenic avian influenza virus H5N1 into Bangladesh. Arch. Virol. 2014, 159, 509–518. [Google Scholar] [CrossRef] [PubMed]
- Globig, A.; Starick, E.; Homeier, T.; Pohlmann, A.; Grund, C.; Wolf, P.; Zimmermann, A.; Wolf, C.; Heim, D.; Schlößer, H.; et al. Epidemiological and molecular analysis of an outbreak of highly pathogenic avian influenza H5N8 clade 2.3.4.4 in a German zoo: Effective disease control with minimal culling. Transbound. Emerg. Dis. 2017, 64, 1813–1824. [Google Scholar] [CrossRef]
- Ferrazzi, V.; Martin, A.M.; Lelli, D.; Gallazzi, D.; Grilli, G. Microbiological and serological monitoring in hooded crow (Corvus corone cornix) in the Region Lombardia, Italy. Ital. J. Anim. Sci. 2007, 6, 309–312. [Google Scholar] [CrossRef]
- Shriner, S.A.; Root, J.J.; Lutman, M.W.; Kloft, J.M.; VanDalen, K.K.; Sullivan, H.J.; White, T.S.; Milleson, M.P.; Hairston, J.L.; Chandler, S.C.; et al. Surveillance for highly pathogenic H5 avian influenza virus in synanthropic wildlife associated with poultry farms during an acute outbreak. Sci. Rep. 2016, 6, 36237. [Google Scholar] [CrossRef]
- Caron, A.; Chiweshe, N.; Mundava, J.; Abolnik, C.; Capobianco Dondona, A.; Scacchia, M.; Gaidet, N. Avian viral pathogens in swallows, Zimbabwe: Infectious diseases in Hirundinidae: A risk to swallows? EcoHealth 2017, 14, 805–809. [Google Scholar] [CrossRef]
- Gronesova, P.; Kabat, P.; Trnka, A.; Betakova, T. Using nested RT-PCR analyses to determine the prevalence of avian influenza viruses in passerines in western Slovakia, during summer 2007. Scand. J. Infect. Dis. 2008, 40, 954–957. [Google Scholar] [CrossRef]
- Zhong, G.; Fan, S.; Lopes, T.J.S.; Le, M.Q.; Van Bakel, H.; Dutta, J.; Smith, G.J.D.; Jayakumar, J.; Nguyen, H.L.K.; Hoang, P.V.M.; et al. Isolation of highly pathogenic H5N1 influenza viruses in 2009–2013 in Vietnam. Front. Microbiol. 2019, 10, 1411. [Google Scholar] [CrossRef]
- Nestorowicz, A.; Kawaoka, Y.; Bean, W.J.; Webster, R.G. Molecular analysis of the hemagglutinin genes of Australian H7N7 influenza viruses: Role of passerine birds in maintenance or transmission? Virology 1987, 160, 411–418. [Google Scholar] [CrossRef]
- Perkins, L.E.L.; Swayne, D.E. Comparative susceptibility of selected avian and mammalian species to a Hong Kong-origin H5N1 high-pathogenicity avian influenza virus. Avian Dis. 2003, 47, 956–967. [Google Scholar] [CrossRef]
- Boon, A.C.M.; Sandbulte, M.R.; Seiler, P.; Webby, R.J.; Songserm, T.; Guan, Y.; Webster, R.G. Role of terrestrial wild birds in ecology of influenza A virus (H5N1). Emerg. Infect. Dis. 2007, 13, 1720–1724. [Google Scholar] [CrossRef]
- Nemeth, N.M.; Thomas, N.O.; Orahood, D.S.; Anderson, T.D.; Oesterle, P.T. Shedding and serologic responses following primary and secondary inoculation of house sparrows (Passer domesticus) and European starlings (Sturnus vulgaris) with low-pathogenicity avian influenza virus. Avian Pathol. 2010, 39, 411–418. [Google Scholar] [CrossRef]
- Qin, Z.; Clements, T.; Wang, L.; Khatri, M.; Pillai, S.P.S.; Zhang, Y.; Lejeune, J.T.; Lee, C.W. Detection of influenza viral gene in European starlings and experimental infection. Influenza Respir. Viruses 2011, 5, 268–275. [Google Scholar] [CrossRef][Green Version]
- Hall, J.S.; Ip, H.S.; TeSlaa, J.L.; Nashold, S.W.; Dusek, R.J. Experimental challenge of a peridomestic avian species, European starlings (Sturnus vulgaris), with novel influenza a H7N9 virus from China. J. Wildl. Dis. 2016, 52, 709–712. [Google Scholar] [CrossRef]
- Root, J.J.; Bosco-Lauth, A.M.; Bielefeldt-Ohmann, H.; Bowen, R.A. Experimental infection of peridomestic mammals with emergent H7N9 (A/Anhui/1/2013) influenza A virus: Implications for biosecurity and wet markets. Virology 2016, 487, 242–248. [Google Scholar] [CrossRef] [PubMed]
- Lipkind, M.A.; Weisman, Y.; Shihmanter, E.; Shoham, D.; Douglas, A.; Skehel, J.J. Characterization of avian influenza viruses isolated in Israel in 1978–1979. Comp. Immunol. Microbiol. Infect. Dis. 1980, 3, 185–192. [Google Scholar] [CrossRef]
- Alexander, D.J. Isolation of influenza a viruses from birds in Great Britain during 1980 and 1981. Vet. Rec. 1982, 111, 319–321. [Google Scholar] [CrossRef]
- Lipkind, M.A.; Weisman, Y.; Shihmanter, E.; Shoham, D. Review of the three-year studies on the ecology of avian influenza viruses in Israel. Avian Dis. 1981, 47, 69–78. [Google Scholar]
- Stallknecht, D.E.; Shane, S.M. Host range of avian influenza virus in free-living birds. Vet. Res. Commun. 1988, 12, 125–141. [Google Scholar] [CrossRef] [PubMed]
- Morishita, T.Y.; Aye, P.P.; Ley, E.C.; Harr, B.S. Survey of pathogens and blood parasites in free-living passerines. Avian Dis. 1999, 43, 549–552. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.D.; Luttrell, M.P.; Berghaus, R.D.; Kistler, W.; Keeler, S.P.; Howey, A.; Wilcox, B.; Hall, J.; Niles, L.; Dey, A.; et al. Prevalence of antibodies to type a influenza virus in wild avian species using two serologic assays. J. Wildl. Dis. 2010, 46, 896–911. [Google Scholar] [CrossRef] [PubMed]
- Račnik, J.; Slavec, B.; Trilar, T.; Zadravec, M.; Dovč, A.; Krapež, U.; Barlič-Maganja, D.; Zorman Rojs, O. Evidence of avian influenza virus and paramyxovirus subtype 2 in wild-living passerine birds in Slovenia. Eur. J. Wildl. Res. 2008, 54, 529–532. [Google Scholar] [CrossRef]
- Lvov, D.K.; Shchelkanov, M.Y.; Prilipov, A.G.; Deryabin, P.G.; Fedyakina, I.T.; Galkina, I.V.; Kireyev, D.Y.; Frolov, A.V.; Akanina, D.S.; Usacheva, O.V.; et al. Interpretation of the epizootic outbreak among wild and domestic birds in the south of the European part of Russia in December 2007. Vopr. Virusol. 2008, 53, 18–23. [Google Scholar]
- Al-Attar, M.Y.; Damal, F.A.; Al-Baroodi, S.Y. Detection of antibodies against avian influenza virus in wild pigeons and starlings. J. Anim. Vet. Adv. 2008, 7, 448–449. [Google Scholar]
- Pearson, H.E.; Lapidge, S.J.; Hernández-Jover, M.; Toribio, J.A.L.M.L. Pathogen presence in European starlings inhabiting commercial piggeries in South Australia. Avian Dis. 2016, 60, 430–436. [Google Scholar] [CrossRef]
- Houston, D.D.; Azeem, S.; Lundy, C.W.; Sato, Y.; Guo, B.; Blanchong, J.A.; Gauger, P.C.; Marks, D.R.; Yoon, K.J.; Adelman, J.S. Evaluating the role of wild songbirds or rodents in spreading avian influenza virus across an agricultural landscape. PeerJ 2017, 5, e4060. [Google Scholar] [CrossRef]
- Fuller, T.L.; Saatchi, S.S.; Curd, E.E.; Toffelmier, E.; Thomassen, H.A.; Buermann, W.; DeSante, D.F.; Nott, M.P.; Saracco, J.F.; Ralph, C.J.; et al. Mapping the risk of avian influenza in wild birds in the US. BMC Infect. Dis. 2009, 10, 187. [Google Scholar] [CrossRef]
- Root, J.J.; Bosco-Lauth, A.M.; Marlenee, N.L.; Bowen, R.A. Viral shedding of clade 2.3.4.4 H5 highly pathogenic avian influenza A viruses by American robins. Transbound. Emerg. Dis. 2018, 65, 1823–1827. [Google Scholar] [CrossRef]
- Fujimoto, Y.; Ito, H.; Shinya, K.; Yamaguchi, T.; Usui, T.; Murase, T.; Ozaki, H.; Ono, E.; Takakuwa, H.; Otsuki, K.; et al. Susceptibility of two species of wild terrestrial birds to infection with a highly pathogenic avian influenza virus of H5N1 subtype. Avian Pathol. 2010, 39, 95–98. [Google Scholar] [CrossRef]
- Guimarães, M.B.; Hurtado, R.; Bello, C.P.; Vanstreels, R.E.T.; Ferreira, A.J.P. Surveillance for newcastle disease virus, avian influenza virus and mycoplasma gallisepticum in wild birds near commercial poultry farms surrounded by Atlantic rainforest remnants, southeastern Brazil. Rev. Bras. Cienc. Avic. 2016, 18, 387–394. [Google Scholar] [CrossRef]
- Brown, J.D.; Stallknecht, D.E.; Berghaus, R.D.; Swayne, D.E. Infectious and lethal doses of H5N1 highly pathogenic avian influenza virus for house sparrows (Passer domesticus) and rock pigeons (Columbia livia). J. Vet. Diagn. Investig. 2009, 21, 437–445. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Hou, G.; Jiang, W.; Han, C.; Liu, S.; Chen, J.; Li, J.; Zhang, P.; Huang, B.; Liu, Y. A survey of avian influenza in tree sparrows in China in 2011. PLoS ONE 2012, 7, e33092. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Ma, J.; Kou, Z.; Pu, J.; Lei, F.; Li, T.; Liu, J. Characterization of a highly pathogenic avian influenza H5N1 clade 2.3.4 virus isolated from a tree sparrow. Virus Res. 2010, 147, 25–29. [Google Scholar] [CrossRef]
- Kou, Z.; Lei, F.M.; Yu, J.; Fan, Z.J.; Yin, Z.H.; Jia, C.X.; Xiong, K.J.; Sun, Y.H.; Zhang, X.W.; Wu, X.M.; et al. New genotype of avian influenza H5N1 viruses isolated from tree sparrows in China. J. Virol. 2005, 79, 15460–15466. [Google Scholar] [CrossRef]
- Amonsin, A.; Choatrakol, C.; Lapkuntod, J.; Tantilertcharoen, R.; Thanawongnuwech, R.; Suradhat, S.; Suwannakarn, K.; Theamboonlers, A.; Poovorawan, Y. Influenza virus (H5N1) in live bird markets and food markets, Thailand. Emerg. Infect. Dis. 2008, 14, 1739–1742. [Google Scholar] [CrossRef]
- Poetranto, E.D.; Yamaoka, M.; Nastri, A.M.; Krisna, L.A.W.; Rahman, M.H.; Wulandari, L.; Yudhawati, R.; Ginting, T.E.; Makino, A.; Shinya, K.; et al. An H5N1 highly pathogenic avian influenza virus isolated from a local tree sparrow in Indonesia. Microbiol. Immunol. 2011, 55, 666–672. [Google Scholar] [CrossRef]
- Zhao, B.; Zhang, X.; Zhu, W.; Teng, Z.; Yu, X.; Gao, Y.; Wu, D.; Pei, E.; Yuan, Z.; Yang, L.; et al. Novel avian influenza A (H7N9) virus in tree sparrow, Shanghai, China, 2013. Emerg. Infect. Dis. 2014, 20, 850. [Google Scholar] [CrossRef]
- Su, S.; Xing, G.; Wang, J.; Li, Z.; Gu, J.; Yan, L.; Lei, J.; Ji, S.; Hu, B.; Gray, G.C.; et al. Characterization of H7N2 avian influenza virus in wild birds and pikas in Qinghai-Tibet Plateau area. Sci. Rep. 2016, 6, 30974. [Google Scholar] [CrossRef]
- Cerda-Armijo, C.; De León, M.B.; Ruvalcaba-Ortega, I.; Chablé-Santos, J.; Canales-Del-Castillo, R.; Peñuelas-Urquides, K.; Rivera-Morales, L.G.; Menchaca-Rodríguez, G.; Camacho-Moll, M.E.; Contreras-Cordero, J.F.; et al. High prevalence of avian influenza virus among wild waterbirds and land birds of Mexico. Avian Dis. 2020, 64, 135–142. [Google Scholar] [CrossRef]
- Gutiérrez, R.A.; Sorn, S.; Nicholls, J.M.; Buchy, P. Eurasian tree sparrows, risk for H5N1 virus spread and human contamination through Buddhist ritual: An experimental approach. PLoS ONE 2011, 6, e28609. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, Y.; Nakamura, K.; Yamada, M.; Mase, M. Pathogenesis in Eurasian tree sparrows inoculated with H5N1 highly pathogenic avian influenza virus and experimental virus transmission from tree sparrows to chickens. Avian Dis. 2013, 57, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Forrest, H.L.; Kim, J.K.; Webster, R.G. Virus shedding and potential for interspecies waterborne transmission of highly pathogenic H5N1 influenza virus in sparrows and chickens. J. Virol. 2010, 84, 3718–3720. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, M.; Yaqub, T.; Mukhtar, N.; Shabbir, M.Z.; McCauley, J.W. Infectivity and transmissibility of H9N2 avian influenza virus in chickens and wild terrestrial birds. Vet. Res. 2013, 44, 100. [Google Scholar] [CrossRef]
- Jones, J.C.; Sonnberg, S.; Koçer, Z.A.; Shanmuganatham, K.; Seiler, P.; Shu, Y.; Zhu, H.; Guan, Y.; Peiris, M.; Webby, R.J.; et al. Possible role of songbirds and parakeets in transmission of influenza A(H7N9) virus to humans. Emerg. Infect. Dis. 2014, 20, 380–385. [Google Scholar] [CrossRef]
- Schnebel, B.; Dierschke, V.; Rautenschlein, S.; Ryll, M.; Neumann, U. Investigations on infection status with H5 and H7 avian influenza virus in short-distance and long-distance migrant birds in 2001. Avian Dis. 2007, 51, 432–433. [Google Scholar] [CrossRef]
- Achenbach, J.E.; Bowen, R.A. Transmission of Avian Influenza A Viruses among Species in an Artificial Barnyard. PLoS ONE 2011, 6, e17643. [Google Scholar] [CrossRef]
| Location | Sampling Year(s) | Serosurvey (N Sampled) | Serosurvey (N Positive) | Virus/RNA Detection (N Sampled) | Virus/RNA Detection (N Positive) | Citation |
|---|---|---|---|---|---|---|
| PA, US * | 1983–1984 | 7 | 0 | -- | -- | [50] |
| VA, US * | 1983–1984 | -- | -- | 62 | 0 | [50] |
| TX, US | 1983–1985 | 440 | 0 | 511 | 0 | [42] |
| USA | 1981–1986 | 210 | 0 | -- | -- | [43] |
| AR, US | 1986 | 44 | 0 | -- | -- | [44] |
| CA, US | 1986–1996 | 383 | 1 | -- | -- | [45] |
| TX, US | 2001 | 70 | 0 | -- | -- | [46] |
| GA, FL, US | 2005–2008 | 19 | 0 | -- | -- | [47] |
| MN, US | 2015 | -- | -- | 84 | 0 | [48] |
| Ontario, Canada | 2011–13, 2015 | -- | -- | 207 | 0 | [49] |
| Totals | 1173 | 1 (0.09%) | 864 | 0 |
| Location | Sampling Year(s) | Serosurvey (N Sampled) | Serosurvey (N Positive) | Virus/RNA Detection (N Sampled) | Virus/RNA Detection (N Positive) | Citation |
|---|---|---|---|---|---|---|
| Israel | 1978–1979 | -- | -- | 42 | 1 H1 | [89] |
| Great Britain | 1981 | -- | -- | ? | 1 H7 | [90] |
| Israel | 1981 | -- | -- | 282 | 1 | [91,92] |
| Australia | 1985 | -- | -- | <208 | 1 H7N7 | [82] |
| Ohio US | 1988? | 868 | 0 | -- | -- | [93] |
| Georgia US | 1999 | 15 | 0 | -- | -- | [94] |
| Slovenia | 2004 | -- | -- | 670 | 1 | [95] |
| Russia | 2007 | -- | -- | 5 | 1 | [96] |
| Iraq | 2007 | 60 | 0 | -- | -- | [97] |
| Ohio US | 2007–2008 | -- | -- | 328 | 21 | [86] |
| Australia | 2008–2009 | -- | -- | 50 | 0 | [98] |
| Iowa US | 2015 | 69 | 6 | 69 | 1 | [78] |
| Iowa US | 2015–2016 | 5 | 0 | 5 | 0 | [99] |
| Totals | 1032 | 6 (0.58%) | 1451 | 26 (1.79%) |
| Location and Species | Sampling Year(s) | Serosurvey (N Sampled) | Serosurvey (N Positive) | Virus/RNA Detection (N Sampled) | Virus/RNA Detection (N Positive) | Citation |
|---|---|---|---|---|---|---|
| Australia | 1985 | ? | 1 H7N7 | -- | -- | [82] |
| Hong Kong, tree sparrow | 2002 | -- | -- | 1 | 1 H5N1 | [43] |
| China, tree sparrow | 2004 | -- | -- | 38 | 4 | [107] |
| Thailand, house sparrow | 2004–2008 | -- | -- | 118 | 0 | [108] |
| China, tree sparrow | 2008 | -- | -- | 68 | 1 H5N1 | [106] |
| California US, house sparrow | 2005–2008 | -- | -- | 77 | 1 | [29] |
| China, tree sparrow | 2011 | 800 | 94 | 1300 | 0 | [105] |
| Indonesia, tree sparrow | 2010 | -- | -- | 1 | 1 | [109] |
| China, tree sparrow | 2013 | -- | -- | ? | 1 | [110] |
| China, tree sparrow | 2006–2009 | -- | -- | ? | 4 | [111] |
| Ohio US, house sparrow | -- | -- | 373 | 0 | [93] | |
| Iowa US, house sparrow | 2015–2016 | 44 | 0 | 44 | 0 | [99] |
| Mexico, house sparrow | 2010–2012 | -- | -- | 9 | 5 | [112] |
| Totals | 844 | 94 (11.14%) | 2029 | 13 (0.64%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shriner, S.A.; Root, J.J. A Review of Avian Influenza A Virus Associations in Synanthropic Birds. Viruses 2020, 12, 1209. https://doi.org/10.3390/v12111209
Shriner SA, Root JJ. A Review of Avian Influenza A Virus Associations in Synanthropic Birds. Viruses. 2020; 12(11):1209. https://doi.org/10.3390/v12111209
Chicago/Turabian StyleShriner, Susan A., and J. Jeffrey Root. 2020. "A Review of Avian Influenza A Virus Associations in Synanthropic Birds" Viruses 12, no. 11: 1209. https://doi.org/10.3390/v12111209
APA StyleShriner, S. A., & Root, J. J. (2020). A Review of Avian Influenza A Virus Associations in Synanthropic Birds. Viruses, 12(11), 1209. https://doi.org/10.3390/v12111209





